Abstract
Balance studies have been carried out to evaluate the influence of vasopressin-induced volume expansion on acid-base equilibrium in normal dogs and in dogs with steady-state metabolic acidosis induced by the administration of 5-7 mmoles/kg per day of hydrochloric acid.
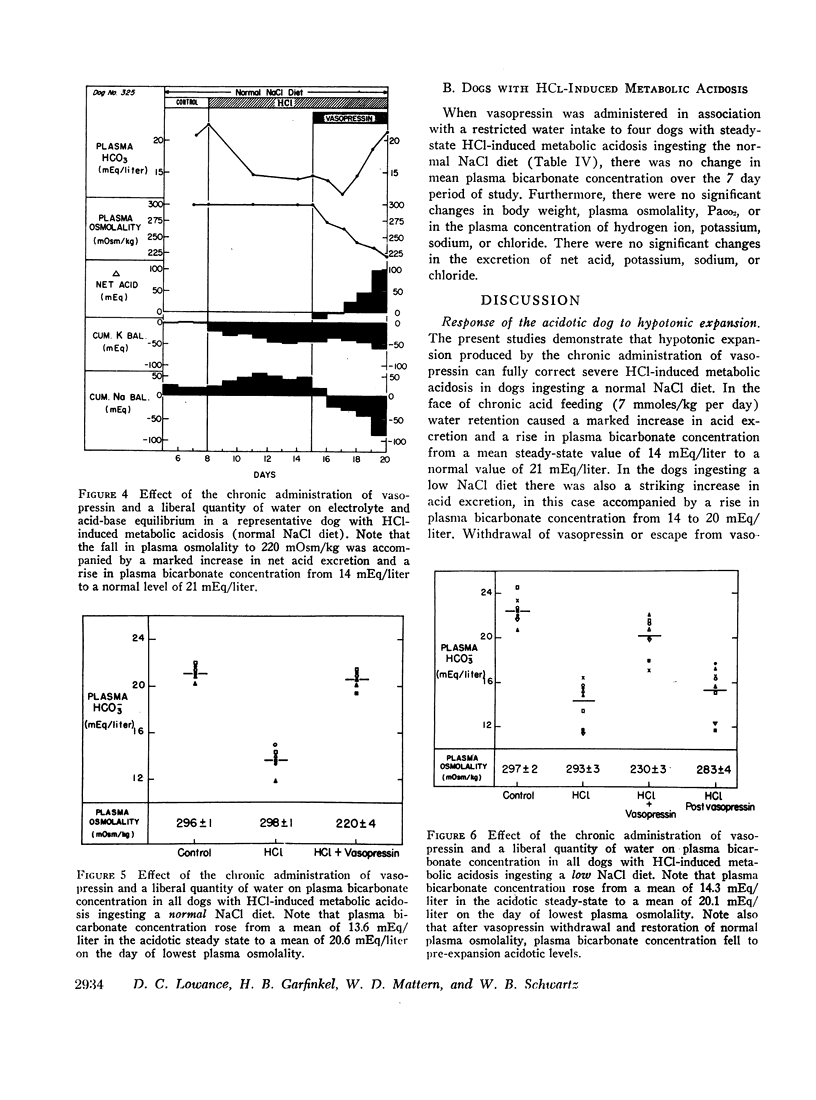
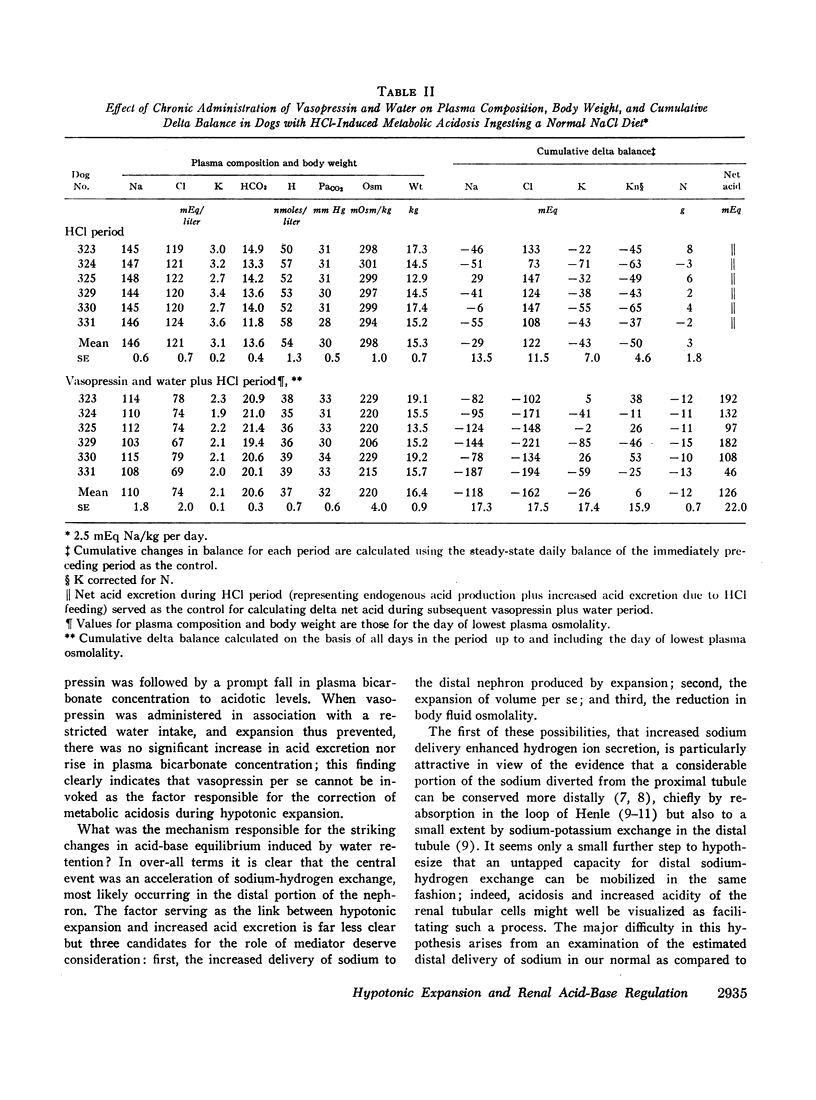
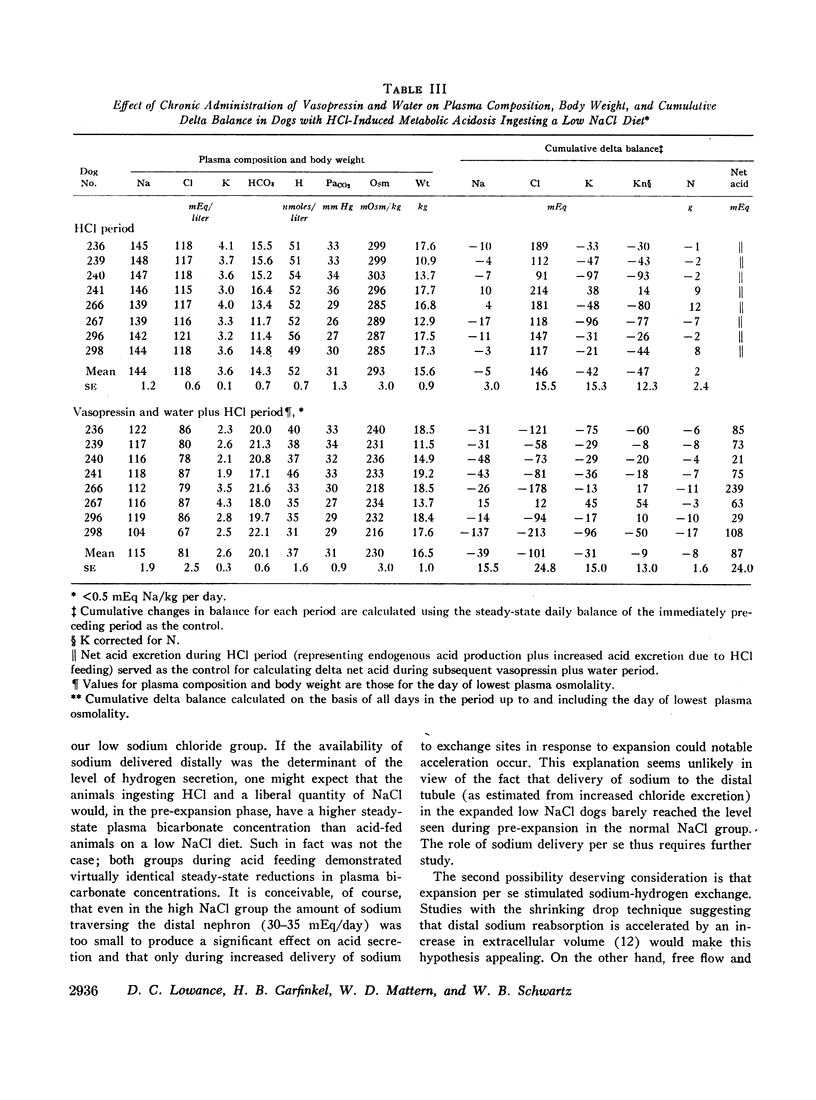
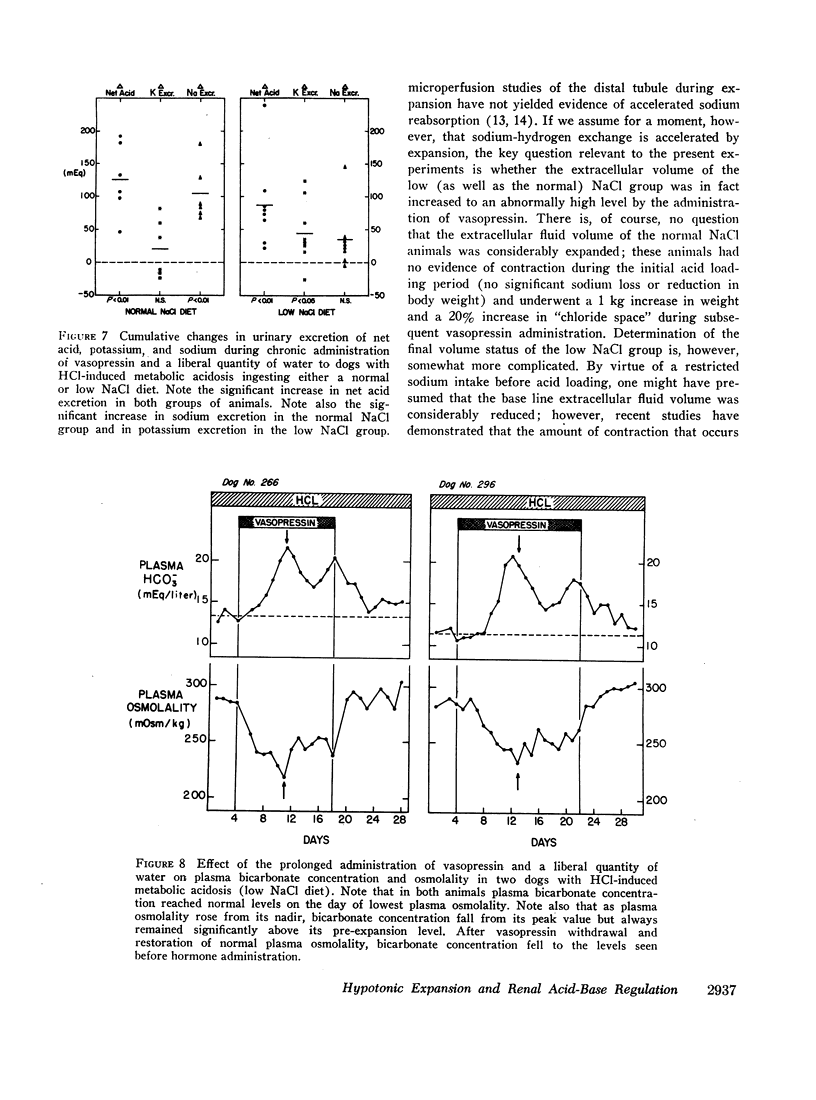
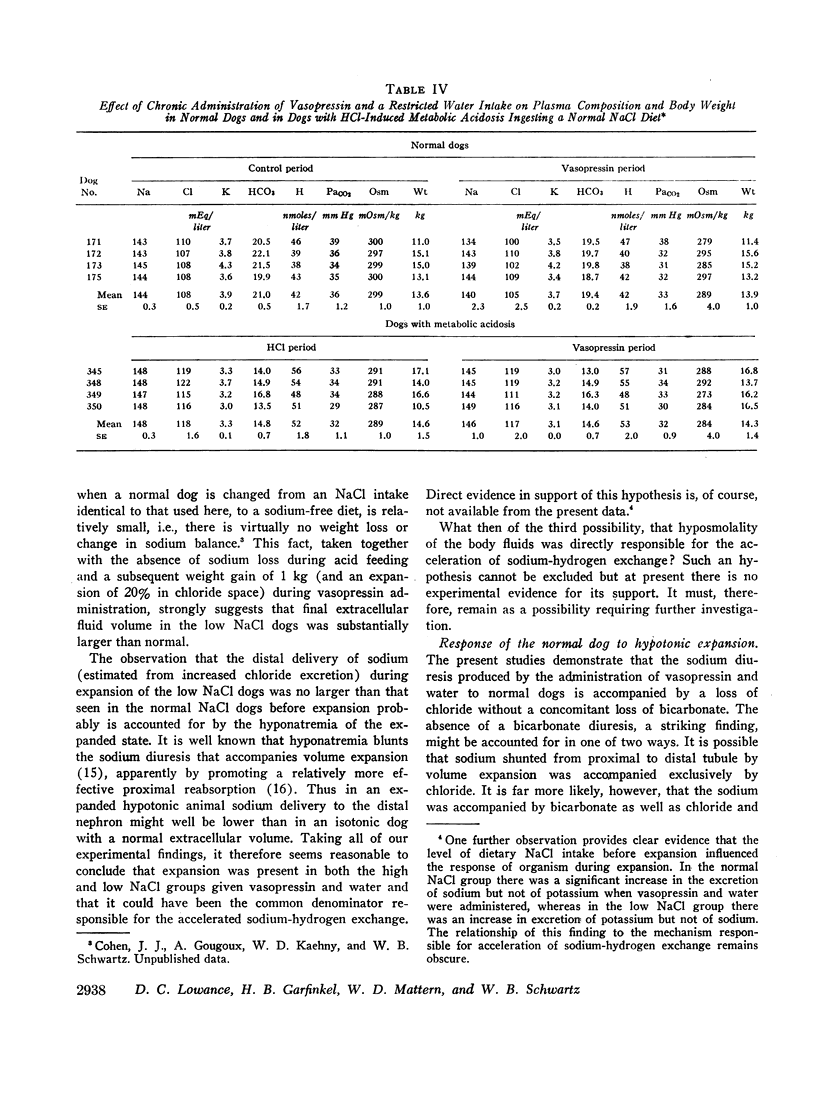
Hypotonic expansion in dogs with metabolic acidosis (mean plasma bicarbonate concentration 14 mEq/liter) produced a marked increase in renal acid excretion that restored plasma bicarbonate concentration to normal (20-21 mEq/liter) despite continued ingestion of acid. When water was restricted during the vasopressin period, and fluid retention thus prevented, no increase in acid excretion or plasma bicarbonate concentration occurred. From these findings we conclude that hypotonic expansion is a potent stimulus to renal hydrogen ion secretion and greatly facilitates the renal removal of an acid load.
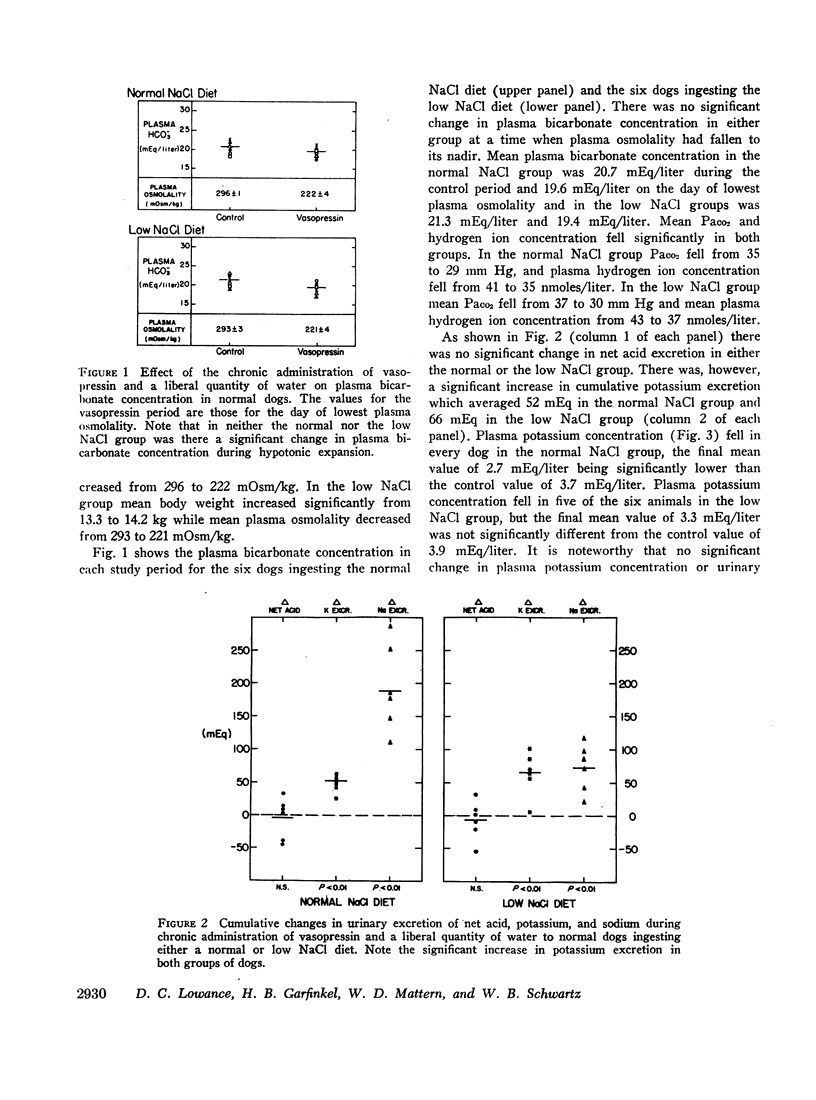
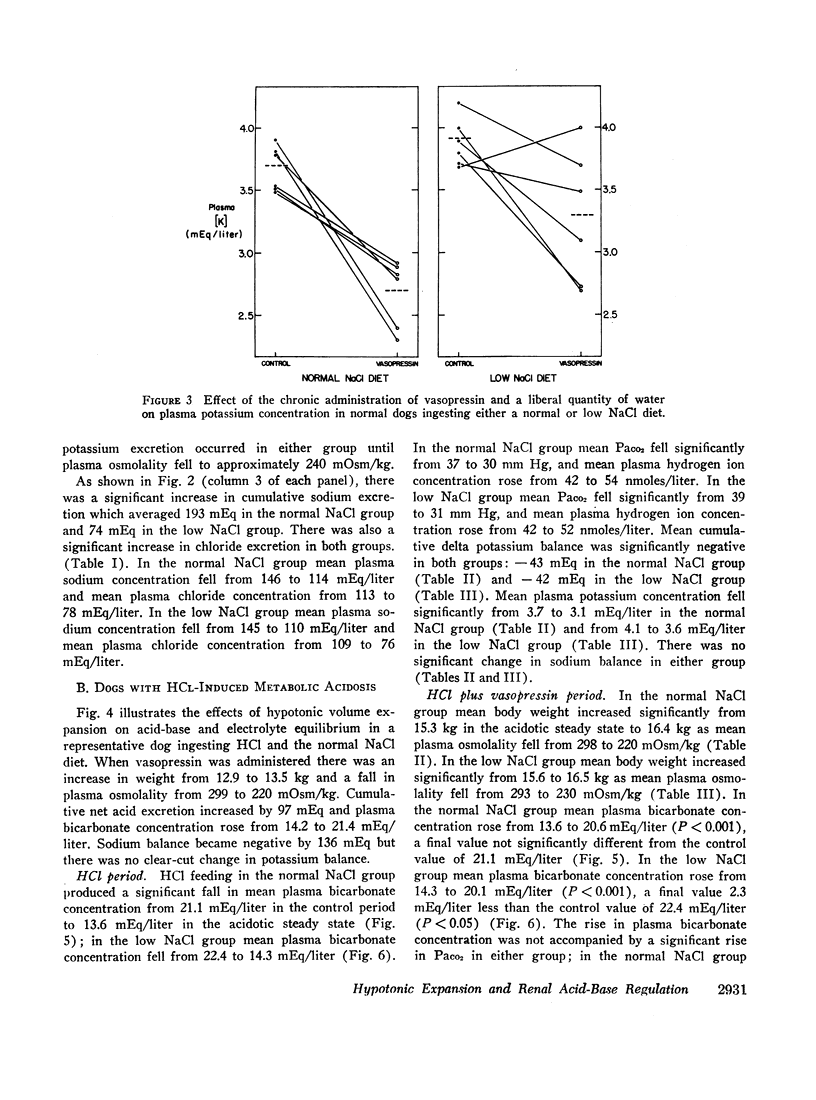
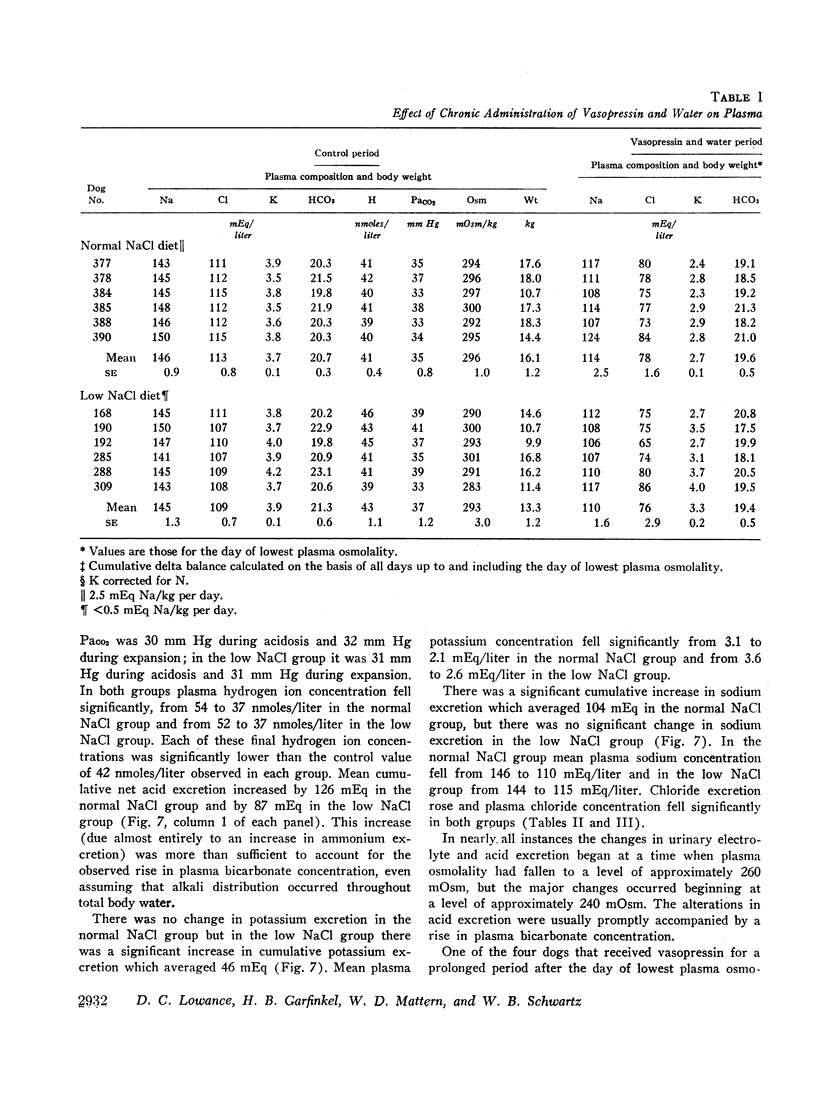
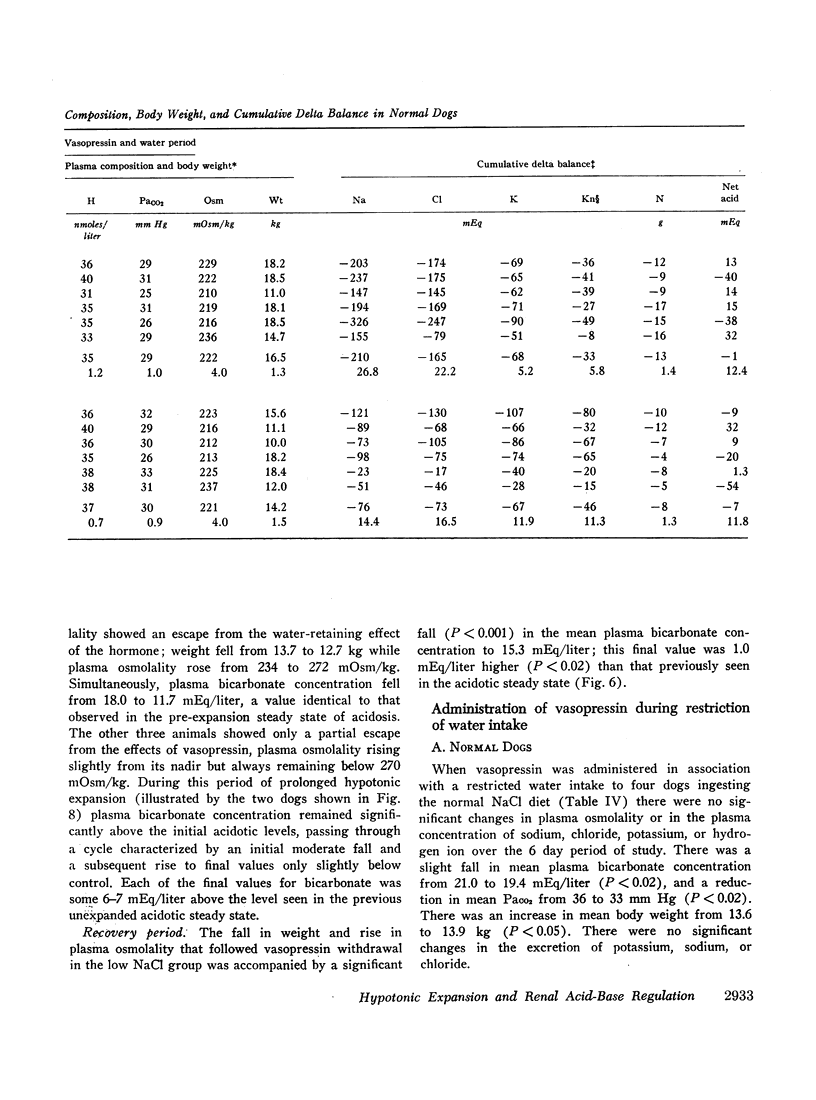
Normal dogs subjected to expansion demonstrated no change in net acid excretion or in plasma bicarbonate concentration even in the face of a marked diuresis of sodium and chloride and a reduction in plasma sodium concentration to approximately 110 mEq/liter. The animals did, however, regularly lose potassium, a finding that clearly indicates an acceleration of distal sodiumcation exchange. On the basis of these observations, and the findings in the expanded acidotic dogs, we suggest that in the expanded normal dogs acceleration of sodium-hydrogen exchange was responsible for preventing a bicarbonate diuresis and for stabilizing plasma bicarbonate concentration.
These studies clearly demonstrate that chronic hypotonic expansion exerts a major influence on the renal regulation of acid-base equilibrium. The exact nature of the mechanism responsible for the increase in sodium-hydrogen exchange during hypotonic expansion remains to be determined.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartter F. C., Schwartz W. B. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967 May;42(5):790–806. doi: 10.1016/0002-9343(67)90096-4. [DOI] [PubMed] [Google Scholar]
- Blythe W. B., Welt L. G. Plasma sodium concentrations and urinary sodium excretion. Trans Assoc Am Physicians. 1965;78:90–96. [PubMed] [Google Scholar]
- Brenner B. M., Berliner R. W. Relationship between extracellular volume and fluid reabsorption by the rat nephron. Am J Physiol. 1969 Jul;217(1):6–12. doi: 10.1152/ajplegacy.1969.217.1.6. [DOI] [PubMed] [Google Scholar]
- Buckalew V. M., Jr, Walker B. R., Puschett J. B., Goldberg M. Effects of increased sodium delivery on distal tubular sodium reabsorption with and without volume expansion in man. J Clin Invest. 1970 Dec;49(12):2336–2344. doi: 10.1172/JCI106452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. B., Knox F. G., Wright F. S., Howards S. S. Effect of expansion of extracellular fluid volume on proximal sodium rabsorption in hyponatremic dogs. Metabolism. 1970 Apr;19(4):291–300. doi: 10.1016/0026-0495(70)90127-7. [DOI] [PubMed] [Google Scholar]
- Gennari F. J., Goldstein M. B., Schwartz W. B. The nature of the renal adaptation to chronic hypocapnia. J Clin Invest. 1972 Jul;51(7):1722–1730. doi: 10.1172/JCI106973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M. B., Gennari F. J., Schwartz W. B. The influence of graded degrees of chronic hypercapnia on the acute carbon dioxide titration curve. J Clin Invest. 1971 Jan;50(1):208–216. doi: 10.1172/JCI106475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayslett J. P., Kashgarian M., Epstein F. H. Changes in proximal and distal tubular reabsorption produced by rapid expansion of extracellular fluid. J Clin Invest. 1967 Jul;46(7):1254–1263. doi: 10.1172/JCI105618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howards S. S., Davis B. B., Knox F. G., Wright F. S., Berliner R. W. Depression of fractional sodium reabsorption by the proximal tubule of the dog without sodium diuresis. J Clin Invest. 1968 Jul;47(7):1561–1572. doi: 10.1172/JCI105848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landwehr D. M., Klose R. M., Giebisch G. Renal tubular sodium and water reabsorption in the isotonic sodium chloride-loaded rat. Am J Physiol. 1967 Jun;212(6):1327–1333. doi: 10.1152/ajplegacy.1967.212.6.1327. [DOI] [PubMed] [Google Scholar]
- Morgan T., Berliner R. W. A study by continuous microperfusion of water and electrolyte movements in the loop of Henle and distal tubule of the rat. Nephron. 1969;6(3):388–405. doi: 10.1159/000179741. [DOI] [PubMed] [Google Scholar]
- POLAK A., HAYNIE G. D., HAYS R. M., SCHWARTZ W. B. Effects of chronic hypercapnia on electrolyte and acid-base equilibrium. I. Adaptation. J Clin Invest. 1961 Jul;40:1223–1237. doi: 10.1172/JCI104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Fedson D. S., Brigham K. L., Mitra R. C., Sack R. B., Mondal A. The ventilatory response to acute base deficit in humans. Time course during development and correction of metabolic acidosis. Ann Intern Med. 1970 May;72(5):633–640. doi: 10.7326/0003-4819-72-5-633. [DOI] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, VANGIESEN G., KIIL F., SELDIN D. W. INFLUENCE OF EXPANSION OF EXTRACELLULAR VOLUME ON TUBULAR REABSORPTION OF SODIUM INDEPENDENT OF CHANGES IN GLOMERULAR FILTRATION RATE AND ALDOSTERONE ACTIVITY. J Clin Invest. 1964 Mar;43:341–348. doi: 10.1172/JCI104919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin J. M., Katz M. A., Rector F. C., Jr, Seldin D. W. Acetazolamide in studying sodium reabsorption in diluting segment. Am J Physiol. 1970 Dec;219(6):1731–1738. doi: 10.1152/ajplegacy.1970.219.6.1731. [DOI] [PubMed] [Google Scholar]
- Sartorius O. W., Roemmelt J. C., Pitts R. F., Calhoon D., Miner P. THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. IV. THE NATURE OF THE RENAL COMPENSATIONS IN AMMONIUM CHLORIDE ACIDOSIS. J Clin Invest. 1949 May;28(3):423–439. doi: 10.1172/JCI102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannen R. L., Bleich H. L., Schwartz W. B. The renal response to acid loads in metabolic alkalosis; an assessment of the mechanisms regulating acid excretion. J Clin Invest. 1966 Apr;45(4):562–572. doi: 10.1172/JCI105370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINTERS R. W., LOWDER J. A., ORDWAY N. K. Observations on carbon dioxide tension during recovery from metabolic acidosis. J Clin Invest. 1958 May;37(5):640–645. doi: 10.1172/JCI103647. [DOI] [PMC free article] [PubMed] [Google Scholar]


