Abstract
Background and objectives: Health-related quality of life (HRQOL) after acute kidney injury (AKI) is an area of great importance to patients. It was hypothesized that HRQOL after AKI would relate to intensity of dialysis during AKI and dialysis dependence at follow-up.
Design, setting, participants, & measurements: The Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study was a multicenter, prospective, randomized trial of intensive versus less intensive renal replacement therapy in critically ill patients with AKI. Of 1124 participants, 415 survived at least 60 days and completed the Health Utilities Index (HUI), which measures 8 health attributes and calculates an overall HRQOL score, also called a utility score. How strongly pre–intensive care unit (ICU) health, severity of illness, hospital course, intensity of dialysis, and outcome were associated with 60-day HUI scores was assessed, after adjustment for demographics.
Results: The overall HUI score was 0.40 ± 0.37, indicating severely compromised health utility and was associated with only admission from home and hospital and ICU length of stay (LOS). Ambulation was better among those with a shorter hospital and ICU LOS. Better cognition was associated with dialysis independence and with fewer comorbid chronic illnesses. Emotion was associated with only hospital LOS. Pain was associated with ICU LOS.
Conclusions: Health utility was low in this cohort of patients after AKI, and intensity of dialysis did not affect subsequent health utility. The effects of a lengthy hospitalization generally outweighed the effects of delayed recovery of kidney function on HRQOL after AKI.
A cute kidney injury (AKI) is common among hospitalized patients and is particularly prevalent among patients cared for in the intensive care unit (ICU) setting (1–3). AKI has been associated with increased morbidity, mortality, and costs (1–4). It remains unclear to what extent treatment of or recovery from AKI influences health-related quality of life in survivors of AKI. There have been several reports of health-related quality of life (HRQOL) among survivors of AKI in the ICU (5–10). However, many of these studies are limited by small sample size and low response rate. In addition, follow-up times are variable among and sometimes within studies, ranging from 3 months to several years. Several measures of HRQOL have been used, including the Medical Outcomes Study Short Form 36-item health survey (SF-36) (9), EuroQol (EQ-5D) (5), and Nottingham Health Profile (6,8,10), and also health utilities by time trade-off (7) or visual analog scale (5) and Activities of Daily Living (7,8). Perhaps because of this variability, results are mixed. On balance, limitations in mobility were fairly common, ranging from 29 to 60% (6,8). However, patients generally reported a favorable health status, with 62 to 77% of patients reporting “good” or “excellent” health status (7,10). Health utility on the EQ-5D index was 0.68 compared with an age- and sex-matched norm of 0.86 (5), but in the same study utility by visual analog scale was not different from the general population. Health utility by the time trade-off method was reported by Hamel et al. to be 0.84, but no normative data were presented (7).
The availability of HRQOL data in a large cohort of survivors of AKI requiring renal replacement therapy (RRT) provides a unique opportunity to study HRQOL and its potential determinants in this population. The Veterans Affairs/National Institutes of Health (VA/NIH) Acute Renal Failure Trial Network (ATN) study (ClinicalTrials.gov, NCT00076219) was a multicenter randomized trial of intensive versus less intensive renal replacement therapy in critically ill patients with acute kidney injury conducted between November 2003 and July 2007 at 27 VA and university-affiliated medical centers (11,12). Although the major goals of the ATN study were to assess the effects of treatment assignment on 60-day mortality, in-hospital mortality, and recovery of renal function, HRQOL was also assessed at 60 days among survivors with the intention of establishing the effect of dialysis intensity on HRQOL and of assigning health utilities to facilitate performance of cost-effectiveness analysis.
We hypothesized that study treatment assignment and ongoing dialysis dependence at 60 days would be potential determinants of HRQOL. Although intensive dialysis did not lead to shorter hospital stays or more rapid recovery of renal function (12), both of which might have contributed to improved HRQOL at 60 days, we postulated that better control of uremia could have direct effects on HRQOL. In addition, given that patients receiving maintenance dialysis routinely report impaired HRQOL (13–15), we also hypothesized that ongoing need for dialysis would be an important determinant of HRQOL at 60 days.
Materials and Methods
Study Population
The ATN study enrolled adults in critical care units who had AKI attributable to acute tubular necrosis plus sepsis or additional organ failure. Full inclusion and exclusion criteria are available elsewhere (12). Of the 1124 patients enrolled, 563 were randomized to the intensive treatment strategy and 561 to the less intensive treatment strategy. All survivors who provided analyzable data on HRQOL 60 days after study enrollment were included in this report. The ATN study was approved by the Human Rights Committee at the West Haven VA Cooperative Studies Program (CSP) Coordinating Center and by the institutional review boards at each of the participating study sites.
Measurement of Health Utility
Health utility was assessed by telephone or an in-person interview among survivors 60 days after randomization using the Health Utilities Index (HUI) Mark 3, which measures eight health attributes, including vision, hearing, speech, ambulation, dexterity, emotion, cognition, and pain and calculates an overall utility score. The HUI asks respondents specific questions about the type and extent of disabilities they have experienced over the past week (16,17). For example, questions in the ambulation attribute ascertain whether the individual can walk around his or her neighborhood without difficulty or equipment, with difficulty but without equipment, with equipment such as a cane or walker, or cannot walk. A preference-based scoring function (derived from preference measurements obtained from the general public) is then used to convert descriptive measures of disability into measures of utility for levels of ability or disability within each attribute, and a measure of overall HRQOL is derived as the product of the individual attribute scales (17). The final score thus represents the utility, or desirability, of the state described by the subject's constellation of abilities and disabilities, on a scale ranging from 0, or equivalent to death, to 1, or perfect health. The HUI has been extensively validated and has been used in numerous studies, which have included persons receiving RRT (17,18). In addition to the overall HRQOL score, we focused on four prespecified attributes that we hypothesized would be most likely to be affected by AKI: ambulation, cognition, emotion, and pain.
The HUI was administered by telephone interview in almost all cases. The 36-question version of the HUI, suitable for administration by an interviewer, was used for all respondents.
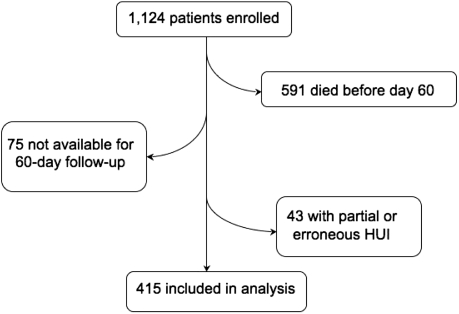
Sixty days after enrollment, 533 patients remained alive (Figure 1). Of these, 299 completed the HUI fully, 159 completed partially, and 75 submitted no form. Twenty percent (82) were completed by a surrogate on behalf of the participant. Where possible, we used inspection and logical deduction to complete missing data elements following the method of Naiem, Keeler, and Mangione (n = 38) (19). Hot-deck imputation was used to impute values in most cases where fewer than four subscales were missing (n = 78), unless too much data in a single subscale were missing to enable reasonable imputation. HUI forms with four or more missing subscales were also treated as unusable (overall n = 43 unusable). Following the imputations there were HUI scores for 415 people, or 78% of survivors to day 60.
Figure 1.
Study enrollment diagram.
Explanatory Variables
Predictor variables included the following: demographic measures such as age, sex, and race; measures of prehospitalization health, including the Charlson Comorbidity Index (CCI) (20) and whether admission was from home or from a skilled nursing facility; severity of acute illness; treatment-related variables; and dialysis dependence or independence at 60 days. The CCI was modified to exclude age because of the nature of the study population and was broken into the following categories for analysis: 0, 1 to 2, 3 to 4, and >4. Severity of acute illness was assessed using the sequential organ failure assessment (SOFA) score (1,21). Treatment-related variables included ICU length of stay (LOS), hospital LOS, treating service (medical versus surgical), and assignment to the intensive versus less intensive study treatment group.
Statistical Analyses
Patient characteristics were reported as mean ± SD for continuous variables that were normally distributed and as median and 25th and 75th percentiles for non-normally distributed variables such as hospital and ICU length of stay. Comparisons between survivors completing the HUI and the rest of the ATN study cohort were made using unpaired t tests for continuous variables and χ2 tests for categorical variables.
Univariate and multivariate linear regression analyses were performed to determine which patient characteristics were associated with overall HUI score and scores on individual attributes. As a sensitivity analysis, multivariate regression was performed using only the subset of individuals with enough HUI data to generate a score without statistical imputation (n = 337). Two-tailed P values <0.05 were considered statistically significant, and analyses were performed using SAS, v9.1 (Cary, NC).
Results
Table 1 shows the characteristics of 60-day survivors who completed the HUI in comparison to the characteristics of the original study cohort and of survivors without HUI data available. Not surprisingly, survivors with or without HUI were younger, more likely to have been admitted from home, had lower SOFA scores, had shorter lengths of hospital and ICU stay, and were more likely to have recovered renal function compared with the whole cohort. Survivors with HUI data were more likely to be white, had shorter hospital and ICU lengths of stay, were less likely to still be hospitalized at 60 days, and were less likely to remain dialysis-dependent compared with survivors without usable HUI data. There were no significant differences in treatment assignment between participants included in this analysis and those who did not survive until 60 days or survived but lacked usable HUI data.
Table 1.
Characteristics of study subjects
| Characteristic | Entire Study Cohort(n = 1124) | 60-Day Survivors without HUI Data (n = 118) | 60-Day Survivors with HUI Data (n = 415) | P |
|---|---|---|---|---|
| Age (years) | 59.6 (15.3) | 55.1 (17.5) | 57.6 (14.9) | 0.13 |
| Sex, n (% men) | 70.6 | 73.7 | 69.2 | 0.34 |
| Race | ||||
| white (%) | 74.3 | 61.9 | 76.4 | 0.002 |
| black (%) | 15.9 | 22.0 | 14.9 | 0.07 |
| other (%) | 9.8 | 16.1 | 8.7 | 0.02 |
| CCI | 2.6 (2.4) | 2.1 (2.6) | 2.4 (2.3) | 0.16 |
| Admitted from home (%) | 82.8 | 84.8 | 90.6 | 0.07 |
| Intensive RRT group (%) | 50.1 | 54.2 | 47.5 | 0.20 |
| Treated by medical service (%) | 50.4 | 38.1 | 48.7 | 0.12 |
| Hospital LOS (days) | 48.0 (17.4) | 46.1 (17.4) | 33.2 (17.5) | <0.0001 |
| ICU LOS (days) | 40.6 (22.4) | 34.6 (21.4) | 18.9 (15.9) | <0.0001 |
| SOFA score | 13.8 (3.9) | 12.5 (3.9) | 12.4 (3.7) | 0.84 |
| Discharged to home (%) | 18.2 | 22.0 | 41.7 | <0.0001 |
| Dialysis-independent (%) | 49.6 | 69.5 | 79.3 | 0.03 |
P values compare those alive at 60 days with HUI data with those surviving but not providing HUI data (i.e., those not included in the analysis).
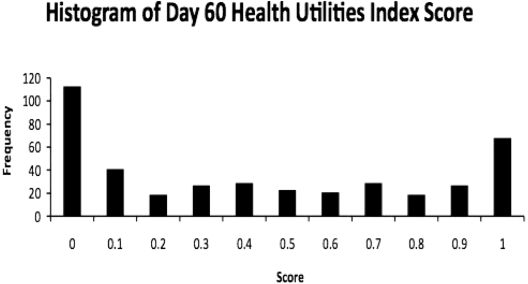
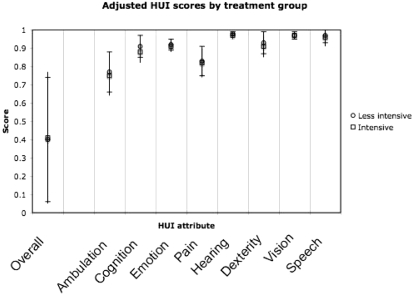
The overall mean HUI score for 60-day survivors of acute kidney injury requiring renal replacement therapy in the ATN study was 0.40 ± 0.37 (mean ± SD). The modal score was 0, with 113 respondents (27%) reporting a comprehensive health state that corresponds to a utility equal to or worse than death (Figure 2). Scores for each attribute are shown in Table 2. Visual inspection of individual scores showed that very low values on the pain and ambulation subscales were the most common causes of low overall scores. In addition, most patients with very low overall HUI scores (82%) were either still hospitalized at 60 days or had been discharged to skilled nursing facilities or assisted living facilities rather than to home. In univariate analysis, there was no effect of assignment to intensive or less intensive renal replacement therapy on 60-day HUI scores (Figure 3).
Figure 2.
Histogram of 60-day HUI scores.
Table 2.
HUI scores
| HUI Score | Mean (SD) | Range |
|---|---|---|
| Overall | 0.40 (0.37) | 0 to 1.0 |
| Ambulation | 0.77 (0.18) | 0.58 to 1.0 |
| Cognition | 0.90 (0.16) | 0.42 to 1.0 |
| Emotion | 0.92 (0.13) | 0.45 to 1.0 |
| Pain | 0.84 (0.18) | 0.55 to 1.0 |
| Hearing | 0.98 (0.07) | 0.61 to 1.0 |
| Dexterity | 0.93 (0.15) | 0.56 to 1.0 |
| Vision | 0.96 (0.07) | 0.61 to 1.0 |
| Speech | 0.97 (0.08) | 0.68 to 1.0 |
Figure 3.
HUI attributes by treatment group. Circles represent the less intensive treatment group; squares represent the more intensive group. Error bars depict SD.
In multivariable analysis, higher overall HUI score was associated with younger age, admission from home, treatment on a surgical service, and shorter hospital and ICU LOS (Table 3). Contrary to our hypotheses, neither treatment group nor dialysis dependence was significantly associated with overall HRQOL at 60 days after initiation of RRT for AKI in the ICU. Treatment assignment was not associated with any of the eight HUI subscales either. Longer hospital and/or ICU LOS was associated with worse HRQOL for all attributes, and older age was associated with worse utility on the ambulation attribute. Somewhat surprisingly, higher burden of comorbidity was associated only with worse cognition. Dialysis dependence was also associated with worse cognition but not with any other HUI attribute. In general, results of the sensitivity analysis excluding statistically imputed HUI results were similar to those of the full study cohort except that the associations between dialysis independence and the emotion and pain attributes of the HUI were stronger (β = 0.037, P = 0.04 for emotion and β = 0.060, P = 0.02 for pain).
Table 3.
Multivariate associations between patient characteristics and HUI scores
| Characteristic | Overall |
Ambulation |
Cognition |
Emotion |
Pain |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient(SEM) | P | Coefficient(SEM) | P | Coefficient(SEM) | P | Coefficient(SEM) | P | Coefficient(SEM) | P | |
| Age, 10 years | −0.036 | 0.003 | −0.002 | 0.0001 | −0.009 | 0.10 | −0.0007 | 0.86 | 0.011 | 0.08 |
| (0.012) | (0.0005) | (0.0005) | (0.004) | (0.006) | ||||||
| Sex (female) | 0.007 | 0.86 | −0.00005 | >0.99 | 0.012 | 0.49 | −0.011 | 0.38 | −0.005 | 0.78 |
| (0.036) | (0.016) | (0.017) | (0.013) | (0.019) | ||||||
| Race | ||||||||||
| white | Reference | Reference | Reference | Reference | Reference | |||||
| black | −0.022 | 0.64 | −0.003 | 0.89 | −0.038 | 0.09 | 0.019 (0.017) | 0.28 | 0.036 | 0.15 |
| (0.048) | (0.022) | (0.022) | (0.025) | (0.025) | ||||||
| other | −0.050 | 0.41 | −0.012 | 0.65 | −0.081 | 0.004 | 0.030 | 0.17 | −0.026 | 0.39 |
| (0.061) | (0.026) | (0.028) | (0.021) | (0.031) | ||||||
| CCI | ||||||||||
| 0 | Reference | Reference | Reference | Reference | Reference | |||||
| >0 and <2 | 0.020 | 0.67 | 0.009 | 0.66 | −0.031 | 0.16 | −0.008 | 0.63 | 0.022 | 0.36 |
| (0.047) | (0.021) | (0.022) | (0.017) | (0.024) | ||||||
| >2 and <4 | −0.047 | 0.35 | −0.010 | 0.65 | −0.049 | 0.04 | −0.045 | 0.01 | −0.007 | 0.78 |
| (0.051) | (0.022) | (0.023) | (0.018) | (0.026) | ||||||
| >4 | −0.045 | 0.44 | −0.027 | 0.28 | −0.082 | 0.002 | −0.028 | 0.17 | −0.024 | 0.42 |
| (0.058) | (0.025) | (0.026) | (0.020) | (0.030) | ||||||
| Admitted from home | 0.141 | 0.02 | 0.008 | 0.76 | 0.035 | 0.19 | 0.012 | 0.57 | 0.053 | 0.08 |
| (0.060) | (0.027) | (0.027) | (0.021) | (0.031) | ||||||
| Intensive RRT group | −0.004 | 0.90 | −0.0003 | 0.98 | −0.025 | 0.11 | −0.011 | 0.35 | 0.002 | 0.91 |
| (0.034) | (0.015) | (0.015) | (0.012) | (0.017) | ||||||
| Treated by medical service | −0.067 | 0.05 | −0.010 | 0.49 | 0.002 | 0.92 | −0.018 | 0.14 | −0.032 | 0.07 |
| (0.034) | (0.015) | (0.016) | (0.012) | (0.018) | ||||||
| Hospital LOS | −0.007 | <0.0001 | −0.004 | <0.0001 | −0.0004 | 0.50 | −0.001 | 0.006 | −0.0004 | 0.52 |
| (0.001) | (0.0006) | (0.0006) | (0.0005) | (0.0007) | ||||||
| ICU LOS | −0.003 | 0.04 | −0.001 | 0.03 | −0.001 | 0.04 | 0.0004 | 0.43 | −0.003 | <0.0001 |
| (0.002) | (0.0007) | (0.0007) | (0.0005) | (0.0008) | ||||||
| SOFA score | 0.001 | 0.77 | −0.003 | 0.25 | −0.0003 | 0.90 | −0.001 | 0.46 | 0.0009 | 0.71 |
| (0.005) | (0.002) | (0.002) | (0.002) | (0.003) | ||||||
| Dialysis-independent | 0.050 | 0.25 | 0.012 | 0.54 | 0.059 | 0.003 | 0.025 | 0.10 | 0.037 | 0.10 |
| (0.043) | (0.019) | (0.020) | (0.015) | (0.022) | ||||||
P < 0.05 are shown in bold text.
Discussion
Health utility following AKI in the ICU requiring RRT in the ATN study was extremely low by any metric, given that 27% of respondents' health states corresponded to utilities considered by the general population to be equivalent to or worse than death. This finding is particularly remarkable because the group who completed the HUI questionnaire had shorter hospital and ICU stays, factors that were associated with more favorable HRQOL, suggesting a bias toward better HRQOL in this cohort. The findings of this randomized clinical trial demonstrate that intensity of dialysis did not have a substantial effect on the overall HUI score, ambulation, cognition, emotion, or pain. Interestingly, dialysis dependence was not associated with overall HUI score or with ambulation. Instead, admission from home and hospital and ICU LOS were important predictors. It is possible that, in the setting of acute illness, muscle wasting related to bed rest is the dominant effector of loss of functioning, and any uremia- or dialysis-associated wasting plays a less critical role. The significant association between dialysis dependence and poor cognition, however, parallels more closely the associations that have been observed among patients receiving chronic hemodialysis (22–24).
The health utility scores reported by ATN study survivors can be compared with norms available for older adults in the United States (25), to individuals with chronic diseases (26) including ESRD (18), and to patients discharged from ICU care after RRT. In a population-based survey designed to be representative of the older half of the U.S. population, Fryback et al. reported that the mean HUI scores ranged from 0.83 for adults aged 35 to 44 years to 0.75 for adults aged 75 to 89 years (25). Another group of investigators proposed that respondents with a HUI score of >0.946 be classified as healthy and those with scores <0.830 (walking with a cane with no other disability, for example) should be considered dysfunctional. By this standard, the vast majority of ATN participants would be considered dysfunctional (Figure 2).
Given that the average CCI for patients included in the ATN study was approximately 2.5, perhaps comparison to populations with chronic diseases would be more informative than comparison to the general population. In a study designed to assess the burden of chronic disease in Ontario, Canada, Manuel et al. performed a population-based telephone survey in which the HUI was administered and respondents were asked about chronic conditions and injuries that limited mobility (26). HUI scores were reported by condition. The lowest scores were reported for those with injuries limiting mobility (0.749 for women and 0.770 for men). Several comorbidities common to patients with kidney disease were evaluated. For example, patients with heart disease had scores of 0.873 for women and 0.863 for men, and patients with diabetes scored a little higher at 0.893 (women) and 0.896 (men). It is perhaps not surprising that surviving ATN study participants had lower health utility than that reported for any specific chronic illness in the Canadian cohort because many ATN study participants had CCI scores indicative of multiple comorbid conditions, whereas the estimates of health utility for each of the conditions in the Manuel et al. study were adjusted to reflect the effect of that condition alone (26). A recent review by Liem et al. (18) found only one study that measured HRQOL among persons with ESRD. Three months after starting conventional (three times weekly) hemodialysis, the 18 patients had a mean HUI score of 0.73 (27), well above the level of ATN survivors, although they had low utility scores (mean 0.34) by an alternative time–trade-off method.
Overall, it appears that HRQOL in 60-day survivors of the ATN study compared unfavorably with previous reports of post-AKI HRQOL (5,8,10,28–30). There are several potential reasons for this. First, 60 days is a shorter interval between acute illness and measurement of HRQOL than has been previously reported in AKI. Longitudinal data on post-RRT HRQOL are not available, but a study of HRQOL after acute respiratory distress syndrome showed substantial improvement in SF-36 scores from 3 to 12 months after acute illness (31). However, it is not clear whether HRQOL would be expected to improve as substantially with time after illness in the ATN study cohort, patients of which are older and have a greater burden of comorbidity. Patients in the ATN study may have been sicker at baseline than patients in other studies. Several of the prior reports were from Europe or were published several years ago, both of which could have led to different patient selection criteria for ICU care or RRT. In addition, the HUI has important differences from other instruments used to assess HRQOL. HUI scores are calculated on the basis of population preferences rather than direct assessment of HRQOL by the affected individuals as are measures used in other studies. Finally, the low response rate of several prior reports might bias toward higher functioning if sicker patients were less likely to respond or institutionalized patients were more difficult to locate. Although our study is also subject to this bias, the magnitude of bias may be smaller as our response rate was higher than those in other studies. The use of interviews may have permitted this report to include patients with a higher burden of comorbidity who would have been overlooked by studies using only self-report. The influence of mode of data collection on the selection of a study sample has been previously reported in the HEMO Study, where patients using interviews were found to be older, were more likely to be diabetic, and had a higher burden of comorbid illness (32).
This study has several important strengths and limitations. Strengths of the study include its large size and high response rate, and also use of an instrument that has been extensively validated in diverse populations of healthy and chronically ill individuals (17,25,33–35). This report includes a greater number of respondents and a higher response rate than any other available report of HRQOL following RRT in the literature. The comparison of respondents to nonrespondents suggests that our estimates of HRQOL are likely overestimates. However, compared with prior studies, our response rate was quite high, and our study is unique in that we collected data on the characteristics of nonresponders, allowing us to confirm the possibility of bias.
Although the HUI has been extensively validated, it has not been used previously to evaluate the HRQOL of patients surviving AKI requiring RRT, which limited our ability to compare our results with available data on this question. Other limitations of this study include a lack of information about pre-illness HRQOL, socioeconomic status, and kidney function at 60 days (beyond the need for ongoing dialysis). Therefore, we were unable to comment on the degree of loss of health utility after ICU care with RRT or the relationship between estimated GFR and HRQOL.
These findings indicate that the intensity of dialysis used in AKI does not improve HRQOL among survivors. This would suggest that clinicians and investigators consider other approaches in the care of these patients, such as improving nutrition, rehabilitation, mood, and pain control. This report presents a particularly daunting finding in that one fourth of survivors report an extremely low HRQOL consistent with that of death, highlighting the importance of HRQOL as an important and quantitative patient-centered outcome variable that may be very useful in the design of subsequent clinical studies. The low post-AKI HRQOL also underscores an urgent need to focus our clinical management of AKI not only on survival in the ICU but also on improvement of HRQOL after discharge. In addition, we believe that these results should be used to counsel patients facing the prospect of RRT in the ICU. It is clear that patients who survive 60 days after initiation of RRT in the ICU can expect to encounter severe limitation in HRQOL, and this information might influence discussions of advance directives and goals of care.
Disclosures
None.
Acknowledgments
This study was supported by the Cooperative Studies Program of the U.S. Department of Veterans Affairs Office of Research and Development and by the U.S. National Institutes of Diabetes and Digestive and Kidney Diseases (interagency agreement Y1-DK-3508-01). This work was presented as a Free Communication at the 42nd Annual Meeting and Scientific Exposition of the American Society of Nephrology; October 27 through November 1, 2009; San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
The views expressed herein are those of the authors and not necessarily those of the U.S. Department of Veterans Affairs.
References
- 1.De Mendonca A, Vincent J, Suter P, Moreno R, Dearden N, Antonelli M, Takala J, Sprung C, Cantraine F: Acute renal failure in the ICU: Risk factors and outcome evaluated by the SOFA score. Intensive Care Med 26: 915–921, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Brivet F, Kleinknecht D, Loirat P, Landais P, Failure FSGoAR: Acute renal failure in intensive care units - causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study: French Study Group on Acute Renal Failure. Crit Care Med 24: 192–198, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Schwilk B, Wiedeck H, Stein B, Reinelt H, Treiber H, Bothner U: Epidemiology of acute renal failure and outcome of haemodiafiltration in intensive care. Intensive Care Med 23: 1204–1211, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Chertow G, Burdick E, Honour M, Bonventre J, Bates D: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Ahlstrom A, Tallgren M, Peltonen S, Rasanen P, Pettila V: Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med 31: 1222–1228, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Gopal I, Bhonagiri S, Ronco S, Bellomo R: Out of hospital outcome and quality of life in survivors of combined acute multiple organ and renal failure treated with continuous venovenous hemofiltration/hemodiafiltration. Intensive Care Med 23: 766–772, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Hamel M, Phillips R, Davis R, Desbiens N, Connors A, Teno J, Wenger N, Lynn J, Wu A, Fulkerson W, Tsevat J, Investigators S: Outcomes and cost-effectiveness of initiating dialysis and continuing aggressive care in seriously ill hospitalized adults. Ann Intern Med 127: 195–202, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Korkeila M, Ruokonen E, Takala J: Costs of care, long-term prognosis and quality of life in patients requiring renal replacement therapy during intensive care. Intensive Care Med 26: 1824–1831, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Maynard S, Whittle J, Chelluri L, Arnold R: Quality of life and dialysis decisions in critically ill patients with acute renal failure. Intensive Care Med 29: 1589–1593, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Morgera S, Kraft A, Siebert G, Luft F, Neumayer H: Long-term outcomes in acute renal failure patients treated with continuous renal replacement therapies. Am J Kidney Dis 40: 275–279, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Palevsky P, O'Connor T, Zhang J, Star R, Smith M: Design of the VA/NIH Acute Renal Failure Trial Network (ATN) Study: Intensive versus conventional renal support in acute renal failure. Clin Trials 2: 423–435, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Network VNARFT, Palevsky P, Zhang J, O'Connor T, Chertow G, Crowley S, Choudhury D, Finkel K, Kellum J, Paganini E, Schein R, Smith M, Swanson K, Thompson B, Vijayan A, Watnick S, Star R, Peduzzi P: Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mapes D, Bragg-Gresham J, Bommer J, Fukuhara S, McKevitt P, Wikstrom B, Lopes A: Health-related quality of life in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 44: 54–60, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Evans R, Manninen D, Garrison L, Hart L, Blagg C, Gutman R, Hull A, Lowrie E: The quality of life of patients with end-stage renal disease. N Engl J Med 312: 553–559, 1985 [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Kader K, Unruh M, Weisbord S: Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol 4: 1057–1064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horsman J, Furlong W, Feeny D, Torrance G: The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes 1: 54, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furlong W, Feeny D, Torrance G, Barr R: The Health Utilities Index (HUI) system for assessing health-related quality of life in clinical studies. Ann Med 33: 375–384, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Liem Y, Bosch J, Hunink M: Preference-based quality of life of patients on renal replacement therapy: A systematic review and meta-analysis. Value Health 11: 733–741, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Naiem A, Keeler E, Mangione C: Options for handling missing data in the Health Utilities Index Mark 3. Med Decis Making 25: 186–198, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Charlson M, Pompei P, Ales K, MacKenzie C: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Vincent J, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart C, Suter P, Thijs L: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Murray A: Cognitive impairment in the aging dialysis and chronic kidney disease populations: An occult burden. Adv Chronic Kidney Dis 15: 123–132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurella M, Chertow G, Luan J, Yaffe K: Cognitive impairment in chronic kidney disease. J Am Geriatr Soc 52: 1863–1869, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Murray A, Tupper D, Knopman D, Gilbertson D, Pederson S, Li S, Smith G, Hochhalter A, Collins A, Kane R: Cognitive impairment in hemodialysis patients is common. Neurology 67: 216–223, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Fryback D, Dunham N, Palta M, Hanmer J, Buechner J, Cherepanov D, Herrington S, Hays R, Kaplan R, Ganiats T, Feeny D, Kind P: US norms for six generic health-related quality-of-life indexes from the National Measurement Study. Med Care 45: 1162–1170, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manuel D, Schultz S, Kopec J: Measuring the health burden of chronic disease and injury using health adjusted life expectancy and the Health Utilities Index. J Epidemiol Community Health 56: 843–850, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heidenheim A, Murihead N, Moist L, Lindsay R: Patient quality of life on quotidian hemodialysis. Am J Kidney Dis 42: 36–41, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Bagshaw S: The long-term outcome after acute renal failure. Curr Opin Crit Care 12: 561–566, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Morgera S, Schneider M, Neumayer H: Long-term outcomes after acute kidney injury. Crit Care Med 36: S193–S197, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Oeyen S, Vandijck D, Benoit D, Decruyenaere J, Annemans L, Hoste E: Long-term outcome after acute kidney injury in critically-ill inpatients. Acta Clin Belg Suppl 2: 337–340, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Angus D, Musthafa A, Clermont G, Griffin M, Linde-Zwirble W, Dremsizov T, Pinsky M: Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1389–1394, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Unruh M, Yan G, Radeva M, Hays R, Benz R, Athienites N, Kusek J, Meyer K, Group HS: Bias in assessment of health-related quality of life in a hemodialysis population: a comparison of self-administered and interviewer-administered surveys in the HEMO study. J Am Soc Nephrol 14: 2132–2141, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Bondini S, Kallman J, Dan A, Younoszai Z, Ramsey L, Nader F, Younossi Z: Health-related quality of life in patients with chronic hepatitis B. Liver Int 27: 1119–1125, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Davison S, Jhangri G, Feeny D: Evidence on the construct validity of the Health Utilities Index Mark 2 and Mark 3 in patients with chronic kidney disease. Qual Life Res 17: 933–942, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Gorodetskaya I, Zenios S, McCulloch C, Bostrom A, Hus C, Bindman A, Go A, Chertow G: Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int 68: 2801–2808, 2005 [DOI] [PubMed] [Google Scholar]