Abstract
Background and objectives: Peritoneal dialysis (PD) patients may be overhydrated especially when inflammation is present. We hypothesized that patients with a plasma albumin below the median value would have measurable overhydration without a proportional increase in plasma volume (PV).
Design, setting, participants, & measurements: We investigated a cross-sectional sample of 46 prevalent PD patients powered to detect a proportional increase in PV associated with whole body overhydration and hypoalbuminemia. PV was determined from 125I-labeled albumin dilution, absolute total body water from D dilution (TBWD), and relative hydration from multifrequency bioimpedance analysis (BIA; Xitron 4200) expressed as the extracellular water (ECW):TBWBIA ratio.
Results: Whereas patients with plasma albumin below the median (31.4 g/dl) were overhydrated as determined both by BIA alone (ECW:TBWBIA 0.49 versus 0.47, P < 0.036) and the difference between estimated TBWBIA and measured TBWD (3.55 versus 0.94 L, P = 0.012), corrected PV was not different (1463 versus 1482 ml/m2, NS). Mean PV was not different from predicted, and its variance did not correlate with any other clinical measures. Multivariate analysis showed that the only independent predictor of whole body overhydration was reduced plasma albumin.
Conclusions: Hypoalbuminemia is an important determinant of tissue overhydration in PD patients. This overhydration is not associated with an increased plasma volume. Attempts to normalize the ECW:TBW ratio in hypoalbuminemic, inflamed PD patients may lead to hypovolemia and loss of residual renal function.
Evidence from various sources suggests that in a significant proportion of peritoneal dialysis (PD) patients there is difficulty in achieving euvolemia. Observational studies have found that low levels of salt and water removal, independent of residual renal function, are associated with worse survival (1,2). It is not clear, however, whether this association is causal, with excess tissue hydration or expanded plasma volume (PV) accelerating organ dysfunction, or simply evidence that there are difficulties in achieving euvolemia due to confounding factors such as cardiac dysfunction, inflammation, or poor dietary intake (3–5). Individual variability in peritoneal membrane function may also contribute to this problem, although there is now increasing evidence that poor ultrafiltration associated with high solute transport rates can be avoided by using automated PD and icodextrin (6,7).
One of the challenges in establishing cause and effect is the difficulty in measuring volume status in PD patients. Bioimpedance analysis (BIA) has been the most widely applied method and has been the main source of evidence that a significant proportion of PD patients are fluid loaded (8). In particular, the abnormal ratio of reactance (an indicator of body cell mass) to resistance (inversely proportional to the total body water [TBW]) implies patients are overhydrated for a given muscle mass. When extrapolated to actual volumes using commercial algorithms, this is frequently expressed as an abnormally high extracellular water (ECW):TBW ratio. This is clearly a relevant biometric as it predicts survival (9) and detects interventions intended to alter fluid status, (5) but suffers a number of problems. First, the ratio will be affected both by muscle wasting and abnormal tissue hydration, and compared with normal subjects, it is the former that is most abnormal (10). Second, the use of algorithms to estimate TBW volume assumes normal hydration of tissues, and we have recently demonstrated in a cohort of hemodialysis (HD) patients, followed over 12 months, that when combining BIA with absolute measurement of TBW using D dilution, patients with greater degrees of comorbidity have overhydrated tissues (11). Third, BIA fails to distinguish between intravascular and interstitial ECW excess. This is especially relevant in PD patients in whom plasma albumin is frequently depressed due to peritoneal protein losses and inflammation when it is possible to hypothesize that mal-distribution of ECW may occur due to lower plasma oncotic pressure. If so, by attempting to normalize the ECW:TBW in hypoalbuminemic patients, it is possible that PV might be reduced below normal, and there is ample evidence from both observational and intervention studies that volume depletion is a risk factor for loss in residual renal function (5,12,13).
The purpose of this study was first to demonstrate that abnormal body composition observed in hypoalbuminemic patients is indeed associated with excess tissue hydration and second to test the hypothesis that this overhydration is not associated with a proportional increase in plasma volume.
Materials and Methods
Patient Population and Study Design
This was a cross-sectional study of prevalent PD patients treated in a single center with a common clinical approach to fluid management that includes appropriate dietary advice (e.g., salt restriction) and use of automated peritoneal dialysis (APD) and icodextrin to optimize fluid removal and prevent overhydration due to dialysate reabsorption. Using this approach, the most recent analysis indicates that peritoneal membrane transport characteristics do not influence patient survival (7). As part of routine clinical assessment, membrane function and adequacy, including sodium removal, is measured at least every 6 months. Comorbidity is evaluated using the externally validated Stoke/Davies score (14,15), which comprises seven domains (noncutaneous malignancy, ischemic heart disease, left ventricular dysfunction, diabetes, systemic collagen vascular disorder, peripheral vascular disease, other life threatening illness); cardiac function was assessed by two-dimensional echocardiography. BIA is not used to inform clinical management of patients.
Sequential patients undergoing routine assessments were approached to participate, and there were no exclusion criteria other than ability and willingness to comply with the study measurements. On the day of study, usually coincident with routine assessments, all subjects underwent BIA, measurement of TBW using D dilution (TBWD) and PV using radiolabeled albumin. The study was peer-reviewed and approved by the local ethics committee, and all patients gave their signed consent.
Body Composition Measurements
PV was determined by the dilution principle following a standard intravenous injection of 10 ml 0.185 Mbq 125I-human serum albumin (HAS) (16). This was followed by blood sampling at 10-, 20-, and 30-minute intervals at a remote venous site. The isotopic decay in each case was plotted against time on a semilogarithmic scale, and the best linear fit line was drawn through these points, extrapolated to time zero to calculate the plasma volume. This is a well-established method in our hospital with validated normal ranges adjusted for gender and body surface area. Blood volume was estimated from PV and hematocrit using published equations including those by Guyton and Hidalgo (17). TBWD was measured by deuterium dilution technique using flowing afterglow mass spectrometry (FA-MS) (11). Following a baseline blood sample, an oral dose (15 to 45 ml according to body weight) of 99.8% deuterium oxide (Cambridge Isotope Laboratories, Andover, MA) was administered. The difference between baseline and equilibrated blood headspace D abundance, measured by FA-MS (11,18,19) was used to determine TBWD after accounting for equilibration with dialysate and 4% D exchange with H in body proteins. BIA was determined using the multifrequency Xitron Hydra device (Model 4200; Xitron Technologies, San Diego, CA). Measurements were performed using the standard bipolar technique with electrodes placed on the dorsum of wrist and anterior aspect of the ankle. The patient was supine for at least 10 minutes before measurements without dialysis fluid being present. TBWBIA, ECW, and percentage fat mass were determined and compared with the normal predicted values as determined by Lindley for a healthy European population, adjusted for gender, weight, and height, (20) and for ECW adjusted for weight and gender, used by Chamney to determine overhydration in HD patients (21).
Analytical Measurements
Routine laboratory measurements were made with a Beckman autoanalyzer. Plasma albumin was estimated using the Bromocreosol Purple colorimetric method, (normal range: 35 to 50 g/L), C-reactive protein (CRP) using a latex enhanced immunoturbidimetric method. Dialysate and urinary sodium were determined using the indirect electrode method.
Statistical Analysis: Primary Endpoint
The study was powered to test the following null hypothesis: The excess ECW associated with low plasma albumin in PD patients is equally distributed between extra- and intravascular space. Given that the expected excess fluid associated with a plasma albumin below the median value is approximately 3 kg and that 20% of this (approximately 0.6 kg) should be intravascular if distributed proportionately, then to detect a significant (P < 0.05) difference (i.e., higher PV in patients with plasma albumin below the mean) with 90% power, assuming a standard deviation (SD) for plasma albumin of 0.7 L, 20 patients were required in each group. Secondary analysis: A prespecified univariate and multivariate linear regression analysis was also undertaken treating the dependent variables (excess tissue hydration and plasma volume) and potential explanatory clinical measures (e.g., plasma albumin, membrane transport status, BP, comorbid score, fluid and sodium removal, log-transformed high-sensitivity CRP) as continuous covariates. The covariates were included in the multivariate model, if on bivariate correlation they were significantly related to either the dependent variables or one of the plausible explanatory variables.
All data are expressed as means (SD) unless the distribution was not parametric, in which case medians (interquartile range; IQR) are shown.
Results
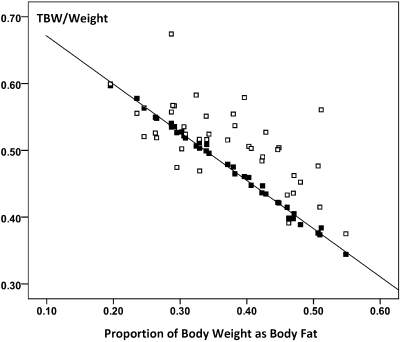
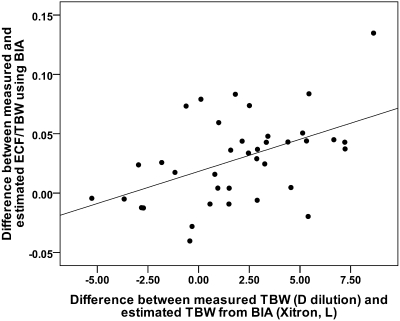
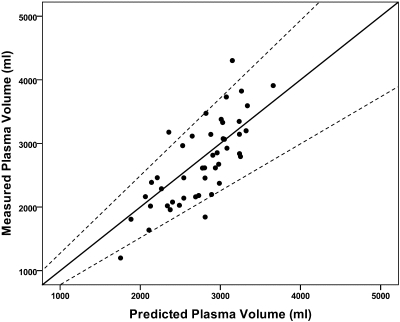
A total of 46 patients (20 female), median time on PD 19.2 months (IQR: 9.2 to 33.7) were recruited to the study to ensure adequate power to detect differences in the primary endpoint. Patient demography and body composition characteristics for the whole study population are described in Table 1. When compared with the normal predicted values, these patients had an elevated ECW:TBW ratio (0.48 versus 0.45, P = 0.001), indicating that they were either fluid-loaded, muscle-wasted, or both. The measured TBWD and the estimated TBWBIA were tightly correlated (r = 0.92, P < 0.001) but significantly different from each other such that the measured TBWD was significantly higher, +2.02 L, (95% CI: 0.96 to 3.07 L, P = 0.001). This difference, expressed as comparative plots of TBWD and TBWBIA both normalized to body weight as this relates to BIA estimated fat mass, is depicted in Figure 1. These two measures of excess tissue hydration were also correlated with each other, r = 0.48, P = 0.002, see Figure 2. In contrast, the measured PV was almost identical to the predicted values using this method, with a mean difference between measured and predicted of −51.3 ml (95% CI −180 to 78 ml, P = 0.428). The SD of the PV was about twice that predicted, indicating a greater variability than would be expected in the normal population (see Figure 3).
Table 1.
Patient demography and comparison by plasma albumin category (primary endpoint)
| All Patients (mean or %/SD) | Plasma albumin <31.4 g/dl | Plasma albumin >31.4 g/dl | 95% CI of difference | P (unpaired t test) | |
|---|---|---|---|---|---|
| N | 46 | 20 | 26 | ||
| Gender split (M:F) | 26:20 | 8:12 | 18:8 | N/A | 0.049 |
| Age (years) | 59.4/17.8 | 63.1 | 56.4 | −17.3 to 4.0 | NS |
| BMI (kg/m2) | 26.5/4.9 | 26.0 | 26.8 | −2.1 to 3.8 | NS |
| BSA (m2) | 1.81/0.23 | 1.71 | 1.88 | 0.04 to 0.3 | 0.021 |
| TBWD (L) | 36.5/7.13 | 35.6 | 37.9 | −2.2 to 6.8 | NS |
| ICWBIA (L) | 18.1/4.8 | 16.3 | 19.4 | 0.36 to 5.6 | 0.028 |
| ECWBIA (L) | 16.6/3.5 | 15.7 | 17.1 | −0.66 to 3.5 | NS |
| Difference between measured TBWD and estimated TBWBIA(L) | 2.02/3.25 | 3.55 | 0.94 | 0.61 to 4.6 | 0.012 |
| Difference between measured and predicted ECW:TBW ratio (20) | 0.029/0.035 | 0.036 | 0.023 | −0.03 to 0.008 | NS |
| Measured ECW:TBW ratio | 0.48/0.04 | 0.495 | 0.472 | −0.001 to −0.05 | 0.036 |
| Difference between measured and predicted ECW (L/kg) (21) | 0.0048/0.029 | −0.003 | +0.018 | −0.0035 to −0.0007 | 0.042 |
| Plasma volume (ml) | 2703/662 | 2551 | 2820 | −124 to 661 | NS |
| Corrected plasma volume (ml/m2) | 1474/254 | 1463 | 1482 | −135 to 173 | NS |
| Plasma volume (% different from predicted) | −2.3/16 | −0.94 | −3.4 | −12.2 to 7.2 | NS |
| Corrected blood volume (ml/m2) | 2124/342 | 2070 | 2165 | −110 to 300 | NS |
| hsCRP, mean g/L (Log CRP) | 13.2/22.90.84/0.5 | 22.3 (1.05) | 6.2 (0.69) | −0.64 to −0.085 | 0.01 |
| Hemoglobin (g/dl) | 11.7/1.34 | 11.1 | 12.3 | 0.52 to 1.96 | 0.001 |
| Solute transport (4 hour dialysate:plasma creatinine) | 0.82/0.15 | 0.84 | 0.73 | −0.18 to −0.025 | 0.011 |
| Peritoneal UF capacity (ml) | 426/194 | 408 | 440 | −85 to 149 | NS |
| Proportion using icodextrin | 63%/— | 60% | 65% | — | NS |
| Plasma sodium (mmol/l) | 137.1/3.4 | 137 | 137.2 | −1.8 to 2.2 | NS |
| Comorbidity score | 1.00/1 | 1.15 | 1.00 | −0.77 to 0.47 | NS |
| Diabetic (%) | 28%/— | 25% | 31% | — | NS |
| Systolic heart failure (%) | 6.5%/— | 10% | 3.8% | — | NS |
| Ejection fraction (%) | 56.4/8.8 | 56.42 | 56.44 | −7 to 7 | NS |
| Systolic BP (mmHg) | 137.9/20 | 137.6 | 138.1 | −11 to 12.6 | NS |
| Diastolic BP (mmHg) | 78.8/13.4 | 75.1 | 81.8 | −1.1 to 14.6 | NS |
| Peritoneal Kt/V (weekly) | 1.51/0.39 | 1.68 | 1.38 | −52 to −0.7 | 0.018 |
| Residual renal Kt/V (weekly) | 0.88/0.79 | 0.71 | 1.00 | −0.18 to 0.7 | NS |
| Peritoneal sodium loss (mmol/day) | 43.2/59.2 | 52.2 | 36.4 | −51.4 to 19.7 | NS |
| Renal sodium loss (mmol/day) | 51.9/44.4 | 37.2 | 63.2 | 0.20 to 51.7 | 0.048 |
UF, ultrafiltration.
Figure 1.
The relationship between TBW normalized to body weight determined from BIA (■) and D dilution (□) and the proportion of body fat determined from BIA. The near perfect correlation for the BIA data are due to the assumption that tissue hydration is the same in all patients, whereas the measured TBW indicates the variability in hydration state with the majority of patients showing evidence of overhydration.
Figure 2.
These two different estimates of overhydration are correlated, (r = 0.48, P = 0.002). The y-axis is a measure of how abnormal the ECW:TBW ratio is, being the difference between the measured value and that predicted from normal subjects, (20), whereas the x-axis is the difference between the estimated and measured TBW (BIA and D dilution, respectively).
Figure 3.
Relationship between measured and predicted PV (in ml). Most patients fall within the predicted normal limits as shown by the broken lines and are equally spread about the line of identity (solid line).
To test the primary endpoint, the population was categorized into those with plasma albumin below or above the median (31.4 g/L). Subjects with albumin below the median were more likely to be women, have a lower hemoglobin, and as predicted from previous studies, have evidence of overhydration, inflammation, and increased solute transport, whereas their absolute and corrected plasma volumes were not different (see Table 1). In view of the hemoglobin discrepancy and the effect this has on hematocrit, blood volume was estimated using several different published equations, but none of these derived measures resulted in a different blood volume corrected for body surface area between the two groups.
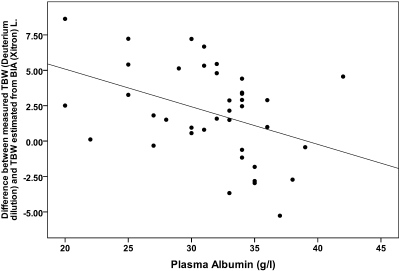
The summary of the bivariate correlations for the whole study group are shown in Table 2 and Figure 4. There were no significant correlations with corrected PV (or derived blood volume, data not shown) so multivariate analysis was not performed. There were a number of associations with excess tissue hydration, including negative correlations with plasma sodium, albumin and urinary sodium losses, positive correlations with solute transport, peritoneal sodium losses, and use of icodextrin. There was an inverse relationship between plasma albumin and inflammatory status. On multivariate analysis, the only significant finding was a negative association between plasma albumin and excess hydration (ANOVA P = 0.011), see Table 3. Substituting ECW:TBWBIA or the difference between the measured and predicted BIA ratio into the multivariate model as the dependent variable gave similar results. No relationship between BP and any of the measures of body composition, fluid status, or other clinical covariates were found except for a negative correlation between diastolic BP and both age and comorbidity. Dialysate and urinary sodium losses are tightly coupled to ultrafiltration and urine volume, respectively, and their substitution into the multivariate model made no difference.
Table 2.
Univariate correlation matrix
| Plasma albumin | High sensitivity CRP | Comorbidity Score | Solute transport | UF Capacity | Dialysate sodium losses | Urinary sodium losses | Using Icodextrin | Plasma sodium | Corrected PV | |
|---|---|---|---|---|---|---|---|---|---|---|
| TBWD:TBWBIA | −0.408, | 0.053, | 0.139, | 0.386, | −0.32, | 0.323, | −0.334, | 0.414, | −0.393, | 0.089, |
| P = 0.011 | P = 0.747 | P = 0.399 | P = 0.015 | P = 0.048 | P = 0.045 | P = 0.038 | P = 0.009 | P = 0.013 | P = 0.588 | |
| Plasma albumin | −0.57, | −0.139 | −0.2, | 0.19, | −0.077, | 0.28, | 0.092, | 0.021, | −0.074, | |
| P < 0.001 | P = 0.399 | P = 0.187 | P = 0.21 | P = 0.615 | P = 0.059 | P = 0.55 | P = 0.89 | P = 0.62 | ||
| High sensitivity CRP | 0.28, | −0.016, | −0.019, | 0.062, | −0.02, | −0.16, | −0.125, | 0.19, | ||
| P = 0.061 | P = 0.92 | P = 0.91 | P = 0.68 | P = 0.9 | P = 0.29 | P = 0.41 | P = 0.2 | |||
| Comorbidity score | −0.068, | −0.15, | 0.214, | −0.27, | 0.136, | −0.3, | −0.215, | |||
| P = 0.66 | P = 0.32 | P = 0.154 | P = 0.06 | P = 0.37 | P = 0.049 | P = 0.152 | ||||
| Solute transport | −0.46, | 0.37, | −0.39, | 0.53, | −0.2, | −0.098, | ||||
| P = 0.001 | P = 0.01 | P = 0.008 | P < 0.001 | P = 0.183 | P = 0.52 | |||||
| UF capacity | −0.19, | 0.232, | −0.48, | 0.41, | 0.06, | |||||
| P = 0.19 | P = 0.12 | P = 0.001 | P = 0.004 | P = 0.69 | ||||||
| Dialysate sodium losses | −0.37, | 0.46, | −0.224, | 0.156, | ||||||
| P = 0.011 | P = 0.001 | P = 0.134 | P = 0.29 | |||||||
| Urinary sodium losses | 0.27, | 0.22, | 0.17, | |||||||
| P = 0.07 | P = 0.145 | P = 0.25 | ||||||||
| Using icodextrin | −0.45, | −0.058 | ||||||||
| P = 0.002 | P = 0.70 | |||||||||
| Plasma sodium | 0.079, | |||||||||
| P = 0.60 |
Figure 4.
Relationship between excess fluid, as determined by the difference between measured and estimated body water, and plasma albumin (R = −0.40, P = 0.011). A similar amount of variance (12% to 25%) was observed, whichever estimate of fluid status was used: ECW:TBWBIA R = −0.49, P = 0.001; difference in ECW:TBW ratio from normal, (20) R = −0.45, P = 0.002; difference in ECW/Wt from normal, (21) R = −0.35, P = 0.018.
Table 3.
Multivariate analysis of clinical measures associated with overhydration (dependent variable is the difference between estimated and measured TBW)
| Covariate | Standard Error | Standardized β | t | Significance |
|---|---|---|---|---|
| Constant | 22.1 | 1.76 | 0.089 | |
| Plasma albumin (g/L) | 0.12 | −0.56 | −3.04 | 0.005 |
| hsCRP (mg/L) | 0.025 | −0.31 | −1.65 | 0.109 |
| Solute transport | 4.1 | 0.09 | 0.50 | 0.62 |
| Comorbid score | 0.51 | 0.096 | 0.60 | 0.55 |
| Use of icodextrin | 1.2 | 0.225 | 1.21 | 0.24 |
| Plasma sodium | 0.15 | −0.211 | −1.32 | 0.197 |
| Dialysate sodium loss | 0.009 | 0.125 | 0.79 | 0.43 |
| Urine sodium loss | 0.012 | 0.049 | 0.29 | 0.77 |
Model summary: r = 0.67, ANOVA P = 0.011.
Discussion
This study confirms that the elevated ECW:TBWBIA previously reported to be associated with hypoalbuminemia and inflammation (4,22,23) is associated with measurable excess body water in PD patients and also supports our hypothesis that this excess fluid is not equally distributed into the intravascular space. By inference, therefore, this excess fluid is within the extravascular space supporting our primary hypothesis that overhydration in PD patients is in part due to the reduced plasma filling as a result of reduced oncotic pressure associated with a low plasma albumin. This is supported by the multivariate analysis, which indicated that plasma albumin overrides the association with hydration status that is seen with a number of other measures. This is in keeping with evidence that inflammation, while important, is not the sole determinant of plasma albumin, which is also a function of peritoneal protein losses (24). The relevance of this is that attempts to normalize hydration measures in the context of hypoalbuminemia exacerbated by inflammation may lead to central volume depletion and loss of residual renal function.
We are aware of only one previous study in which both PV and hydration status of PD patients were measured simultaneously (25). Using the disappearance rate of Dextran 70, Konings et al. found an average PV of 3200 ml, rather greater than observed in this study (2700 ml). This might be due to differences in the technique, which was why we also chose to express our data as both absolute levels as well as in comparison to the predicted values using the 125I-albumin method, but most likely this reflects the different gender split (Konings: 75% male compared with 56% in this study) and thus patient sizes between these studies: Once corrected for body surface area (BSA), the values are more similar (1684 versus 1474 ml) and the remaining difference could be due to gender, as men have a relatively higher PV for their BSA than women. Nevertheless, there could be some real differences, as the patients in the Konings study might have been more overhydrated since they were studied before using icodextrin in a randomized trial and a proportion were using 1.36% glucose in the long exchange (13). These authors did divide their patients into two subgroups according to their hydration status as determined from bromide dilution, but did not report their relative plasma volumes or albumin levels. They also observed that the more overhydrated a patient was, the more the BIA underestimated it (26).
One of the advantages of the 125I-albumin method for determining plasma volume, not possible using the Dextran 70 technique, is that it can be used in patients using icodextrin, so providing the first reported measurements in PD patients using this dialysis solution. It has been proposed that use of icodextrin might either lead to loss in residual renal function due to excessive salt and water removal or relatively preserve it for a given fall in ECW by ameliorating a precipitant fall in PV (5,27). In this study, as might be expected, icodextrin was selectively used in patients with higher solute transport and was associated with higher peritoneal ultrafiltration and sodium removal and reduced plasma sodium. We observed no differences in corrected plasma volume, gender split, or any other clinical variables associated with icodextrin use with the exception of a positive correlation with overhydration on univariate analysis. This disappeared on multivariate analysis however, underlining the complex relationship between peritoneal solute transport, ultrafiltration, plasma albumin, isotonic hyponatremia, and residual and peritoneal sodium losses in PD patients. In particular, the opposite direction of the relationships between overhydration and urinary versus peritoneal sodium losses is of interest; a likely explanation is that preserved residual renal function enables better fluid status (28), whereas the higher peritoneal sodium losses represent the clinicians attempt to improve the ECW when there is high solute transport by using icodextrin as demonstrated in randomized controlled trials (13,29). The end result is that solute transport is not independently associated with overhydration. The observation that low plasma albumin is correlated with urinary but not dialysate sodium removal and that these patients lose less urinary sodium for a given residual renal clearance may reflect activation of the renin-aldosterone system in hypoalbuminemic patients.
Several previous studies have found an inverse relationship between the plasma albumin and abnormal body composition as measured by BIA (4,22,23). Usually, an increased ECW:TBW ratio is taken as evidence of overhydration when using BIA, despite the fact that both an increase in ECW and/or a decrease in TBW—effectively muscle mass—could be responsible. To account for this problem, we combined the estimated measures of TBW using BIA with an absolute measure determined from D dilution. This approach is based on the model developed by Chamney in which he demonstrated the constant levels of tissue compartment hydration independent of size and body fat (thus gender) and argued that any measured extra fluid is a measure of overhydration (30). As can readily be seen in Figure 1, the TBW estimates from BIA assume a fixed hydration constant for lean body tissues, whereas in the majority of these PD patients this underestimates tissue hydration, especially when plasma albumin is low, further supporting our hypothesis. This is strong independent evidence that a low ECW:TBW or increased ECW/weight as shown previously by Chamney (21) can indeed reflect excess fluid, as shown in Figure 2, and is in keeping with previous studies using sodium bromide dilution in PD patients (26). Using the same approach to estimate excess extracellular fluid in a longitudinal study of HD patients, we found that the average overhydration at baseline postdialysis was a little less (1.62 kg) than that observed here (2.02 kg), the main difference being that comorbidity rather than plasma albumin was the most important clinical predictor of overhydration (11). This probably reflects the fact that the variance in comorbidity is greater in HD patients, whereas the spread of plasma albumin levels is greater in PD patients.
The main limitation of this study is its cross-sectional design, which enables associations but not cause and effect to be determined with certainty. It can be argued that a low plasma albumin is in fact a consequence of intravascular dilution rather than a cause of extravascular overhydration. This would seem unlikely, however, as this would have led to higher than predicted normal plasma volumes, and we observed no correlation between measured plasma volumes and either plasma albumin concentration or its rate of disappearance from the vascular compartment. By necessity, prevalent patients were studied to observe a significant spread in residual renal function, known to influence fluid status (28). We were able to recruit sufficient patients to test our primary hypothesis, and the predicted difference in overhydration between our predefined groups and SD of the PV were similar to the values used in our power calculation; the degree of overhydration was a little less, but the identical values for the PV between groups combined with a slightly lower SD than expected makes a type 2 error very unlikely. The unequal distribution of gender and hemoglobin between the groups were not anticipated but were taken into account in the subsequent analysis. By comparing measured PV with gender-matched predicted values, still no difference was observed. If the lower hemoglobin in the overhydrated group had been dilutional, then if anything this patient group would have had a lower blood volume, but on correction for this no significant difference was found. It should be acknowledged however that the study may have had insufficient power to identify multiple other factors associated with overhydration on secondary analysis. Our failure to find any relationship between our measures of body composition and BP may have been masked by the fact that the patients continued to take antihypertensive drugs.
In summary, PD patients may be overhydrated, although not substantially more so than HD patients. The excess fluid is not necessarily associated with an expanded PV and is associated with a low plasma albumin, which potentially encourages extra vascular ECW accumulation. This may well have an adverse effect in organ function, but the risk of normalizing may adversely impact intravascular volume, patient well-being, and residual renal function. The risk to benefit ratio of normalizing the ECW:TBWBIA should be tested in carefully designed clinical trials.
Disclosures
None.
Acknowledgments
The consumable costs for this research were funded by the North Staffordshire Medical Institute Renal Research Fund. The development of the Flowing Afterglow Mass Spectometer was supported by the Wellcome Trust, (GR067160MA): Dr. Kay Tan is supported by the Baxter Renal Discoveries Extramural Grant Programme.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Brown EA, Davies SJ, Rutherford P, Meeus F, Borras M, Riegel W, Divino Filho JC, Vonesh E, Van Bree M: Survival of functionally anuric patients on automated peritoneal dialysis: The European APD Outcome Study. J Am Soc Nephrol 14: 2948–2957, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Paniagua R, Amato D, Mujais S, Vonesh E, Ramos A, Correa-Rotter R, Horl WH: Predictive value of brain natriuretic peptides in patients on peritoneal dialysis: Results from the ADEMEX trial. Clin J Am Soc Nephrol 3: 407–415, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies SJ, Brown E, Riegel W, Clutterbuck E, Heimburger O, Vega Diaz N, Mellotte G, Perez-Contreras J, Scanziani R, D'Auzac C, Kuypers D, Divino Fiho JC: What is the link between poor ultrafiltration and increased mortality in anuric APD patients? Analysis of data from EAPOS. Perit Dial Int 26: 458–465, 2006 [PubMed] [Google Scholar]
- 4.Avila-Diaz M, Ventura MD, Valle D, Vicente-Martinez M, Garcia-Gonzalez Z, Cisneros A, Furlong MD, Gomez AM, Prado-Uribe MD, Amato D, Paniagua R: Inflammation and extracellular volume expansion are related to sodium and water removal in patients on peritoneal dialysis. Perit Dial Int 26: 574–580, 2006 [PubMed] [Google Scholar]
- 5.Davies SJ, Garcia Lopez E, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, Bosselmann HP, Heimburger O, Simonsen O, Davenport A, Lindholm B, Tranaeus A, Divino Filho JC: Longitudinal relationships between fluid status, inflammation, urine volume and plasma metabolites of icodextrin in patients randomized to glucose or icodextrin for the long exchange. Nephrol Dial Transplant 23: 2982–2988, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Fang W, Bargman JM, Oreopoulos DG: High peritoneal permeability is not associated with higher mortality or technique failure in patients on automated peritoneal dialysis. Perit Dial Int 28: 82–92, 2008 [PubMed] [Google Scholar]
- 7.Perl J, Huckvale K, Chellar M, John B, Davies SJ: Peritoneal protein clearance and not peritoneal membrane transport status predicts survival in a contemporary cohort of peritoneal dialysis patients. Clin J Am Soc Nephrol 4: 121–126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plum J, Schoenicke G, Kleophas W, Kulas W, Steffens F, Azem A, Grabensee B: Comparison of body fluid distribution between chronic haemodialysis and peritoneal dialysis patients as assessed by biophysical and biochemical methods. Nephrol Dial Transplant 16: 2378–2385, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Guo LJ, Wang T: Extracellular water/intracellular water is a strong predictor of patient survival in incident peritoneal dialysis patients. Blood Purif 25: 260–266, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Woodrow G, Oldroyd B, Wright A, Coward WA, Truscott JG: The effect of normalization of ECW volume as a marker of hydration in peritoneal dialysis patients and controls. Perit Dial Int 25: S49–S51, 2005 [PubMed] [Google Scholar]
- 11.Chan C, McIntyre C, Smith D, Spanel P, Davies SJ: Combining near-subject absolute and relative measures of longitudinal hydration in hemodialysis. Clin J Am Soc Nephrol 4: 1791–1798, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies SJ: Preserving residual renal function in peritoneal dialysis: Volume or biocompatibility? Nephrol Dial Transplant 24: 2620–2622, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Konings CJ, Kooman JP, Schonck M, Gladziwa U, Wirtz J, Van Den Wall Bake AW, Gerlag PG, Hoorntje SJ, Wolters J, Van Der Sande FM, Leunissen KM: Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: A randomized study. Kidney Int 63: 1556–1563, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Davies SJ, Phillips L, Naish PF, Russell GI: Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant 17: 1085–1092, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Van Manen JG, Korevaar JC, Dekker FW, Boeschoten EW, Bossuyt PM, Krediet RT: Adjustment for comorbidity in studies on health status in ESRD patients: Which comorbidity index to use? J Am Soc Nephrol 14: 478–485, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Schultz AL, Hammarsten JF, Heller BI, Ebert RV: A critical comparison of the T-1824 dye and iodinated albumin methods for plasma volume measurement. J Clin Invest 32: 107–112, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyton AC, Coleman TG, Granger HJ: Circulation: Overall regulation. Annu Rev Physiol 34: 13–46, 1972 [DOI] [PubMed] [Google Scholar]
- 18.Spanel P, Smith D: Accuracy and precision of flowing afterglow mass spectrometry for the determination of the deuterium abundance in the headspace of aqueous liquids and exhaled breath water. Rapid Commun Mass Spectrom 15: 867–872, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Asghar RB, Diskin AM, Spanel P, Smith D, Davies SJ: Measuring transport of water across the peritoneal membrane. Kidney Int 64: 1911–1915, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindley E, Devine Y, Hall L, Cullen M, Cuthbert S, Woodrow G, Lopot F: A ward-based procedure for assessment of fluid status in peritoneal dialysis patients using bioimpedance spectroscopy. Perit Dial Int 25: S46–S48, 2005 [PubMed] [Google Scholar]
- 21.Chamney PW, Kramer M, Rode C, Kleinekofort W, Wizemann V: A new technique for establishing dry weight in hemodialysis patients via whole body bioimpedance. Kidney Int 61: 2250–2258, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Jones CH, Smye SW, Newstead CG, Will EJ, Davison AM: Extracellular fluid volume determined by bioelectric impedance and serum albumin in CAPD patients. Nephrol Dial Transplant 13: 393–397, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Jones CH, Wells L, Stoves J, Farquhar F, Woodrow G: Can a reduction in extracellular fluid volume result in increased serum albumin in peritoneal dialysis patients? Am J Kidney Dis 39: 872–875, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Kaysen GA, Yeun J, Depner T: Albumin synthesis, catabolism and distribution in dialysis patients. Miner Electrolyte Metab 23: 218–224, 1997 [PubMed] [Google Scholar]
- 25.Konings CJ, Kooman JP, Schonck M, Dammers R, Cheriex E, Palmans Meulemans AP, Hoeks AP, van Kreel B, Gladziwa U, van der Sande FM, Leunissen KM: Fluid status, blood pressure, and cardiovascular abnormalities in patients on peritoneal dialysis. Perit Dial Int 22: 477–487, 2002 [PubMed] [Google Scholar]
- 26.Konings CJ, Kooman JP, Schonck M, Cox-Reijven PL, van Kreel B, Gladziwa U, Wirtz J, Gerlag PG, Hoorntje SJ, Wolters J, Heidendal GA, van der Sande FM, Leunissen KM: Assessment of fluid status in peritoneal dialysis patients. Perit Dial Int 22: 683–692, 2002 [PubMed] [Google Scholar]
- 27.Konings CJ, Kooman JP, Gladziwa U, van der Sande FM, Leunissen KM: A decline in residual glomerular filtration during the use of icodextrin may be due to underhydration. Kidney Int 67: 1190–1191, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Konings CJ, Kooman JP, Schonck M, Struijk DG, Gladziwa U, Hoorntje SJ, Van Der Wall Bake AW, Van Der Sande FM, Leunissen KM: Fluid status in CAPD patients is related to peritoneal transport and residual renal function: Evidence from a longitudinal study. Nephrol Dial Transplant 18: 797–803, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, Bosselmann HP, Heimburger O, Simonsen O, Davenport A, Tranaeus A, Divino Filho JC: Icodextrin improves the fluid status of peritoneal dialysis patients: Results of a double-blind randomized controlled trial. J Am Soc Nephrol 14: 2338–2344, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Chamney PW, Wabel P, Moissl UM, Muller MJ, Bosy-Westphal A, Korth O, Fuller NJ: A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr 85: 80–89, 2007 [DOI] [PubMed] [Google Scholar]