Abstract
Background and objectives: Hyperuricemia is associated with hypertension, inflammation, renal disease progression, and cardiovascular disease. However, no data are available regarding the effect of allopurinol in patients with chronic kidney disease.
Design, setting, participants, & measurements: We conducted a prospective, randomized trial of 113 patients with estimated GFR (eGFR) <60 ml/min. Patients were randomly assigned to treatment with allopurinol 100 mg/d (n = 57) or to continue the usual therapy (n = 56). Clinical, biochemical, and inflammatory parameters were measured at baseline and at 6, 12, and 24 months of treatment. The objectives of study were: (1) renal disease progression; (2) cardiovascular events; and (3) hospitalizations of any causes.
Results: Serum uric acid and C-reactive protein levels were significantly decreased in subjects treated with allopurinol. In the control group, eGFR decreased 3.3 ± 1.2 ml/min per 1.73 m2, and in the allopurinol group, eGFR increased 1.3 ± 1.3 ml/min per 1.73 m2 after 24 months. Allopurinol treatment slowed down renal disease progression independently of age, gender, diabetes, C-reactive protein, albuminuria, and renin-angiotensin system blockers use. After a mean follow-up time of 23.4 ± 7.8 months, 22 patients suffered a cardiovascular event. Diabetes mellitus, previous coronary heart disease, and C-reactive protein levels increased cardiovascular risk. Allopurinol treatment reduces risk of cardiovascular events in 71% compared with standard therapy.
Conclusions: Allopurinol decreases C-reactive protein and slows down the progression of renal disease in patients with chronic kidney disease. In addition, allopurinol reduces cardiovascular and hospitalization risk in these subjects.
In patients with renal disease, there is decreased uric acid (UA) urinary excretion, and whether this will give rise to hyperuricemia depends on the gastrointestinal excretory compensation. By this, the prevalence of elevated serum UA in patients with chronic kidney disease (CKD) is higher (1). Elevated serum UA has been related to increased risk for the development of hypertension and cardiovascular disease (2). Chronic hyperuricemia would stimulate the renin-angiotensin system and inhibit release of endothelial nitric oxide, contributing to renal vasoconstriction and increasing BP, at the same time, high levels of UA may have a pathogenetic role in interstitial inflammation and progression of renal disease (3,4).
Allopurinol decreases serum UA level by inhibiting the enzyme xanthine oxidase. For animal models of established renal diseases, correction of the hyperuricemic state can significantly improve BP control, decreasing proteinuria and slowing the progression of renal disease (4). There are few data on patients with CKD that confirm these findings.
Recently, two studies of patients with CKD have been published that show a relationship between serum UA levels and cardiovascular mortality (5,6). However, prospective studies are necessary to show that reduction of UA levels prevent cardiovascular events.
The primary objective of this study was to analyze the effect of allopurinol in patients with moderate CKD in reduction of inflammatory markers and renal disease progression. The secondary objective was to analyze the effect of allopurinol treatment in cardiovascular and hospitalization risk.
Materials and Methods
The study was approved by the institutional ethics committee, and each participating patient gave written informed consent before enrollment.
One hundred thirty-five patients were followed up in our renal clinic from January 2007 to May 2007 and screened for eligibility to participate in the study. Included subjects had to fulfill the following inclusion criteria: (1) presence of renal disease, defined as having an estimated GFR (eGFR) lower than 60 ml/min; (2) stable clinical condition in terms of no hospitalizations nor cardiovascular events within the 3 months before screening; and (3) stable renal function (baseline serum creatinine had not increased by 50% in the 3 months before screening).
We excluded patients with a history of allopurinol intolerance, those who were already on allopurinol treatment, with active infections or inflammatory diseases, with HIV infection, with chronic hepatopathy, and patients who received immunosuppressive therapy.
One hundred thirteen patients satisfied these criteria and were screened.
Patients were randomly assigned according to a computer-generated list into a control group or a treatment group. Treatment group patients were administered a dose of 100 mg/d of allopurinol. The dosage of antihypertensive drugs, lipid-lowering agents, and antiplatelet drugs were continued and adjusted according to the individual patient's clinical condition.
Follow-Up Assessment
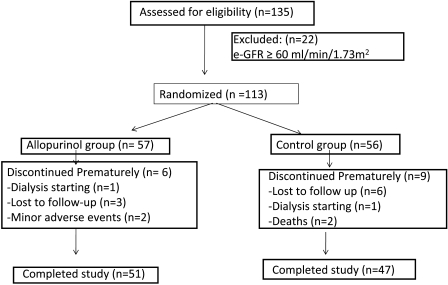
The mean time of follow-up was 23.4 ± 7.8 months. A patient flow chart is showed in Figure 1. Systolic BP (SBP), diastolic BP (DBP), and previous cardiovascular diseases were recorded. Serum creatinine, daily urinary protein excretion, hemoglobin level, erythrocyte sedimentation rate, C-reactive protein (CRP), C-cystatin, serum fibrinogen, and serum albumin were checked basally and 6 and 12 months after treatment. Modification of the Diet in Renal Disease (MDRD)-4 equation was used to estimate glomerular filtration. Renal function was measured basally and at 6, 12, and 24 months after allopurinol treatment.
Figure 1.
Patient flow chart.
Routine clinical and biochemical variables were measured by standardized methods on autoanalyzers. High sensitive C-reactive protein (hs-CRP) plasma level was measured with a latex-based turbidimetric immunoassay on a Hitachi analyzer (Sigma Chemical Co, St. Louis, MO). Daily urinary albumin excretion was measured with an immunonephelometric method.
Adverse Events
Any adverse events considered to be related to the use of allopurinol were recorded during the follow-up assessment. For serious adverse events, allopurinol therapy would be discontinued.
Outcome Analysis
The patient's clinical outcome was analyzed after the follow-up time. We defined study end points as followed: (1) hospitalizations; (2) cardiovascular events; (3) end-stage renal disease requiring dialysis therapy; and (4) mortality.
Cardiovascular event was considered if the patient had a myocardial infarction, coronary revascularization, or angina pectoris. Congestive heart failure (CHF) was diagnosed by x-ray examination (pulmonary edema) and echocardiogram with left ventricular dysfunction. This diagnostic was considered as the patients were symptomatic and in New York Heart Association (NYHA) class II to IV with a left ventricular ejection fraction ≤45%. Cerebrovascular disease was established if the patient had a history of transient ischemic attacks, whenever stroke could be verified by computer tomography or carotid artery stenosis >70% could be verified by doppler ultrasound. Peripheral vascular disease was diagnosed by intermittent claudication, stenosis of the major arteries of the lower limbs angiographically or sonographically proven, and the presence of ulcers caused for atheroesclerotic disease or by surgery was used for diagnosis.
Death and hospitalizations of any causes were accurately recorded. Each event was reviewed by physicians. This information always included study hospitalization records and in the case of an out-of-hospital death, family members were interviewed by telephone to better ascertain the circumstances surrounding death.
Blinding
The laboratory researcher was unaware of the baseline clinical status of the patients. Clinical data, including baseline and the outcomes of the patients were recorded by clinicians who were unaware of the laboratory results.
Statistical Analysis
The statistical analysis was performed by intention to treat.
All statistical analysis were performed using the SPSS program, version 16.0 (SPSS Inc, Chicago, IL) for Windows XP. Values are expressed as mean ± SD, mean ± SEM, or median (interquartile range). Categorical data were compared by means of Chi-square test and continous variables by means of t test. ANOVA test was used when several parameters of the two groups were compared. Cox proportional hazard models were used to evaluate the risk of cardiovascular events and renal disease progression, adjusted for several groups of covariates. Statistical significance is defined as two-tailed P less than 0.05.
Results
A total of 113 patients were enrolled in the study. Fifty-six patients were randomized to the control group and 57 patients to the allopurinol group. Baseline characteristics, previous cardiovascular diseases, concomitant medication, and laboratory parameters are listed in Tables 1 and 2.
Table 1.
Baseline analytical in control and allopurinol groups
| Control Group (n = 56) | Allopurinol Group (n = 57) | |
|---|---|---|
| Age (years) | 71.4 ± 9.5 | 72.1 ± 7.9 |
| C cystatine (mg/L) | 1.9 ± 0,7 | 1.9 ± 0.5 |
| Serum creatinine (mg/dl) | 1.8 ± 0.6 | 1.7 ± 0.4 |
| eGFR (ml/min per 1.73 m2) | 39.5 ± 12.4 | 40.6 ± 11.3 |
| Uric acid (mg/dl) | 7.3 ± 1.6 | 7.9 ± 2.1 |
| hsCRP (mg/L) | 3.4 (4.7) | 4.4 (4.5) |
| Serum fibrinogen (mg/dl) | 374 ± 78 | 381 ± 79 |
| ESR (mm/h) | 15 (21) | 17 (23) |
| Hemoglobin (g/dl) | 14.5 ± 4.6 | 13.6 ± 1.7 |
| Serum albumin (g/dl) | 4.4 ± 0.3 | 4.3 ± 0.3 |
| Albuminuria (mg/d) | 35 (47) | 36 (343) |
Variables are expressed as a mean ± SD or median (interquartile range).
ESR, erythrocyte sedimentation rate.
No significant differences were observed between the different analyzed variables.
Table 2.
Baseline characteristics in the two groups
| Control Group (n = 56) | Allopurinol Group (n = 57) | |
|---|---|---|
| Renal pathology, % (n) | ||
| Diabetes mellitus | 18 (10) | 16 (9) |
| Vascular nephropathy | 45 (25) | 49 (28) |
| Glomerulonephritis | 9 (5) | 2 (1) |
| Polycystic kidney disease | 2 (1) | 3 (2) |
| Interstitial nephropathy | 3 (2) | 14 (8) |
| Systemic vasculitis | 3 (2) | 0 (0) |
| Unknown etiology renal disease | 20 (11) | 16 (9) |
| Diabetes mellitus, % (n) | 36 (20) | 39 (22) |
| Ischemic cardiopathy, % (n) | 18 (10) | 29 (16) |
| Cerebrovascular disease, % (n) | 2 (2) | 9 (2) |
| Periferic vascular disease, % (n) | 4 (1) | 4 (5) |
| Diuretics use, % (n) | 54 (30) | 63 (36) |
| Thiazide diuretics, n | 13 | 15 |
| Loop diuretics, n | 17 | 21 |
| RAAS blockers, % (n) | 75 (41) | 85 (47) |
| Calcium-channel blockers, % (n) | 36 (20) | 23 (13) |
| Statins treatment, % (n) | 44 (24) | 49 (27) |
| Antiplatelet treatment, % (n) | 33 (18) | 27 (15) |
| Double treatment, % (n) | 50 (28) | 56 (32) |
| Triple treatment, % (n) | 20 (11) | 14 (8) |
No significant differences were observed between the different analyzed variables.
Double treatment, RAAS blockers and statins or antiplatelet treatment.
Triple treatment, RAAS blockers and statins and antiplatelet treatment.
Biochemical, Inflammatory Parameters, and BP Control
BP control was similar in both groups, and no significant differences were observed in the follow-up period in SBP and DBP (Table 3).
Table 3.
BP control in the two groups
| Control Group (n = 56) | Allopurinol Group (n = 57) | |
|---|---|---|
| Baseline (mmHg) | 146 ± 17/76 ± 13 | 147 ± 20/77 ± 11 |
| 6 months (mmHg) | 144 ± 16/77 ± 9 | 145 ± 17/76 ± 9 |
| 12 months (mmHg) | 141 ± 15/75 ± 8 | 142 ± 16/74 ± 9 |
| 24 months (mmHg) | 143 ± 13/74 ± 10 | 144 ± 15/73 ± 10 |
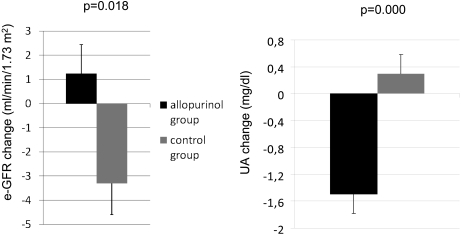
After 24 months of allopurinol treatment, serum UA levels were significantly decreased in subjects treated with allopurinol, from 7.8 ± 2.1 mg/dl to 6.0 ± 1.2 mg/dl (P = 0.000), whereas serum UA levels for subjects in the control group remain unchanged throughout the study period (7.3 ± 1.6 mg/dl at baseline and 7.5 ± 1.7 mg/dl at 24 months) (P = 0.016 between groups and time period) (Table 4). The change in UA levels at 24 months was +0.3 ± 0.27 mg/dl in the control group in comparison to −1.6 ± 0.27 mg/dl in the allopurinol group (P = 0.000) (Figure 2).
Table 4.
Effect of allopurinol in UA levels and renal function estimated by MDRD-4
| Uric Acida (mg/dl) | P1 | eGFRb (ml/min per 1.73 m2) | P2 | |
|---|---|---|---|---|
| Control group | ||||
| Basal | 7.3 ± 1.6 | 39.5 ± 12.4 | ||
| 6 months | 7.0 ± 1.6 | ns | 37.2 ± 14.3 | ns |
| 12 months | 7.4 ± 2.0 | ns | 35.6 ± 13.4 | ns |
| 24 months | 7.5 ± 1.7 | ns | 35.9 ± 12.3 | ns |
| Allopurinol group | ||||
| Basal | 7.8 ± 2.1 | 40.8 ± 11.2 | ||
| 6 months | 6.2 ± 1.5 | 0.000 | 41.1 ± 12.9 | ns |
| 12 months | 6.0 ± 1.8 | 0.000 | 41.1 ± 13.2 | ns |
| 24 months | 6.0 ± 1.2 | 0.000 | 42.2 ± 13.2 | ns |
P = 0.016 between groups.
P = 0.000 between groups.
P1, differences in comparison to baseline period within each experimental group.
P2, differences in comparison to baseline period within each experimental group.
Figure 2.
Change in UA levels and change in eGFR at the end of study. Values are expressed as mean ± SEM.
hs-CRP median levels decreased significantly after 12 months of allopurinol treatment (from 4.4 mg/L to 3.0 mg/L) (P = 0.04 in comparison to baseline values), whereas the control group remained unchanged in the follow-up period (from 3.4 to 3.2 mg/L). C-cystatin decreased significantly in the allopurinol group from 1.9 ± 0.5 to 1.4 ± 0.4 mg/L after 12 months of treatment. In the control group, C-cystatin levels remained unchanged (P = 0.008 between groups). There were no changes in serum hemoglobin, serum fibrinogen, erythrocyte sedimentation rate, and serum albumin levels after the 12-month study period in both groups (Table 5).
Table 5.
Allopurinol effect in inflammatory parameters
| hsPCRa (mg/L) | C Cystatinb (mg/L) | Albuminuria (mg/d) | Fibrinogen (mg/dl) | |
|---|---|---|---|---|
| Control group | ||||
| Basal | 3.4 (5.2) | 2.0 ± 0.7 | 32 (383) | 384 ± 104 |
| 6 months | 3.0 (7.6) | 2.0 ± 0.8 | 43 (417) | 373 ± 112 |
| 12 months | 3.2 (10.8) | 1.9 ± 1.0 | 51 (296) | 402 ± 98 |
| Allopurinol group | ||||
| Basal | 4.4 (4.5) | 1.9 ± 0.5 | 36 (388) | 381 ± 78 |
| 6 months | 3.0 (4.0)c | 1.8 ± 0.6 | 15 (103) | 367 ± 58 |
| 12 months | 3.0 (2.5)c | 1.4 ± 0.4 | 16 (166) | 369 ± 49 |
P = 0.018 between groups and time periods (two-way ANOVA).
P = 0.008 between groups (two-way ANOVA).
P = 0.04 differences in comparison to baseline period in allopurinol group.
Data are expressed as mean ± SD or median (interquartile range). No differences were observed in hemoglobin, ESR, and serum albumin levels between groups and periods.
Progression of Renal Disease
In the allopurinol group, there was no significant change in eGFR (MDRD-4) after 24 months (from 40.8 ± 11.2 to 42.2 ± 13.2 ml/min per 1.73 m2), whereas in the control group, there was worsening by the end of the study (from 39.5 ± 12.4 to 35.9 ± 12.3 ml/min) (P = 0.000 between groups) (Table 4). In the control group, eGFR decreased 3.3 ± 1.2 ml/min per 1.73 m2, and in allopurinol group, eGFR increased 1.3 ± 1.3 ml/min per 1.73 m2 after 24 months (P = 0.018) (Figure 2).
We have evaluated the correlation between UA levels and eGFR in the whole data and within each experimental group. There is a significant inverse correlation between UA levels and eGFR in all cases. The change in UA levels at 24 months has been plotted against the change in eGFR (Figure 2), and we found a significant inverse correlation between changes (r = −0375; P = 0001).
Allopurinol treatment slowed renal disease progression (defined as a decrease higher than 0.2 ml/min per 1.73 m2 per month) in comparison with control groups in a cox regression model adjusted for age, gender, diabetes, UA, hs-CRP levels, renin-angiotensin system blockers, CKD etiology, and albuminuria (hazard ratio [HR], 0.53; [0.28 to 0.99]; P = 0.048).
Cardiovascular Events, Hospitalizations, and Death
There were two deaths in the control group and two patients required dialysis (one from the control group and one from the allopurinol group).
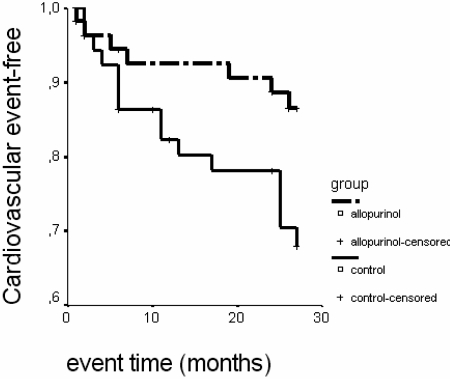
After a mean follow-up time of 23.4 ± 7.8 months, 22 patients suffered a cardiovascular event: 15 in the control group and seven in allopurinol group. Cardiovascular events were: 8 congestive heart failures, 7 ischemic coronary events, 5 cerebrovascular accidents, 1 peripheric arteriopathy, and 1 arrythmia. Kaplan-Meier survival showed that patients in the allopurinol group had lower cardiovascular risk than patients in the control group (log rank: 4.25; P = 0.039) (Figure 3).
Figure 3.
Effect of allopurinol treatment in cardiovascular events. Log rank: 4.25; P = 0.039.
Regression cox analysis adjusted for age, eGFR change, and UA levels showed that diabetes (P = 0.004), CRP levels (P = 0.031), and previous coronary arteriopathy (P = 0.005) increased the risk of cardiovascular events. Allopurinol treatment decreased the risk of cardiovascular events in 71% (P = 0.026) (Table 6).
Table 6.
Cox regression analysis. Risk of new cardiovascular events
| HR | CI (95%) | P | |
|---|---|---|---|
| Diabetes | 4.38 | 1.59 to 12.09 | 0.004 |
| Previous coronary heart disease | 4.49 | 1.56 to 12.86 | 0.005 |
| C-Reactive protein (mg/L) | 2.83 | 1.09 to 7.32 | 0.031 |
| Allopurinol treatment | 0.29 | 0.09 to 0.86 | 0.026 |
Model adjusted for age, eGFR change, and serum UA.
HR, hazard rates for new cardiovascular events; CI, confidence interval.
New cardiovascular events: Congestive heart failure, cerebrovascular accidents, ischemic coronary events, and peripheric arteriopathy.
Twenty-two patients from the control group and 12 from the allopurinol group were hospitalized (P = 0.032). Allopurinol treatment reduced the risk of hospitalization in 62% in a cox regression model that included age, eGFR, presence of diabetes mellitus, and coronariopathy (HR, 0.378; [0.154 to 0.927]; P = 0.033).
Adverse Events
Allopurinol was withdrawn in two patients for gastrointestinal symptoms. No abnormalities in liver function test were attributed to allopurinol treatment. No hematologic alterations or serious adverse events in relation to allopurinol treatment appeared in the follow-up study. Six patients in the control group and three in the allopurinol group were lost during the study period.
Discussion
Patients with CKD develop hyperuricemia as GFR declines. In different small randomized controlled trials, allopurinol treatment resulted in the improvement of oxidative stress, endotelial function (7,8), and progression of CKD (9).
In this study, we showed that allopurinol treatment decreases CRP levels, slows the progression of renal disease, decreases the number of hospitalizations, and reduces cardiovascular risk.
Allopurinol Treatment and Inflammation
A correlation of CRP, a marker of subclinical inflammation related to atherosclerosis, and serum UA levels has been described (10). A significant independent association was found between UA and inflammatory markers, such as a white blood cell count, CRP, interleukins, and TNFα levels. There is also evidence that hyperuricemia per se impairs endothelial function-dependent vasodilation by the reduction in nitric oxid synthase in animal experiments (11). There are no data regarding the effect of allopurinol treatment in these inflammatory markers in moderate CKD. In this work, we showed that allopurinol decreases hs-CRP levels after 12 months compared with the control group.
Allopurinol Treatment and Progression of Renal Disease
An elevated UA level has been associated with a greater incidence of end-stage renal disease. Hyperuricemia induces high BP, renal afferent arteriopathy, increased glomerular hydrostatic pressure, and renal scarring. Kang et al. found that hyperuricemic rats showed greater proteinuria, greater BP, and greater serum creatinine levels than controls that were treated with allopurinol to decrease serum UA levels.
In our study, we demonstrated that allopurinol is able to slow the progression of renal disease after a mean time of 23.4 ± 7.8 months. No changes in BP or in albuminuria induced by allopurinol have been observed. Although, there is a relationship between hypertension and hyperuricemia, it is not known whether lowering UA levels with allopurinol will be effective in people with longstanding hypertension. The only study that showed that allopurinol treatment reduces BP was performed in adolescent subjects with initial hypertension (12).
Allopurinol may, by diminishing serum UA levels, serve as an agent to decrease glomerular hydrostatic pressure indirectly and thus help alleviate renal damage. In our study, there is a significant inverse correlation between UA levels and eGFR in the whole data and within each experimental group. The change in UA levels at 24 months has been plotted against the change in eGFR (Figure 2), and we found a significant inverse correlation between changes (r = −0375; P = 0001). By the mean, the beneficial effect of allopurinol slowing down the progression of renal disease could be related to the decrease of UA level.
Recent studies suggest that lowering levels of UA may slow progression of renal disease, especially in patients with hyperuricemia. Kanbay et al. reported that treatment of asymptomatic hyperuricemia improved renal function (13). Likewise, Siu et al. reported that the treatment of asymptomatic hyeruricemia delayed disease progression (9). The results of our study are similar, but with more patients and a longer follow-up time.
Allopurinol Treatment and Cardiovascular Risk
The relation of UA to cardiovascular disease in the general population is controversial, with studies showing conflicting results. The National Health and Nutrition Éxamination Survey (NHANES I) study demonstrated a positive correlation (14). An analysis of the Framingham data showed no relation between UA and cardiovascular disease after adjustment for diuretic use (15). Subanalysis of the Atherosclerosis Risk in Communities (ARIC) study has demonstrated that hyperuricemia is associated with insulin resistance and mortality in the non-CKD population. However, the presence of CKD attenuates the associations of UA with mortality (16). A recent work in renal transplant recipients demonstrated an association of hyperuricemia with the composite outcome of cardiovascular events and chronic allograft nephropathy (17). Larger prospective studies are required to understand this complex interrelation in the general population.
Actually, there are no data about the allopurinol effect in decreasing cardiovascular risk in the general population or in CKD. In this study, preliminary results showed that allopurinol treatment reduces cardiovascular events (relative risk [RR] 71%) and hospitalizations (RR 62%) compared with the usual therapy.
Although in our study, no serious adverse effects appeared, allopurinol therapy can produce serious reactions, such as Stevens-Johnson syndrome, and there is no evidence to treat all patients with asymptomatic hyperuricemia with this drug.
There are several limitations to our study. First, it was not designed in a double-blinded fashion. Second, all patients were advised about the dietary composition, although the potential role of dietary factors in the results has not been evaluated. Therefore, we assume that there were no differences in the diet between the two groups, and the possibility that a stricter diet restricting the intake of protein and/or salt could influence CKD progression is only probable. Finally, the results of our study may be limited by the concomitant use of statins, antiplatelet, and renin-angiotensin-aldosterone system (RAAS) blocker drugs. Although there were no baseline differences in the use of these drugs between the groups, these treatments could have been modified during the study according to good clinical practices, and we could not delineate completely the possible beneficial effect contributed by these drugs in the decrease of cardiovascular risk and preservation of kidney function.
We conclude that allopurinol treatment decreases inflammation and slows the progression of renal disease in patients with moderate CKD. In addition, allopurinol reduces cardiovascular and hospitalization risk. These results have to be confirmed in larger prospective trials and are the basis for a hypothesis that still needs to be tested.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Edwards NL: The role of hyperuricemia and gout in kidney and cardiovascular disease. Clev Clin J Med suppl 5: S13–S16, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Gagliardi A, Miname M, Santos R: Uric acid: A marker of increased cardiovascular risk. Atherosclerosis 202: 11–17, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Feig DI, Kang D, Johnson R: Uric acid and cardiovascular risk. N Engl J Med 358: 1811–1821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, Tuttle KR, Rodriguez-Iturbe B, Herrera-Acosta J, Mazzali M: Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 41: 1183–1190, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Suliman ME, Johnson RJ, Garcia-Lopez E, Qureshi AR, Molinaei H, Carrero JJ, Heimbürger O, Bárány P, Axelsson J, Lindholm B, Stenvinkel P: J-shaped mortality relationship for uric acid in CKD. Am J Kidney Dis 48: 761–771, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, Levey AS, Menon V: Uric acid and long-term outcomes in CKD. Am J kidney Dis 53: 796–803, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farquharson CA, Butler R, Hill A, Belch JJ, Struthers A: Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation 106: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 8.George J, Carr E, Davies J, Belch JJ, Struthers A: High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 114: 2508–2516, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Siu YP, Leung KT, Tong Mk, Kwan THl: Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 47: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Ruggiero C, Cherubini A, Ble A, Bos AJ, Maggio M, Dixit VD, Lauretani F, Bandinelli S, Senin U, Ferrucci L: Uric acid and inflammatory markers. Eur J Heart 27: 1174–1181, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ: Hyperuricemia induces endothelial dysfunction and vasoconstriction. Kidney Int 67: 1739–1742, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Feig DL, Soletsky B, Johnson RJ: Effect of allopurinol on the blood pressure of adolescents with newly diagnosed essential hypertension. JAMA 330: 94–132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N, Uz E, Akcay A, Yigitoglu R, Covic A: Effect of treatment of hiperuricemia with allopurinl on blood pressure, creatinine clearance, and proteinuria in patients with normal renal function. Int Urol Nephrol 39: 1227–1233, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Fang J, Aderman M: Serum uric acid and cardiovascular mortality: The NHANES I epidemiologic follow-up study, 1971-1972. JAMA 283: 2404–2410, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Culleton BJ, Larson MG, Kannel WB, Levy D: Serum uric acid and risk for cardiovascular disease and death: The Framingham Heart Study. Ann Intern Med 131: 7–13, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Navaneethan SD, Beddhu S: Associations of serum uric acid with cardiovascular events and mortality in moderate chronic kidney disease. Nephrol Dial Transplant 24: 1260–1266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akalin E, Ganesthan SV, Winston J, Muntner P: Hyperuricemia is associated with the development of the composite outcomes of new cardiovascular events and chronic allograft nephropathy. Transplantation 86: 652–658, 2008 [DOI] [PubMed] [Google Scholar]