Abstract
Mechanosensitive ion channels (MSCs) exist in all cells, but mechanosensitivity is a phenotype not a genotype. Specialized mechanoreceptors such as the hair cells of the cochlea require elaborate mechanical impedance matching to couple the channels to the external stress. In contrast, MSCs in nonspecialized cells appear activated by stress in the bilayer local to the channel—within about three lipids. Local mechanical stress can be produced by far-field tension, amphipaths, phase separations, the cytoskeleton, the extracellular matrix, and the adhesion energy between the membrane and a patch pipette. Understanding MSC function requires understanding the stimulus.
The first requirement for understanding stretch-activated channels (SACs) is the knowledge that the term “SACs” was never intended as an abbreviation of my name. Second, this review will never mention the role of an amino acid. The literature includes many synonyms for SACs including mechanosensitive channels (MSC), mechanogated channels, pressure-sensitive, pressure-induced, or mechanical channels. Exercising my independence as the author, I will use the term MSC. The term “pressure sensitive” comes from the method of stretching the patch by applying pressure to the pipette, but membrane tension, not pressure, is the relevant variable. MSCs are identified by currents that increase with mechanical stress in the membrane they may also inactivate (64). There is currently no unique protein structure for chemically tracking expression of MSCs, although many have been associated, correctly or incorrectly (21), with the TRP superfamily.
Mechanical sensitivity, much like voltage or ligand sensitivity, is a general property of channels as well as other proteins. Channels previously labeled as “voltage-gated” like K, Na, HCN (33, 34, 37, 47) or “ligand-gated” (11, 13, 39, 54) are also mechanically sensitive. The only requirement for mechanical sensitivity is that the channels change shape between closed and open states and that membrane stress can reach the channels (40, 44). Mechanical sensitivity is a property as general as voltage sensitivity (40, 44). Whether the mechanical sensitivity of a particular channel is physiologically useful or whether it simply serves as a biophysical tool has to be determined.
In this review, I will arbitrarily consider MSCs to be those channels for which mechanical stress alone can drive them over their full dynamic range. Stress can modulate many channels and involves the same properties as MSCs, but lower energies. For example, the kinetics of voltage-sensitive channels can be changed by orders of magnitude by mechanical stress, but the voltage dependence dominates the net behavior. MSCs are not strongly gated by voltage, although they can be effectively gated by amphipathic ligands such as unsaturated fatty acids and general anesthetics (12, 45, 54–57). The most difficult problem in studying MSCs is that the local stimulus, the stresses at the channel, is not easily measured. In what follows, I will use the terms tension and stress interchangeably (note: stress refers to force and strain to distance).
Consider the simplest case of a lipid bilayer of fixed area, A. If you stretch it, the tension will increase linearly with the area strain [T = kA(δA/A)] where the area stiffness (kA; like the Hooke’s law stiffness) is ~100–1,000 mN/m (59). MSCs from bacteria have been studied in lipid vesicles (46), but that technology has not yet worked for eukaryotic MSCs. It is not simple to measure the area of a patch, and harder yet to measure the change in area with stretch (let alone simultaneously perform electrophysiology) so that defining the mean stress in a patch is complicated. If you apply suction (or pressure) to a patch, the tension will increase according to Laplace’s law:
| (1) |
where T is the cortical tension, P is the pressure drop across the patch, and r is the radius of curvature. P is easily measurable (4), although r is not (46, 61). T is the stimulus for the channels. In the simplest model of a channel in a bilayer (a cylindrical plug), if the closed channel has an in-plane area () and the open channel has an in-plane area (, where rc,o are the radii in their respective states), the free-energy difference between the states is:
| (2) |
The larger the difference in area, the higher the sensitivity. In the case of TREK-1, if the closed channel is 5 nm in diameter, the observed stretch sensitivity can be accounted for if the radius increases by 0.2 nm (25). These considerations apply to any pair of states, not just to open and closed. For example, a tension-sensitive rate could connect two closed states, as well as states of different conductance.
It is important to be clear about the meaning of sensitivity. As with voltage-gated channels, the gating curve is a sigmoidal Boltzmann function with two parameters (Eq. 3): the stimulus strength at which the system is half activated (the midpoint, chord sensitivity, equal probability tension, or T1/2) and the steepness of the curve at that point (or the slope sensitivity, δA/kBT, where kBT = Boltzmann’s constant <chem degrees Kelvin = 4.1 pN <chem nm at room temperature). The chord sensitivity or midpoint tension is readily modulated by prestressing the channel with amphipaths, the cytoskeleton, or the adherence of the membrane to a patch pipette (66). The slope sensitivity, however, primarily reflects the dimensional changes of the channel that occur during gating and is expected to be independent of the chord sensitivity.
Notice that the simple gating model of Eq. 2 has no reference to the material properties of the membrane. Weird. How could channel activity be the same in a diamond membrane and a lipid membrane? The answer is that there is an additional energy term, the line tension (42), that contains all the chemistry. The line tension is a circumferential tension at the perimeter of the channel where it meets the bilayer. It reflects the change in chemical potential as one moves from the lipid into the protein and summarizes the local chemistry.
How can different lipids change channel gating? If the closed and open states have different interaction energies with the lipids, that will create a difference in line tension between the closed open states, and there will be a shift in the midpoint of the gating curve. Additional energy can come from a hydrophobic mismatch when the channel is thicker or thinner than the bilayer (57, 70). Luckily, the simple model of Eq. 2 appears to contain the dominant energy term.
There are other ways to put energy into the channel. You can bend it, squeeze it, stretch it, etc. (70). Amphipaths affect MSC gating by bending the lipids in the boundary layer surrounding the channel (67). The boundary layer is the lipid equivalent to the hydration layer of water-soluble proteins. Depending on the affinity of a channel for particular lipids, channels can segregate specific lipids around the channel. A single hydrogen bond, for example, can create a concentration gradient of nearly 10:1, so the composition of the local environment is not likely to be the same as the average membrane (2, 23, 38). Membrane stresses decay within about three lipids (2) so that the channel will not be affected by the average properties of the membrane except for the mean tension. With these caveats in mind, let’s return to the simple model of a homogenous bilayer membrane and discuss what we mean by membrane tension.
If we had a bilayer with a fixed number of lipids, as one might have in a Langmuir-Blodgett trough, stretching it will increase the area/molecule (decrease the number density). Tension is the force/length required to hold together the edges of a slit in the membrane. However, the standard assay for channel activity is not a Langmuir-Blodgett trough but a patch-clamped membrane, and in a patch the number of lipids is not fixed. In cell-attached mode, lipids can flow from the cell along the pipette walls and into the patch dome, and they can also reposition themselves in excised patches (66). The physical constraint for modeling the patch (the boundary condition) is not a fixed number of lipids but a fixed tension. The tension is produced by membrane adhesion to the glass and the hydrostatic pressure gradient across the patch.
Tension can be measured by the angle at which the patch membrane contacts the glass (51) (FIGURE 1). The adhesion energy density (which has the units of tension) determines the resting tension of the patch. That tension is large, perhaps 30–40% of the lytic strength of the bilayer (66). Thus all patch recordings have been made in highly stressed membranes. Applying suction will increase the tension, but the increment is usually less than the resting tension. The resting tension is sufficient open many MSCs (25), and for particular channels opening can also lead to inactivation, making the patch appear to contain no MSCs.
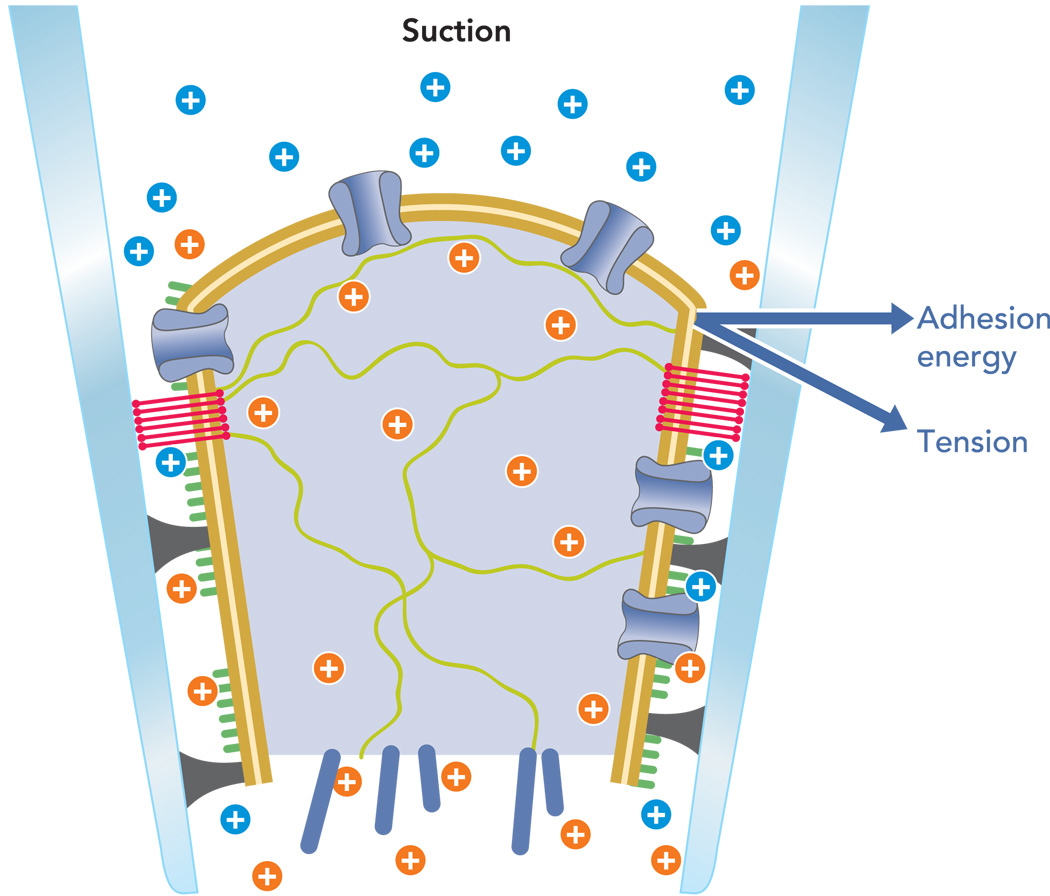
FIGURE 1. Cartoon structured of an excised patch illustrating the complex geometry and the origin of the forces.
The bilayer is tan, the cytoplasm is light blue with green and dark blue filaments, ion channels are blue tubes crossing the bilayer, the glycocalyx is green, proteins denatured against the glass are black, extracellular cations are in blue, intracellular cations are in orange, and the blue vectors show the force resolution into membrane tension and adhesion energy. Not shown is the upward component of force parallel to the pipette that leads to creep. The postulated lipid seal region where the membrane lipids bind directly to the glass is shown as red bands.
The difference in physical state between the resting cell membrane and the patch was recently emphasized to us by an experiment showing that patches are insensitive to the detergent Trixon-X100, not even lowering the seal resistance (Gottlieb P, Sachs F, unpublished observations). How can this occur since Triton easily dissolves the membrane of the host cell? We are not sure, but either Triton cannot enter the patch membrane or Triton in the patch cannot form a micelle. We prefer the latter explanation. To make a spherical micelle from a planar membrane (to “dissolve” it), you have to increase the total area of the system: Atotal = Apatch disk + Amicelle =<rpatch2 + 4πrmicelle2. We know from Eq. 2 that to increase the area of a membrane under tension requires energy, and apparently the resting tension of the patch is sufficient to raise the energy of micelle formation well above kBT. Enough already: Is that all we have to know about membrane stress? No.
In a bilayer patch, only the outer monolayer touches the glass, and the inner monolayer floats viscously over the outer monolayer. The stress is not equal in the two monolayers (17, 48, 72). In steady state, the outer monolayer bears all the tension (also noted far-field tension), and the inner monolayer has no tension. The mean tension estimated from Laplace’s law is actually the tension in the outer monolayer. There will be a large gradient in tension normal to the membrane or, equivalently, a gradient of intramembrane pressure (10). If the gating hardware of a channel were located in the inner half of that bilayer, as proposed for potassium channels (6, 31), the transitions might appear insensitive to tension. If a channel were to gate with motion in the extracellular monolayer, as is likely to be true for endogenous cationic MSCs (58, 67), they would appear tension sensitive.
In addition to generating the resting tension, the adhesion energy keeps the patch from flying up the pipette when you apply suction. If there is excess lipid available as in a cell-attached patch, the patch can creep up the pipette indefinitely since the bilayer is a liquid. As it creeps, the patch changes geometry, creating time-dependent changes in channel activity that might be classed as run-up, run-down, or desensitization (66). The adhesion energy plays one more critical role in forming a patch. The differential adhesion of different membrane components for the glass acts as a chromatograph to separate membrane components and affect which ones will arrive in the dome.
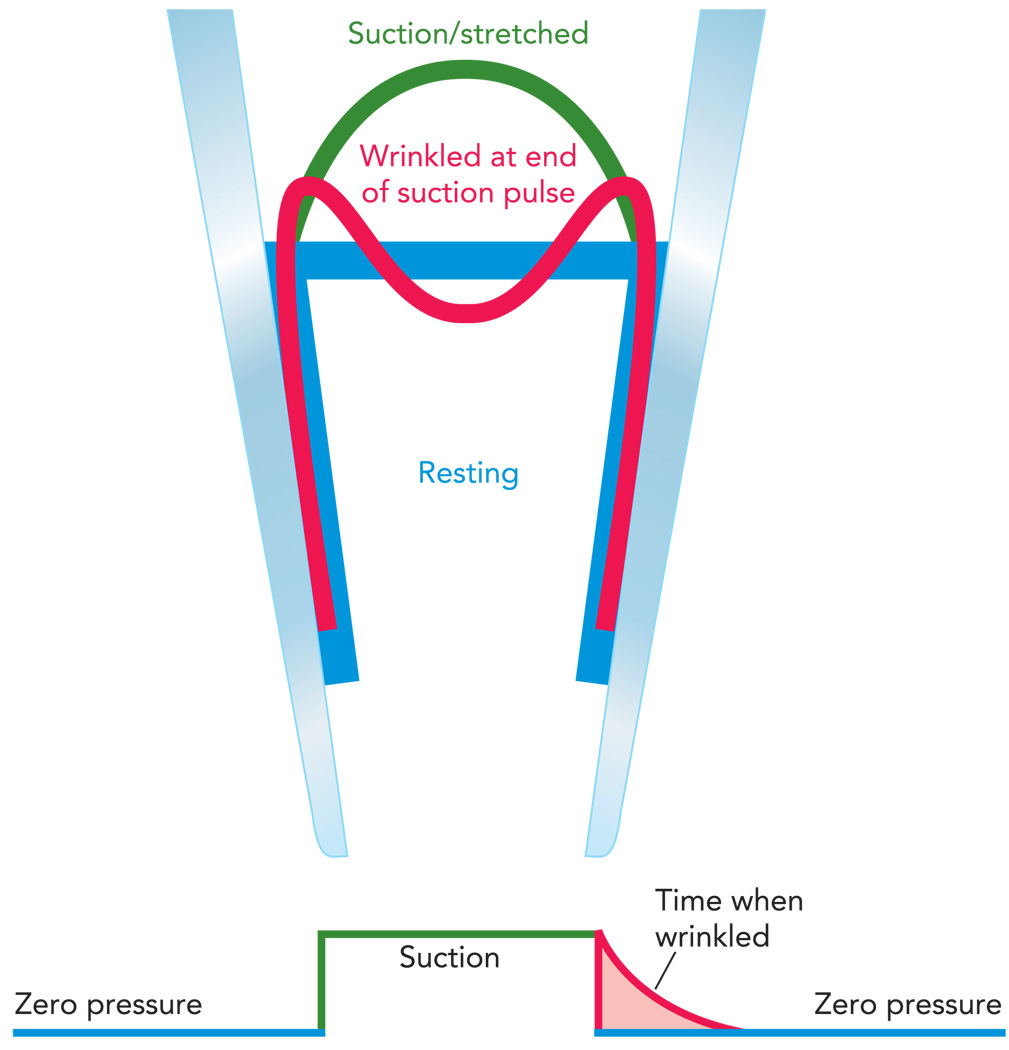
To study the stress-dependent changes in channel activity, we would like to be able to measure activity with no baseline tension rather than the arbitrary value set by the adhesion energy. It is possible to create a tension-free patch, although only transiently. When we stretch a patch, it forms a spherical cap with an area of Acap = 2πrh, where r is the radius of curvature and h is the height of the cap. If we then suddenly step back to zero pressure, the patch will attempt to return to a flat disk that spans the pipette. But that only requires an area of Adisk = πrh, about one-half that of the cap. Where does the excess area go? Initially, it just wrinkles the patch (FIGURE 2), and this wrinkled patch has approximately zero tension (25). The wrinkling has a spatial period of ~200–500 nm, which is insufficient to create significant bending energy in the channel to cause it to open. Within ~1 s after wrinkling, the membrane reanneals to the glass, restoring the resting tension to 3–4 mN/m. Thus, by using a high-speed pressure clamp (4) and examining channel behavior just after release of suction, one can explore channel behavior under minimal (although time-dependent) tension.
FIGURE 2. How a pressure clamp can generate a transient tension-free patch.
The resting patch (blue trace) is approximately normal to the pipette due to the adhesion energy and has an area of πr2. Applying a step of suction stretches the patch by bringing in new membrane and peeling it off the wall (green trace) creating a spherical cap of area of ~2πrh. Returning the pressure to zero causes the excess membrane area to wrinkle (red trace) until it reanneals to the glass returning to the resting configuration (blue trace). This peeling and reannealing can be seen from changes in patch capacitance (c.f. Figure 3). Thus, although it is wrinkled, the patch has less than resting (baseline) tension.
In a patch of a bilayer, a change in pipette pressure will cause slippage of one monolayer against the other (17, 72), creating a time-dependent normal component of tension. Initially following a step in pressure, the tension is shared by both monolayers, but in equilibrium only one. If we did not pay attention to the details of the local stimulus, we could mistakenly ascribe the effect of this elaborate physical kinetics to a “novel” property of the channel. If the mechanics of a pure bilayer are so complicated, how about stresses in a biological membrane?
The predominant change in going from a bilayer to a biological patch is the presence of the cytoskeleton that forms a plug behind the patch dome (66). Patches are actually samples of the cell cortex, not bilayers. The patch cytoskeleton shares stresses with the bilayer, thereby reducing the stress in the bilayer. The stresses in biological patches are actually distributed in three dimensions, not two. These normal stresses are easily visualized by curvature of the patch since the equilibrium shape of an adherent membrane is normal to the walls (65). Stresses in the cytoskeleton relax with time (an effective viscosity) and hence create time-dependent stresses in the bilayer so that a step of pipette pressure is not equivalent to a step of membrane tension. This nonuniform distribution of mechanical stress limits the speed with which one can change the stimulus.
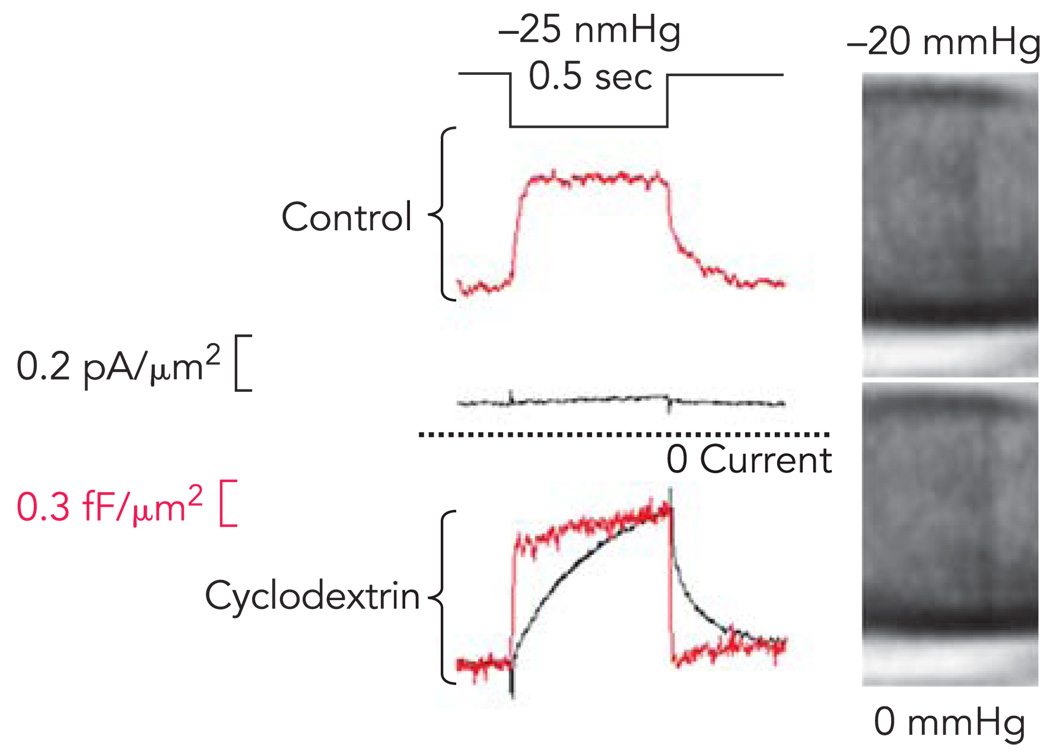
Patches can be simplified to better approximate a bilayer in a number of ways. Blebs have less cytoskeleton than the surrounding membrane (74); cytoskeletal reagents like latruculin and cytochalasin can reduce the population of cycling actin, although they are not effective on cortical actin (65); the cytoskeleton can be broken down with repeated suction/pressure pulses (66). We have found that removing cholesterol from the membrane with cyclodextrin leads to complete delamination of the cytoskeleton from the bilayer and produces patches that behave as expected for a lipid bilayer (FIGURE 3).
FIGURE 3. Cholesterol depletion in mouse myotubes uncouples the cytoskeleton from the bilayer and increases stress on MSCs.
Top traces shows the response of a control cell to a step of suction. The MSC current is small and activates and relaxes slowly (bottom black trace). The patch capacitance, a measure of bilayer stress, increases faster than current, but at a clearly resolvable speed (red trace). Bottom: the response in a patch after treatment of the cells for 2 h with 5mM β methylcyclodextrin. The micrographs at the right of this patch show that patch becomes almost invisible due to the loss of bound proteins. (The images have been digitally enhanced to improve contrast, and the pipette is shown horizontal with the tip to the right). The MSC current is now much larger, although still displaying the activation and deactivation kinetics. The local stress viewed through the patch capacitance (red trace) now changes as fast as the stimulus pulse. The large resting current observed with cyclodextrin is the result of MSC activation by resting tension (Suchyna T, Sachs F, unpublished observations).
Finally, what about osmotic pressure as a stimulus? Contrary to dogma, nucleated cells can live in distilled water for hours at estimated osmotic pressure of 7 Atm (69). If osmotic pressure is so far above the hydrostatic pressure applied to patches (100 mmHg = 0.13 Atm), why do swollen cells not saturate their MSCs in the open state and generate huge whole currents? There are two reasons. The traditional view of cell osmotic pressure as arising cleanly from the pressure of small solute molecules against the membrane is untrue. The cytoskeleton bears a substantial amount of the osmotic stress (62), as is rather obvious, in retrospect, from the nonspherical shape of adherent cells under osmotic stress. The osmotic stress is borne in three dimensions by the cytoskeleton acting as a sponge, and only a small component is borne by the membrane. The sponge-like properties of the cytoskeleton are emphasized by the fact that cells get softer, not harder, when they are swollen (62). Second, the relevant radius of curvature for Laplace’s law is not that of the cell but that of the cytoskeletal lattice, ~50 nm. If the membrane becomes delaminated from the cytoskeleton, however, the system becomes two dimensional, and the channels should activate and maybe inactivate. Osmotic stimuli cannot create uniform tension in the cell membrane since the curvature is not constant and the compliance is not constant. Even worse, in most cells, osmotic stress induces a pronounced Ca2+ elevation (50), which is a serious perturbation for a many currents, and the exchange of osmolytes during volume regulation will change the chemical environment of the cytoskeleton and the channels. Osmotic stress and mechanical stress are not the same stimuli.
Having established the fundamentals of the mechanical stimulus, I will explain some of the problems in identifying the molecular entities of MSCs. The most critical problem is that all cells, including red cells (68), have cationic MSCs so that there is no null background. If you apply patch suction looking for MSCs in a transfected cell, how do you know if that current came from the transfected DNA or from the endogenous channels (21)? You don’t. The minimal requirement for such an experiment is that they be performed in double blind so that the experimenter does not know the source of the cells. Cells that appear to have no MSCs will often display them after treatment with cytochalasin; the channels were present but shielded. The patch is a dismal sampler of the average cell properties (66), especially in actively remodeling cells like COS and HEK. Ideally, one would like to see the transfection cause a massive expression of a new kind of conductance, and that does happen with the 2P channels (25) where one can easily generate K currents of several nA from a patch. Of course if the patch behavior were representative of the whole cell membrane, one should be able to generate microamps from a cell, but that never happens. Possibly, in the resting cell membrane, the 2P channels are shrouded in parallel elastic elements that provide mechanoprotection.
A more subtle issue for examining MSC activity is that channel expression is known to cause reorganization of the cytoskeleton, regardless of whether the channels are active (35). Reorganization means that the stresses changed from the control cells. Proper controls are not cells transfected with GFP or some other innocuous protein or even a nonconducting version of channel under study. It is far from obvious what is a proper control. Changes in structure mean changes in stress, and that can affect the response from endogenous as well as exogenously expressed MSCs. Any pharmacological agent that might affect any cytoskeletal structure can change the stress on an MSC. Does anyone believe that amphipathic and lipophilic agents cannot affect the cytoskeleton? Do not freely apply drugs to cells unless you know the effects on the local stimulus. Genistein, for example is an excellent modulator of gramicidin channel gating in bilayers even though there is no kinase or substrate (29). Those nonspecific effects may explain the discrepancy between the reported cell-attached mechanosensitivity of TRPC-6 (63) and its insensitivity when measured directly (66).
The all-encompassing ability of the cytoskeleton to control MSC activity is clear from whole cell recordings on adult heart cells (5). Squeezing a cell with the side of a second pipette at 1 Hz/1 µm produced no current for minutes at a time. No current at all. After about 5 min, there was a large step of inward current, as though something had snapped, and the cell became mechanically responsive to every subsequent compression. That sensitivity slowly decreased over minutes as the cytoskeleton presumably repaired itself. Understanding this nonlinear hysteretic behavior is critical for interpreting the effects of mechanical transduction as is likely to occur in the study of mechanosensitive pain (1, 26, 27, 52). Actin reorganizes in seconds at sites of stress producing a “work hardening.” A mechanical stimulus given at one time will not be sensed to same degree as a supposed identical stimulus at a later time, and that changes with the history of stimulation. The cytoskeleton can transmit signals as fast as nerves (49), so reorganization may not be local and will depend on the history of the stimulus: magnitude, waveshape, and repetition rate. The dynamic changes in compliance will also depend on the metabolic state of the cell and could clearly change with the time out of the incubator. There is no gold standard for mechanical stimulation.
Pharmacology
We showed a number of years ago that micromolar Gd3+ would block MSCs (71) through a decrease in the opening rate and the unitary conductance. The lanthanides in general work similarly, although the active species, Gd3+ ions or various hydrates or carbonates (9), is not known. Gd3+ compresses lipid bilayers (16) and that may be the origin of the inhibition, but Gd3+ is not a specific inhibitor and should be used, therefore, with caution. GsMTx4, a peptide we isolated from tarantula venom, is a much more selective inhibitor of cationic MSCs and works from the extracellular side (8). It is not a universal MSC inhibitor since it has no effect on the 2P channels and possibly other MSCs (15). There is a persistent question about the endogenous activity of MSCs in cells, and this cannot be answered using a patch since resting stress begs the question. However, if a process can be inhibited GsMTx4 in a cell, it probably began with a cationic MSC, and this is what is observed in dystrophic muscle (65).
With all these caveats, MSCs are real, and the physiological function of their mechanosensitivity remains to be determined. In bacteria, some channels like MscL and MscS function as safety valves for relieving hydrostatic pressure (32). Some animal cells may use them for volume sensing (28). MSCs may have evolved as sensors of amphiphiles (41) and incidentally exhibited mechanosensitivity. My own unsubstantiated hypothesis is that they serve as cellular pain sensors, indicating to the cell where the local cytoskeleton needs to be reinforced. The channels also seem to be component of systemic pain sensing since mechanical pain sensation in rats is inhibited by GsMTx4, a specific blocker of cationic MSCs (53). Levine’s laboratory found that subcutaneous GsMTx4 did not alter normal nociceptive mechanical thresholds but did inhibit the mechanical thresholds after inflammation (1). This result may reflect the cytoskeletal reorganization associated with inflammation (30).
Regardless of the “intended” physiological function, there are many correlations between MSC behavior and pathologies involving MSCs. Dystrophic muscle has stretch-sensitive MSCs that are blocked by GsMTx4 (65, 73), and GsMTx4 also inhibits activation of calpains in dystrophic muscle (18). It also suppress the generation of reactive oxygen species in stretched lung cells (14). Cardiac arrhythmias are known to be correlated with mechanical stress, and dilation-induced atrial fibrillation is reversibly inhibited by 170 nM GsMTx4 (7). The mechanosensitive 2P channels that are involved in blood pressure regulation (60) will have a lot to say about MSC function. There are undoubtedly many more pathologies that modify MSC activation since they are present in all cells and can be modified by changes in the cytoskeleton, the extracellular matrix, and second messengers. There are many review papers on the possible structure of MSCs in eukaryotic cells, typically those of the TRP family, and the interested reader should examine those papers directly (3, 19, 20, 22, 24, 36, 75).
Acknowledgments
This research was supported by the National Institutes of Health.
References
- 1.Alessandri-Haber N, Dina OA, Chen XJ, Levine JD. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci. 2009;29:6217–6228. doi: 10.1523/JNEUROSCI.0893-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen OS, Nielsen C, Maer AM, Lundb’k JA, Goulian M, Koeppe RE. Ion channels as tools to monitor lipid bilayer-membrane protein interactions: gramicidin channels as molecular force transducers. Methods Enzymol. 1999;294:208–224. doi: 10.1016/s0076-6879(99)94013-2. [DOI] [PubMed] [Google Scholar]
- 3.Beech DJ. TRPC1: store-operated channel and more. Pflügers Arch. 2005;451:53–60. doi: 10.1007/s00424-005-1441-3. [DOI] [PubMed] [Google Scholar]
- 4.Besch SR, Suchyna T, Sachs F. High-speed pressure clamp. Pflügers Arch. 2002;445:161–166. doi: 10.1007/s00424-002-0903-0. [DOI] [PubMed] [Google Scholar]
- 5.Bett GCL, Sachs F. Whole-cell mechanosensitive currents in rat ventricular myocytes activated by direct stimulation. J Membr Biol. 2000;173:255–263. doi: 10.1007/s002320001024. [DOI] [PubMed] [Google Scholar]
- 6.Beyder A, Sachs F. Electromechanical coupling in the membranes of Shaker-transfected HEK cells. Proc Natl Acad Sci USA. 2009;106:6626–6631. doi: 10.1073/pnas.0808045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bode F, Sachs F, Franz MR. Tarantula peptide inhibits atrial fibrillation during stretch. Nature. 2001;409:35–36. doi: 10.1038/35051165. [DOI] [PubMed] [Google Scholar]
- 8.Bowman CL, Gottlieb PA, Suchyna TM, Murphy YK, Sachs F. Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon. 2007;49:249–270. doi: 10.1016/j.toxicon.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caldwell RA, Clemo HF, Baumgarten CM. Using gadolinium to identify stretch-activated channels: technical considerations. Am J Physiol Cell Physiol. 1998;275:C619–C621. doi: 10.1152/ajpcell.1998.275.2.C619. [DOI] [PubMed] [Google Scholar]
- 10.Cantor RS, Twyman KS, Milutinovic PS, Hasenedes R. A kinetic model of ion channel electrophysiology: bilayer-mediated effects of agonists and anesthetics on protein conformational transitions. Soft Matter. 2005;5:3266–3278. [Google Scholar]
- 11.Casado M, Ascher P. Opposite modulation of NMDA receptors by lysophospholipids and arachidonic acid: common features with mechanosensitivity. J Physiol. 1998;513:317–330. doi: 10.1111/j.1469-7793.1998.317bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chemin J, Patel AJ, Delmas P, Sachs F, Lazdunski M, Honore E. Up- and downegulation of the mechano-gated K-2P channel TREK-1 by PIP2 and other membrane phospholipids. Pflügers Arch. 2007;455:97–103. doi: 10.1007/s00424-007-0250-2. [DOI] [PubMed] [Google Scholar]
- 13.Chemin J, Sachs F, Patel AA, Lazdunski M, Honore E. Mechanosensitive Ion Channels, Part B, Vol. 59. Current Topics in Membranes. New York: Academic; 2007. Polymedal regulation of the mechano-gated K 2P channel 2P channel TREK-1 by PIP 2 and other membrane phospholipids; pp. 155–170. [DOI] [PubMed] [Google Scholar]
- 14.Chess PR, O’Reilly MA, Sachs F, Finkelstein JN. Reactive oxidant and p42/44 MAP kinase signaling is necessary for mechanical strain-induced proliferation in pulmonary epithelial cells. J Appl Physiol. 2005;99:1226–1232. doi: 10.1152/japplphysiol.01105.2004. [DOI] [PubMed] [Google Scholar]
- 15.Drew LJ, Rugiero F, Cesare P, Gale JE, Abrahamsen B, Bowden S, Heinzmann S, Robinson M, Brust A, Colless B, Lewis RJ, Wood JN. High-threshold mechanosensitive ion channels blocked by a novel conopeptide mediate pressure-evoked pain. PLoS One. 2007;2:e515. doi: 10.1371/journal.pone.0000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ermakov YA, Averbakh AZ, Yusipovich AI, Sukharev S. Dipole potentials indicate restructuring of the membrane interface induced by gadolinium and beryllium ions. Biophys J. 2001;80:1851–1862. doi: 10.1016/S0006-3495(01)76155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans E, Yeung A. Hidden dynamics in rapid changes of bilayer shape. Chem Phys Lipids. 1994;73:39–56. [Google Scholar]
- 18.Gailly P, De Backer F, Van Schoor M, Gillis JM. In situ measurements of calpain activity in isolated muscle fibres from normal and dystrophin-lacking mdx mice. J Physiol. 2007;582:1261–1275. doi: 10.1113/jphysiol.2007.132191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallo C, Shapovalov G, Dorchies OM, Petermann O, Zanou N, Gailly P, Ruegg UT, Roulet E. Role of the cationic channel TRPC1 in Duchenne muscular dystrophy: analysis of double knock-out TRPC1(−/−) dystrophin(−) mice. Neuromuscular Disorders. 2009;19:582–582. [Google Scholar]
- 20.Gevaert T, Vandepitte J, Hutchings G, Vriens J, Nilius B, De Ridder D. TRPV1 is involved in stretch-evoked contractile changes in the rat autonomous bladder model: a study with piperine, a new TRPV1 agonist. Neurourol Urodyn. 2007;26:440–450. doi: 10.1002/nau.20343. discussion 451–453. [DOI] [PubMed] [Google Scholar]
- 21.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honore E. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflügers Arch. 2008;455:1097–1103. doi: 10.1007/s00424-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb P, Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill O, Bowman CB, Folgering JHA, Patel A, Sachs F, Honore E. Revisiting TRPC1 mechanosensitivity. Pflügers Arch. 2007;455:1097–1103. doi: 10.1007/s00424-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 23.Goulian M, Mesquita ON, Fygenson DK, Nielsen C, Andersen OS, Libchaber A. Gramicidin channel kinetics under tension. Biophys J. 1998;74:328–337. doi: 10.1016/S0006-3495(98)77790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- 25.Honore E, Patel AJ, Chemin J, Suchyna T, Sachs F. Desensitization of mechano-gated K-2P channels. Proc Nat Acad Sci USA. 2006;103:6859–6864. doi: 10.1073/pnas.0600463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu J, Lewin GR. Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J Physiol. 2006;577:815–828. doi: 10.1113/jphysiol.2006.117648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu J, Milenkovic N, Lewin GR. The high threshold mechanotransducer: A status report. Pain. 2006;120:3–7. doi: 10.1016/j.pain.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Hua SZ, Gottlieb P, Sachs F. Mechanosensitive ion channels don’t control cell volume regulation. 2008. [Google Scholar]
- 29.Hwang TC, Koeppe RE, Andersen OS. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry. 2003;42:13646–13658. doi: 10.1021/bi034887y. [DOI] [PubMed] [Google Scholar]
- 30.Inoue R, Jian Z, Kawarabayashi Y. Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol Therap. 2009;123:371–385. doi: 10.1016/j.pharmthera.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. The open pore conformation of potassium channels. Nature. 2002;417:523–526. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- 32.Kouwen TR, Trip EN, Denham EL, Sibbald MJ, Dubois JY, van Dijl JM. The large mechanosensitive channel MscL determines bacterial susceptibility to the bacteriocin sublancin 168. Antimicrob Agents Chemother. 2009;53:4702–4711. doi: 10.1128/AAC.00439-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laitko U, Juranka PF, Morris CE. Membrane stretch slows the concerted step prior to opening in a Kv channel. J Gen Physiol. 2006;127:687–701. doi: 10.1085/jgp.200509394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laitko U, Morris CE. Membrane tension accelerates rate-limiting voltage-dependent activation and slow inactivation steps in a shaker channel. J Gen Physiol. 2004;123:135–154. doi: 10.1085/jgp.200308965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lauritzen I, Chemin J, Honore E, Jodar M, Guy N, Lazdunski M, Patel AJ. Cross-talk between the mechano-gated K-2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 2005;6:642–648. doi: 10.1038/sj.embor.7400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SY, Holt JR, Vollrath MA, Garcia-Anoveros J, Geleoc G, Kwan K, Hoffman MP, Zhang DS, Corey DP. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Biophys J. 2005;88:287A–288A. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- 37.Lin W, Laitko U, Juranka PF, Morris CE. Dual stretch responses of mHCN2 pacemaker channels: accelerated activation, accelerated deactivation. Biophys J. 2007;92:1559–1572. doi: 10.1529/biophysj.106.092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundbaek JA, Andersen OS. Spring constants for channel-induced lipid bilayer deformations. Estimates using gramicidin channels. Biophys J. 1999;76:889–895. doi: 10.1016/S0006-3495(99)77252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J Biol Chem. 2000;275:10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- 40.Markin VS, Sachs F. Thermodynamics of mechanosensitivity. Phys Biol. 2004;1:110–124. doi: 10.1088/1478-3967/1/2/007. [DOI] [PubMed] [Google Scholar]
- 41.Markin VS, Martinac B. Mechanosensitive ion channels as reporters of bilayer expansion. A theoretical model. Biophys J. 1991;60:1120–1127. doi: 10.1016/S0006-3495(91)82147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markin VS, Sachs F. Thermodynamics of mechanosensitivity. In: Hamill OP, editor. Mechanosensitive Ion Channels, Part A, Vol. 58, Current Topics in Membranes. New York: Academic; 2007. pp. 87–119. [Google Scholar]
- 44.Markin VS, Martinac B. Mechanosensitive ion channels as reporters of bilayer expression. A theoretical model. Biophys J. 1991;60:1120–1127. doi: 10.1016/S0006-3495(91)82147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinac B, Adler J, Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990;348:261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- 46.Moe P, Blount P. Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemistry. 2005;44:12239–12244. doi: 10.1021/bi0509649. [DOI] [PubMed] [Google Scholar]
- 47.Morris CE, Juranka PF. Nav channel mechanosensitivity: activation and inactivation accelerate reversibly with stretch. Biophys J. 2007;93:822–833. doi: 10.1529/biophysj.106.101246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukhin SI, Baoukina SV. Inter-layer slide and stress relaxation in a bilayer lipid membrane in the patch-clamp setting. Biologicheskie Membrany. 2004;21:506–517. [Google Scholar]
- 49.Na S, Collin O, Chowdhury F, Tay B, Ouyang MX, Wang YX, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Nat Acad Sci USA. 2008;105:6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niggel J, Sigurdson W, Sachs F. Mechanically induced calcium movements in astrocytes, bovine aortic endothelial cells and C6 glioma cells. J Membr Biol. 2000;174:121–134. doi: 10.1007/s002320001037. [DOI] [PubMed] [Google Scholar]
- 51.Opsahl LR, Webb WW. Lipid-glass adhesion in giga-sealed patch-clamped membranes. Biophys J. 1994;66:75–79. doi: 10.1016/S0006-3495(94)80752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Padilla F, Couble ML, Coste B, Maingret F, Clerc N, Crest M, Ritter AM, Magloire H, Delmas P. Expression and localization of the Nav1.9 sodium channel in enteric neurons and in trigeminal sensory endings: Implication for intestinal reflex function and orofacial pain. Mol Cell Neurosci. 2007;35:138–152. doi: 10.1016/j.mcn.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Park SP, Kim BM, Koo JY, Cho H, Lee CH, Kim M, Na HS, Oh U. A tarantula spider toxin, GsMTx4, reduces mechanical and neuropathic pain. Pain. 2008;137:208–217. doi: 10.1016/j.pain.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 54.Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- 55.Patel AJ, Lazdunski M, Honore E. Lipid and mechano-gated 2P domain K+ channels. Curr Opin Cell Biol. 2001;13:422–428. doi: 10.1016/s0955-0674(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 56.Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 2002;418:942–948. doi: 10.1038/nature00992. [DOI] [PubMed] [Google Scholar]
- 57.Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nature Struct Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- 58.Posokhov YO, Gottlieb PA, Morales MJ, Sachs F, Ladokhin AS. Is lipid bilayer binding a common property of inhibitor cysteine knot ion-channel blockers? Biophys J. 2007;93:L20–L22. doi: 10.1529/biophysj.107.112375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rawicz W, Smith BA, McIntosh TJ, Simon SA, Evans E. Elasticity, strength, and water permeability of bilayers that contain raft microdomain-forming lipids. Biophys J. 2008;94:4725–4736. doi: 10.1529/biophysj.107.121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharif-Naeini R, Folgering JHA, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, Retailleau K, Loufrani L, Patel A, Sachs F, Delmas P, Peters DJM, Honore E. Polycystin-1 and-2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 61.Sokabe M, Sachs F. The structure and dynamics of patch-clamped membranes: a study by differential interference microscopy. J Cell Biol. 1990;111:599–606. doi: 10.1083/jcb.111.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spagnoli C, Beyder A, Besch S, Sachs F. Atomic force microscopy analysis of cell volume regulation. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;78 doi: 10.1103/PhysRevE.78.031916. 031916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Nat Acad Sci USA. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suchyna T, Sachs F. Dynamic regulation of mechanosensitive channels; capacitance used to monitor patch tension in real time. Phys Biol. 2004;1:1–18. doi: 10.1088/1478-3967/1/1/001. [DOI] [PubMed] [Google Scholar]
- 65.Suchyna T, Sachs F. Mechanical and electrical properties of membranes from dystrophic and normal mouse muscle. J Physiol. 2007;581:369–387. doi: 10.1113/jphysiol.2006.125021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suchyna TM, Markin VS, Sachs F. Biophysics and structure of the patch and the gigaseal. Biophys J. 2009;97:738–747. doi: 10.1016/j.bpj.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suchyna TM, Tape SE, Koeppe RE, Andersen OS, Sachs F, Gottlieb PA. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430:235–240. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- 68.Vandorpe DH, Xu C, Shmukler BE, Otterbein L, Trudel M, Sachs F, Gottlieb PA, Brugnara C, Alper S. Hypoxia activates a Ca2+-permeable cation conductance sensitive to carbon monoxide and to GsMTx-4 in human and mouse sickle erythrocytes. PLoS Biol. doi: 10.1371/journal.pone.0008732. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wan X, Harris JA, Morris CE. Responses of neurons to extreme osmomechanical stress. J Membr Biol. 1995;145:21–31. doi: 10.1007/BF00233304. [DOI] [PubMed] [Google Scholar]
- 70.Wiggins P, Phillips R. Membrane-protein interactions in mechanosensitive channels. Biophys J. 2005;88:880–902. doi: 10.1529/biophysj.104.047431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang XC, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989;243:1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- 72.Yeung A. Mechanics of Intermonolayer Coupling in Fluid Surfactant Bilayers. British Columbia: British Columbia, Canada: Univ.; 1994. [Google Scholar]
- 73.Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005;562:367–380. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Gao F, Popov VL, Wen JW, Hamill OP. Mechanically gated channel activity in cytoskeleton-deficient plasma membrane blebs and vesicles from Xenopus oocytes. J Physiol. 2000;523:117–130. doi: 10.1111/j.1469-7793.2000.t01-1-00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou XL, Batiza AF, Loukin SH, Palmer CP, Kung C, Saimi Y. The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proc Natl Acad Sci USA. 2003;100:7105–7110. doi: 10.1073/pnas.1230540100. [DOI] [PMC free article] [PubMed] [Google Scholar]