Abstract
Background and objectives: Little is known about the performance of plasma cystatin C (CysC) in patients undergoing cardiopulmonary bypass (CPB) and its utility in the early diagnosis of acute kidney injury (AKI). In this post hoc analysis, the goal was to determine whether plasma cystatin C, measured 2 hours after the conclusion of CPB, is a reliable marker of AKI.
Design, setting, participants, & measurements: Plasma CysC was measured in 150 patients undergoing CPB at the following times: preoperatively, 2 hours after the conclusion of CPB, postoperative day 1, and postoperative day 2. Plasma CysC levels were related to the development of AKI as defined by an increase in serum creatinine of ≥50% or ≥0.3 mg/dl from baseline up to 3 days postoperative. Mixed linear models were used to evaluate the relationship of serial plasma CysC values with AKI. The discriminatory capacity of plasma CysC was estimated using receiver operating characteristic curves. Logistic regression was utilized to assess the adjusted relationship between plasma CysC and subsequent AKI.
Results: AKI developed in 47 (31.3%) patients. Plasma CysC was higher at all times among patients who developed AKI compared with those who did not (P < 0.0001). The discriminatory capacity of plasma CysC measured preoperatively and 2 hours after the conclusion of CPB was modest.
Conclusions: Serial measures of plasma CysC are highly correlated with the development of AKI. However, the discriminatory capacity of plasma CysC as an early marker of AKI remains limited.
Acute kidney injury (AKI) is a common and serious complication of cardiac surgery and cardiopulmonary bypass (CPB) (1,2). Current diagnostic criteria for AKI are based on the presence of relative or absolute increments in serum creatinine (sCr) (3). Although readily available and widely used, sCr is a suboptimal marker of AKI for several reasons: sCr concentration is affected by nonrenal factors (e.g., muscle mass and distribution volume); creatinine is secreted by the renal tubules and serum concentrations do not entirely reflect glomerular filtration; and in the setting of acute injury, elevations in sCr usually do not occur until well after the time of the actual insult. Discovery of an alternative marker that reliably reflects kidney function in the acute setting and enables the early detection of AKI would be very desirable.
Cystatin C (CysC) is a 13-kD cysteine protease inhibitor that is synthesized in all nucleated cells at a steady state. It is freely filtered by the glomerulus, not secreted by renal tubules, and completely metabolized at the level of the renal tubules. These properties have made it an attractive marker of the GFR in chronic kidney disease (4,5). However, receipt of glucocorticoids, age, gender, and C-reactive protein are nonrenal factors that may affect the measurement of plasma CysC (6,7). There is a relative paucity of information on the plasma levels of CysC after acute kidney insults such as those associated with CPB. We therefore undertook a three-center cohort study that evaluated the performance of plasma CysC as an early diagnostic marker for AKI as compared with a reference standard based on sCr elevation from baseline. We also assessed the ability of plasma CysC to discern patients with AKI from those without AKI after cardiac surgery requiring CPB.
Materials and Methods
Study Design and Participants
We performed a cohort study of patients undergoing on-pump cardiac surgery at three tertiary care academic centers (Tufts Medical Center (Boston, MA), Caritas St. Elizabeth's Medical Center (Boston, MA), and St. Michael's Hospital (Toronto, ON, Canada). The study protocol was approved by the Research Ethics Board at each site and informed consent was obtained from all participants. Patients >18 years of age who were undergoing cardiac surgery with the use of CPB were eligible for recruitment. This work is a post hoc analysis of a study that was primarily designed to evaluate the relationship between single nucleotide polymorphisms and AKI in the setting of CPB (8). Pregnant women, patients with end-stage renal disease on maintenance dialysis, solid organ or bone marrow transplant recipients, and those undergoing off-pump or “minimally invasive” coronary artery bypass grafting were not included. At St. Michael's Hospital, the study was performed as an ancillary study to the Blood Conservation Using Antifibrinolytics in a Randomized Trial and eligibility criteria were selected to enroll a population of patients that was at higher risk of postoperative bleeding (Appendix A) (9).
Data and Sample Collection
A wide array of prespecified demographic and clinical data were recorded for each patient by the investigators and trained study coordinators. Serum creatinine was measured preoperatively and daily for 3 days after surgery. We also obtained venous blood samples at the following time points: preoperatively (either at a preoperative clinic visit, on the ward, or in the operating room after induction of anesthesia and before the start of CPB), 2 hours after the conclusion of CPB (corresponding to arrival in the intensive care unit [ICU]), postoperative day 1, and postoperative day 2. After being drawn, the blood was kept on ice and then centrifuged at 4°C at 1000 × g for 15 minutes. The resulting supernatant was then centrifuged at 10,000 × g for 10 minutes. Plasma was stored at −80°C.
Cystatin C Assay
Plasma cystatin C was measured by immunonephelometry using the BN II System (Siemens Healthcare Diagnostics, Deerfield, IL). The lower limit of detection for cystatin C was 0.05 mg/L, and the average intra- and interassay coefficient of variation was <2.5 and <3.5%, respectively.
Definition of Acute Kidney Injury
AKI was defined as an increase of ≥50% or ≥0.3 mg/dl in sCr from the preoperative value during the first 3 days after surgery. This definition differs slightly from the Acute Kidney Injury Network criteria that require the sCr rise to occur within 48 hours (3). Because of the possibility of hemodilution associated with CPB, we anticipated an early postoperative decline in sCr concentration in some patients and a delay in achieving the Acute Kidney Injury Network threshold for a diagnosis of AKI (1,10). We therefore adopted a 3-day window for the rise in sCr. Serum creatinine was assayed using the modified Jaffe method. The Acute Kidney Injury Network criteria also incorporates oliguria as evidence of AKI (urine output <0.5 ml/kg per hour for 6 hours) (3). We elected to not use this criterion for the ascertainment of AKI as in the highly controlled postoperative period in intensive care, the urine output may be kept stable through intravenous fluid and diuretic administration. Therefore, oliguria is a rare occurrence in our cohort and not useful for the detection of AKI. Furthermore, whereas urine output is reliably captured in the ICU, this is not the case on postsurgical wards. Because patients are often discharged from the ICU on the first postoperative day, it would be impossible to rely on urine output criteria for the diagnosis of AKI.
Statistical Analyses
We used descriptive statistics to delineate the characteristics of the cohort by AKI status. Normally distributed continuous variables were compared using the unpaired t test and non-normally distributed variables were evaluated with the Wilcoxon test. Normality was designated if skewness and kurtosis were close to 0. Categorical variables were compared using the χ2 test and Fisher's exact test as appropriate. Plasma CysC levels were compared at each discrete time point using the unpaired t test. Serial measures of plasma CysC were compared in patients with and without AKI using mixed linear models to account for intrapatient correlations. We generated receiver operating characteristic curves to describe the performance characteristics of plasma CysC, measured preoperatively and 2 hours after CPB. The area under the curve (AUC) with associated 95% confidence intervals (CIs) served as a measure of the discriminatory capacity of plasma CysC to predict AKI.
We used logistic regression to evaluate whether ascending quartiles of plasma CysC, measured 2 hours after the conclusion of CPB, were independently associated with the development of AKI. We considered key preoperative variables that are known to be associated with postoperative AKI (11) as potential confounders: chronic kidney disease (CKD), as defined by an estimated GFR <60 ml/min per 1.73 m2 using the four-variable Modification of Diet in Renal Disease equation (12) based on the preoperative serum creatinine, diabetes mellitus requiring medication, systolic ejection fraction <40%, the receipt of any surgery other than coronary artery bypass graft (CABG), previous cardiac surgery, use of an intra-aortic balloon pump preoperatively, and the nonelective nature of the surgery. The final model was fit using a stepwise selection procedure that permitted the inclusion of any variable with P < 0.10 in the model.
Because plasma CysC may be elevated at baseline in patients with CKD, we performed a sensitivity analysis that included only patients who were free of CKD preoperatively (preoperative estimated GFR >60 ml/min per 1.73 m2).
As this was a preliminary study using a convenience sample that explored the properties of plasma cystatin C in AKI, sample sizes were not calculated a priori. All analyses were performed using SAS Version 9.1.3 (SAS Institute, Cary, NC). A two-sided P value of <0.05 was considered statistically significant.
Results
We recruited 150 patients undergoing CPB between February 2004 and October 2007 (57 at Tufts Medical Center, 77 at St. Elizabeth's Medical Center, and 16 at St. Michael's Hospital). Postoperative AKI occurred in 47 (31.3%) patients and was diagnosed a median of 1 day (interquartile range, 1 to 2 days) from surgery. AKI was diagnosed in 29 patients on the first postoperative day, 11 patients on the second postoperative day, and in 7 patients on the third postoperative day. Three patients required renal replacement therapy and 6 died in the hospital.
The characteristics of patients who developed AKI and those who did not are displayed in Table 1. Patients who developed AKI were more likely to have pre-existing CKD, had a lower preoperative estimated GFR, and were more likely to undergo surgeries other than isolated CABG.
Table 1.
Patient characteristics
| No AKI Group (n = 103) | AKI Group(n = 47) | P | |
|---|---|---|---|
| Patient characteristics | |||
| men (%) | 71 (68.9) | 32 (68.1) | 0.91 |
| age (years) | 67.0 ± 11.8 | 69.5 ± 9.2 | 0.20 |
| baseline sCr (mg/dl) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.3) | 0.04 |
| baseline eGFR (ml/min per 1.73 m2) | 75.5 ± 17.1 | 63.9 ± 18.9 | <0.0001 |
| chronic kidney disease (%) | 16 (15.5) | 17 (36.2) | 0.005 |
| diabetes mellitus (%) | 32 (31.1) | 17 (36.2) | 0.54 |
| left ventricular ejection fraction <40% (%) | 17 (18.1) | 9 (20.5) | 0.74 |
| peripheral vascular disease (%) | 17 (16.5) | 11 (23.4) | 0.31 |
| chronic obstructive pulmonary disease (%) | 15 (14.6) | 14 (29.8) | 0.03 |
| Surgery characteristics | |||
| surgery type (%) | |||
| CABG only | 57 (55.3) | 16 (34.0) | 0.02 |
| valve only | 14 (13.6) | 11 (23.4) | |
| combined CABG and valve | 27 (26.2) | 20 (42.6) | |
| other | 5 (4.9) | 0 (0) | |
| CPB time (minutes) | 109.1 ± 39.3 | 116.9 ± 43.5 | 0.29 |
| crossclamp time (minutes) | 85.1 ± 33.7 | 86.6 ± 28.0 | 0.77 |
| Interventions and outcomes | |||
| receipt of furosemide within 24 hours of CPB (%) | 77 (76.2) | 43 (93.5) | 0.01 |
| receipt of pressors >24 hours postoperatively | 17 (16.8) | 7 (15.2) | 0.81 |
| ICU length of stay (days) | 2 (2, 3) | 3 (2, 5) | 0.04 |
| length of stay from surgery to discharge (days) | 7 (5, 9) | 8 (7, 13) | 0.0002 |
| in-hospital death (%) | 4 (3.9) | 2 (4.3) | 1.00 |
Continuous variables are displayed as means ± standard deviation or medians with interquartile range in parentheses, as appropriate. Categorical variables are displayed as a number (%). Chronic kidney disease defined as preoperative estimated GFR <60 ml/min per 1.73 m2. Data on ejection fraction missing for 12 patients. Data on receipt of furosemide and pressors missing for 3 patients. eGFR, estimated GFR.
Perioperative Plasma Cystatin C and AKI
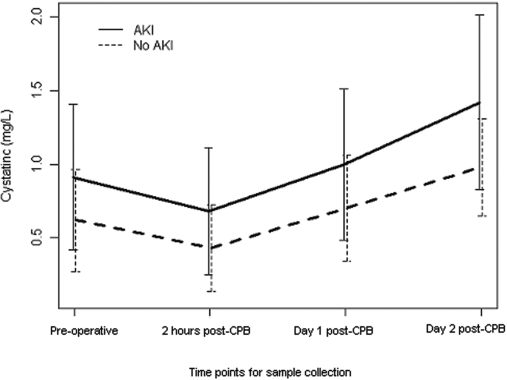
As shown in Figure 1, plasma CysC levels were higher preoperatively in patients who developed postoperative AKI, as compared with those who did not. In both groups, plasma CysC declined 2 hours after the conclusion of CPB and then rose above baseline on postoperative day 1 and even further on postoperative day 2. At all perioperative time points, plasma CysC concentrations were higher among patients who developed AKI and this was confirmed when serial plasma CysC values were evaluated using a mixed linear model (P < 0.0001).
Figure 1.
Perioperative plasma cystatin C concentrations in patients with and without acute kidney injury.
Performance of Cystatin C in the Diagnosis of Subsequent AKI
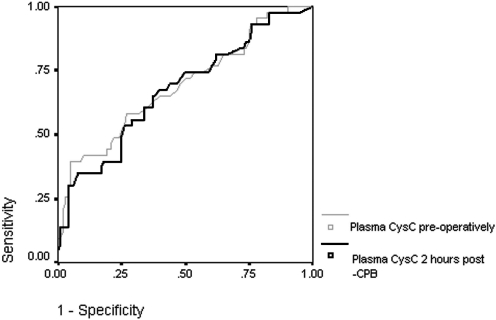
Plasma cystatin C was available for 149 subjects preoperatively and 144 subjects 2 hours after the conclusion of CPB. The ability of plasma CysC to predict the development of subsequent AKI was modest at all time points. Preoperatively, the area under the receiver operator curve was 0.67 (95% CI 0.57 to 0.77) for CysC and 0.60 for serum creatinine (95% CI 0.51 to 0.70). Two hours after CPB, the AUC for CysC was 0.68 (95% CI 0.58 to 0.78) (Figure 2).
Figure 2.
Receiver operating characteristic curve depicting the performance characteristics of plasma cystatin C concentration measured preoperatively and 2 hours after cardiopulmonary bypass in the diagnosis of acute kidney injury.
We then examined the relationship between plasma CysC measured 2 hours after CPB, evaluated in quartiles, and the development of postoperative AKI. As compared with individuals with plasma CysC values in the lowest quartile, patients with values in the highest quartile had a significantly elevated risk of developing subsequent AKI (odds ratio (OR) 2.66, 95% CI 1.02 to 6.92) (Table 2). After accounting for other potential confounders of the relationship between plasma CysC and postoperative AKI in a stepwise logistic regression model, this relationship persisted (OR 2.33, 95% CI 1.00 to 5.45). CKD (OR 2.53, 95% CI 1.08 to 5.92) and the receipt of surgery other than isolated CABG (OR 2.59, 95% CI 1.22 to 5.51) were also associated with a heightened risk of AKI.
Table 2.
Unadjusted and adjusted relationship between quartiles of plasma cystatin C measured 2 hours after cardiopulmonary bypass and acute kidney injury
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | |
|---|---|---|
| Plasma cystatin C | ||
| quartile 1 (lowest) | 1.00 | 1.00 |
| quartile 2 | 0.68 (0.23 to 1.99) | |
| quartile 3 | 1.35 (0.51 to 3.58) | |
| quartile 4 (highest) | 2.66 (1.02 to 6.92) | 2.33 (1.00 to 5.45) |
| Chronic kidney disease | 3.08 (1.39 to 6.85) | 2.53 (1.08 to 5.92) |
| Diabetes mellitus | 1.26 (0.61 to 2.60) | |
| Left ventricular systolic function <40%b | 1.20 (0.49 to 2.93) | |
| Surgery other than CABG alone | 2.40 (1.17 to 4.92) | 2.59 (1.22 to 5.51) |
| Previous cardiac surgery | 2.15 (0.95 to 4.84) | |
| Nonelective nature of surgery | 0.78 (0.39 to 1.57) |
Odds ratios reported for variables remaining in the stepwise logistic regression model. OR, odds ratio.
Among 12 individuals in whom ejection fraction could not be ascertained, ejection fraction was assumed to be ≥40%.
Performance Characteristics of Plasma Cystatin C in Patients without Pre-existing CKD
We performed a sensitivity analysis that was restricted to individuals without CKD (preoperative estimated GFR ≥60 ml/min per 1.73 m2). As shown in Table 3, preoperative plasma CysC did not differ among patients with and without subsequent AKI. However, at 2 hours after CPB and at subsequent postoperative time points, plasma CysC was significantly higher in individuals who developed AKI.
Table 3.
Plasma cystatin C concentrations at various perioperative time points among patients with preoperative estimated GFR ≥60 ml/min per 1.73 m2
| Cystatin C values in mg/L |
|||
|---|---|---|---|
| Perioperative time point | No AKI Group(n = 87) | AKI Group(n = 30) | Pa |
| Preoperative | 0.58 ± 0.32 | 0.70 ± 0.32 | 0.08 |
| 2 hours after CPB | 0.40 ± 0.26 | 0.54 ± 0.35 | 0.03 |
| Postoperative day 1 | 0.66 ± 0.29 | 0.88 ± 0.39 | 0.007 |
| Postoperative day 2 | 0.92 ± 0.23 | 1.27 ± 0.44 | 0.001 |
Comparisons of cystatin C values were performed with the unpaired t test.
Data are presented as means ± SD.
The discriminatory capacity of plasma CysC as an early marker of AKI remained limited in individuals without pre-existing CKD (AUC 0.57, 95% CI 0.48 to 0.70 preoperatively, and 0.61, 95% CI 0.48 to 0.74, 2 hours after CPB).
Discussion
Plasma CysC concentrations, measured serially during the postoperative course of individuals undergoing cardiac surgery with CPB, were positively associated with the development of AKI. Two hours after the completion of CPB, the highest values of plasma CysC were independently associated with the subsequent development of AKI. However, plasma CysC values had limited discriminatory capacity for the early diagnosis of AKI.
This post hoc analysis is the largest study to date to examine the role of plasma CysC in the context of CPB-associated AKI. We used a well-established assay for the measurement of plasma CysC that has been shown to correlate best with the GFR (13). The assays were performed by individuals who were blinded to the clinical data and, specifically, the development of AKI. We also utilized a widely accepted definition for the development of AKI. The generalizability of our findings is enhanced by the recruitment of participants from three centers.
Our study also has important limitations. For logistic reasons, we could not screen and enroll a consecutive sample of patients, thereby raising the possibility of selection bias. However, there was no systematic pattern to the recruitment or exclusion of eligible participants. As in most biomarker studies to date, our reference standard for AKI was the achievement of a biochemical threshold as reflected by a change in sCr. Although widely accessible, sCr is a marker of glomerular filtration and sCr elevations may be insensitive to subtle manifestations of kidney injury occurring at the level of the renal tubule. We also focused on a 2-hour after CPB time point for early AKI detection and did not explore a wider array of time points during the first 12 hours after CPB. Although this may have limited our ability to identify the utility of CysC at different time points, we believe that the 2-hour time point is clinically relevant. This time point roughly corresponds to the patient's arrival in the ICU and is sufficiently close to the time of the CPB-associated insult. Identification of a reliable marker of AKI at this critical juncture would permit the institution of a strategy for the early treatment of AKI. Because we relied on sCr assessments from the clinical laboratory, we did not analyze sCr at the 2-hour after CPB time point and cannot compare its performance to plasma CysC. Previous work has suggested that plasma CysC measurements may be altered by nonrenal factors including the receipt of glucocorticoids (7) or changes in thyroid function (14). Unfortunately, we did not collect data on these variables. Finally, most cases of AKI were of modest severity and only three subjects developed a need for renal replacement therapy. The predictive capacity of CysC for more severe AKI, including the need for renal replacement therapy, cannot be inferred from our results.
Our findings are compatible with other studies that confirm the reliability of CysC as a marker of kidney function in the acute setting (15,16) and particularly in the setting of CPB (17–19). These data support the extension of CysC as a marker for glomerular filtration from the chronic to acute setting. Plasma CysC is an attractive marker for the assessment of the GFR (13). CysC is synthesized by all nucleated cells, and unlike creatinine, which is produced by muscle, it is not influenced by interindividual differences and intraindividual changes in muscle mass. Moreover, CysC is freely filtered at the level of the glomerulus and completely metabolized at the level of the renal tubule without being secreted or reabsorbed. It is notable that although plasma CysC concentrations differed in patients with and without AKI at all time points, an initial decline from baseline and a subsequent increase above baseline were evident in both groups. The rise in plasma CysC concentrations in patients without a sCr-based diagnosis of AKI may indicate a subcohort of individuals in whom kidney injury occurred that was undetectable by conventional sCr-based criteria. Alternatively, plasma CysC may be affected by CPB-induced inflammation (2). This relationship is suggested by data showing an association between serum CysC concentration and that of C-reactive protein, a well-known marker on inflammation (6).
Two other studies have examined the utility of cystatin C as an early AKI biomarker (Table 4). Our finding of a limited utility for plasma CysC as an early marker of AKI is consistent with the observations of Koyner et al. (20). In a cohort of 72 patients undergoing CPB, plasma CysC upon arrival in the ICU was associated with an AUC for the development of AKI (defined as a sCr rise of 25% from baseline) of 0.62 (95% CI, 0.48 to 0.75). CPB-associated AKI is mediated by renal tubular injury (2,21), whereas CysC is a marker of glomerular filtration, rather than tubular damage per se. In the setting of tubular injury, glomerular filtration eventually becomes impaired as a consequence of tubular obstruction and tubuloglomerular feedback and this lag may explain the modest performance of plasma CysC as an early marker of AKI.
Table 4.
Comparison of studies examining cystatin C as an early marker of acute kidney injury
| Koyner et al. (20) | Haase-Fielitz et al. (23) | This Study | |
|---|---|---|---|
| No. patients | 72 | 100 | 150 |
| No. centers | 1 | 1 | 3 |
| Baseline time point | Preoperatively, after anesthesia induction | In the operating room, before start of surgery | Preoperatively in clinic or on ward, or before anesthesia induction |
| Subsequent time points | After CPB | 6 hours after CPB commencement (usually corresponding with arrival in ICU) | 2 hours after CPB conclusion |
| Arrival in ICU | 24 hours after CPB commencement | Postoperative day 1 | |
| 6 hours after arrival in ICU | Postoperative day 2 | ||
| Daily for 1 week | |||
| Weekly for 28 days | |||
| Discharge from hospital | |||
| Cystatin C assay | ELISA | Nephelometry | Nephelometry |
| Definition of AKI | >25% sCr increase from preoperative baseline or RRT within first 3 postoperative days | >50% increase in creatinine from baseline to peak value within the first 5 postoperative days | ≥50% or ≥0.3 mg/dl increase in sCr from baseline within first 3 postoperative days |
| Patients with AKI (%) | 34 (47.2) | 23 (23.0) | 47 (31.3) |
| Patients with CKDa (%) | Unclear (mean eGFR was 70 ml/min per 1.73 m2) | 27 (27.0) | 33 (22.0) |
Defined as preoperative eGFR (estimated GFR) <60 ml/min per 1.73 m2.
However, other studies have supported the utility of CysC as an early predictor of AKI. In a population of critically ill patients, serum cystatin C predated the identification of creatinine-based AKI by 1 to 2 days (22). In a recently published study of 100 patients undergoing CPB, the predictive capacity of plasma CysC on arrival in the ICU was excellent (AUC 0.83, 95% CI 0.68 to 0.98) for the subsequent development of AKI (23). The reasons for the discrepancy between these findings as compared with ours and the work of Koyner et al. (20) are not entirely clear. In our cohort and Koyner's, there was an early decline in CysC concentration immediately after surgery, as noted above. This suggests a contribution from hemodilution related to priming of the CPB circuit and center-related fluid management practices in the operating room. This early drop in plasma CysC related to nonrenal factors may obscure the ability of this marker to discern AKI early. This theory cannot be rigorously tested in our data set as fluid balance data were not systematically collected for this study.
Plasma CysC is a robust marker of GFR and it is notable that in our cohort and the one described by Haase-Fielitz et al. (23,24) CysC values were elevated preoperatively in patients who would later develop AKI as compared with those who would not. This suggests that at least part of the predictive capacity of plasma CysC is a carryover effect of the well-known association between low GFR at baseline and subsequent AKI.
The identification of novel biomarkers for the diagnosis of AKI may facilitate the initiation of early interventions for this devastating condition. Plasma levels of CysC, measured serially in the postoperative course in patients undergoing CPB, are closely associated with the development of postoperative AKI. However, CysC performs modestly as a stand-alone predictor of AKI when measured preoperatively and at approximately 2 hours after the conclusion of CPB. Other biomarkers (23,25–27) and combinations of biomarkers (28) may hold promise as early predictors of CPB-associated AKI, although studies examining these biomarkers have been small and the results nondefinitive. We expect that the ongoing study by the Translational Research Investigating Biomarkers and Endpoints for Acute Kidney Injury (TRIBE-AKI) Consortium (29), a multicenter cohort that plans to enroll 1800 patients undergoing CPB, will substantially advance knowledge in this area. However, the utility of AKI biomarkers will only be fully realized if it is demonstrated that their integration into clinical care serves to guide management that leads to improved patient-relevant outcomes.
Appendix A
Eligibility criteria for patients enrolled in this study as an ancillary to Blood Conservation Using Antifibrinolytics in a Randomized Trial (adapted from “Appendix A. Description of the study population,” available online with reference (9)).
Inclusion Criteria
Urgent or elective surgery requiring cardiopulmonary bypass including one of the following:
reoperation for CABG;
reoperation for aortic valve replacement;
reoperation for mitral valve replacement or repair;
initial mitral valve replacement;
aortic and/or mitral valve replacement/repair with a CABG;
multiple-valve replacement/repair (initial or reoperation);
ascending aortic artery procedures (including Bental procedures, etc.).
Exclusion Criteria
Patients were excluded if they
were <19 years of age;
refused consent (refusal from patient or physician);
had a terminal illness with a life expectancy of <3 months;
were previously enrolled in this study;
were currently enrolled in another perioperative interventional study;
were unable to receive blood products (i.e., difficulty with crossmatching, inability to receive blood product, including Jehovah's Witnesses, or a past history of unexplained severe transfusion reaction);
exposed to aprotinin within 6 months before randomization;
have had a thrombocytopenia defined as a platelet count <100,000/mm3;
have had a coagulopathy defined as an international normalized ratio >1.5 before surgery or the immediate preoperative use of tissue plasminogen activator or streptokinase;
have had a known or suspected adverse reaction to any of the study medications;
were scheduled for a primary operation or reoperation for adult congenital heart procedures;
had the possibility of mitral valve repair only.
Disclosures
None.
Acknowledgments
These findings were presented in part at the 2008 American Society of Nephrology Renal Week; November 4 through 9, 2008; Philadelphia, PA. The authors are grateful for the statistical insights provided by Dr. Rosane Nisenbaum (St. Michael's Hospital). Dr. Wald is supported by a Randomized Controlled Trial Mentoring Program award from the Canadian Institutes of Health Research and by an unrestricted educational grant from Amgen. Dr. Liangos was supported in part by a grant from the American Heart Association. Dr. Jaber was supported by grants from the National Institutes of Health (DK065102 and DK077751). The authors thank Robert W. MacKinnon, RN, and Douglas Michaud for assistance with enrollment of study participants, and Dr. Mary Lou Ganzer (Siemens Healthcare Diagnostics, Deerfield, IL) for loaning the BN II System to the investigators. We are also grateful to the participants.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M: Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol 15: 1597–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Rosner MH, Okusa MD: Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 1: 19–32, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A: Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens LA, Levey AS: Chronic kidney disease in the elderly—how to assess risk. N Engl J Med 352: 2122–2124, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE: Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 65: 1416–1421, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Xu H, Lu Y, Teng D, Wang J, Wang L, Li Y: Assessment of glomerular filtration rate in renal transplant patients using serum cystatin C. Transplant Proc 38: 2006–2008, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Liangos O, Kolyada A, Perianayagam MC, Tighiouart H, Wald R, MacKinnon R, Warner K, Dolan N, Jaber BL: Increased plasma IL-8 level is associated with IL-8-251 AA genotype and with acute kidney injury following cardiopulmonary bypass. J Am Soc Nephrol 18: 798A, 2007 [Google Scholar]
- 9.Fergusson DA, Hebert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, Teoh K, Duke PC, Arellano R, Blajchman MA, Bussieres JS, Cote D, Karski J, Martineau R, Robblee JA, Rodger M, Wells G, Clinch J, Pretorius R: A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med 358: 2319–2331, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Swaminathan M, Phillips-Bute BG, Conlon PJ, Smith PK, Newman MF, Stafford-Smith M: The association of lowest hematocrit during cardiopulmonary bypass with acute renal injury after coronary artery bypass surgery. Ann Thorac Surg 76: 784–791: discussion 792, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Wijeysundera DN, Karkouti K, Dupuis JY, Rao V, Chan CT, Granton JT, Beattie WS: Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA 297: 1801–1809, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Greene T, Kusek J, Beck G: A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]
- 13.Dharnidharka VR, Kwon C, Stevens G: Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis 40: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Fricker M, Wiesli P, Brandle M, Schwegler B, Schmid C: Impact of thyroid dysfunction on serum cystatin C. Kidney Int 63: 1944–1947, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Ahlstrom A, Tallgren M, Peltonen S, Pettila V: Evolution and predictive power of serum cystatin C in acute renal failure. Clin Nephrol 62: 344–350, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Villa P, Jimenez M, Soriano MC, Manzanares J, Casasnovas P: Serum cystatin C concentration as a marker of acute renal dysfunction in critically ill patients. Crit Care 9: R139–R143, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu-Omar Y, Mussa S, Naik MJ, MacCarthy N, Standing S, Taggart DP: Evaluation of Cystatin C as a marker of renal injury following on-pump and off-pump coronary surgery. Eur J Cardiothorac Surg 27: 893–898, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Momeni M, Baele P, Jacquet L, Mourad M, Waterloos H, Wallemacq P: Cystatin C in cardiac surgery. Acta Anaesthesiol Belg 58: 107–112, 2007 [PubMed] [Google Scholar]
- 19.Zhu J, Yin R, Wu H, Yi J, Luo L, Dong G, Jing H: Cystatin C as a reliable marker of renal function following heart valve replacement surgery with cardiopulmonary bypass. Clin Chim Acta 374: 116–121, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, Kasza KE, O'Connor MF, Konczal DJ, Trevino S, Devarajan P, Murray PT: Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int 74: 1059–1069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moran SM, Myers BD: Pathophysiology of protracted acute renal failure in man. J Clin Invest 76: 1440–1448, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herget-Rosenthal S, Marggraf G, Husing J, Goring F, Pietruck F, Janssen O, Philipp T, Kribben A: Early detection of acute renal failure by serum cystatin C. Kidney Int 66: 1115–1122, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, Haase M: Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery–a prospective cohort study. Crit Care Med 37: 553–560, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Haase M, Bellomo R, Devarajan P, Ma Q, Bennett MR, Mockel M, Matalanis G, Dragun D, Haase-Fielitz A: Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg 88: 124–130, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL: Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int 70: 199–203, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Coca SG, Yalavarthy R, Concato J, Parikh CR: Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int 73: 1008–1016, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Han WK, Wagener G, Zhu Y, Wang S, Lee HT: Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 4: 873–882, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu JC, Coca SG, Patel UD, Cantley L, Parikh CR: Searching for genes that matter in acute kidney injury: A systematic review. Clin J Am Soc Nephrol 4: 1020–1031, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]