Summary
Antibiotics containing a β-lactam ring (e.g., ceftriaxone) display anti-glutamate effects that underlie their efficacy in animal models of CNS diseases (Rothstein et al., 2005). We hypothesized that the structurally related β-lactamase inhibitors (clavulanic acid, tazobactam)- which also contain a β-lactam ring - will mimic ceftriaxone efficacy in an invertebrate (planarian) assay designed to screen for anti-seizure activity (Rawls et al., 2009). Glutamate or cocaine administration produced planarian seizure-like activity (pSLA). Glutamate- or cocaine-induced pSLA was inhibited by ceftriaxone, clavulanic acid, or tazobactam, but not by the non-β-lactam antibiotic vancomyocin. The present findings indicate β-lactamase inhibitors display efficacy, and mimic ceftriaxone activity, in an invertebrate anti-seizure screen. These results suggest β-lactamase inhibitors particularly ones such as clavulanic acid that display enhanced brain penetrability, oral bioavailability, and negligible anti-bacterial activity might offer an attractive alternative to direct antibiotic therapy for managing CNS diseases caused by increased glutamate transmission and provide a solution to the growing concern that ceftriaxone will be of only limited utility as a CNS-active therapeutic because of its intolerable side effects.
Keywords: clavulanic acid, tazobactam, ceftriaxone, β-lactam antibiotic, glutamate, planaria
β-lactam antibiotics enhance cellular glutamate uptake through activation of glutamate transporter subtype 1 (GLT-1), a predominantly astrocytic transporter that is responsible for about 90% of glutamate uptake in the brain and widely recognized by neuroscientists as a promising target to manage CNS diseases caused by excessive glutamate transmission. The representative β-lactam antibiotic ceftriaxone has already been successfully used as a pharmacological probe. It is active in preclinical models of CNS diseases such as amyotrophic lateral sclerosis, multiple sclerosis, stroke, depression, tolerance, addiction, and seizure (Rothstein et al., 2005; Chu et al., 2007; Lipski et al., 2007; Miller et al., 2008; Rawls et al., 2010a, b; Knackstedt et al., 2010; Sari et al., 2009). The main problem with ceftriaxone is that its broad-spectrum preclinical efficacy may not actually translate into a clinically useful CNS-active therapeutic. Its poor brain penetrability requires the administration of high concentrations to achieve anti-glutamate effects in animals and humans, a requirement that exacerbates the risk of adverse effects. For example, the standard ceftriaxone concentration of 200 mg/kg, given for 5–10 days, to achieve anti-glutamate effects in rats and mice equates to about 13 g/day in a human, approximately 5–6 times greater than ceftriaxone concentration prescribed for meningitis. It is not surprising that many patients receiving ceftriaxone to treat amyotrophic lateral sclerosis have discontinued its use due to intolerable side effects, an outcome that underscores the need to identify more patient-friendly therapies. The structurally-related β-lactamase inhibitor clavulanic acid may be a particularly attractive option. Clavulanic acid possesses a β-lactam ring, an apparent structural requirement for the anti-glutamate effects of the β-lactam antibiotics. It is also orally active and stable, with a bioavailability of 64–75%, a distinct advantage over ceftriaxone which is normally administered intravascularly or intramuscularly (Bolton et al., 1986). Clavulanic acid also displays excellent blood-brain barrier penetrability (CSF/plasma ratio of 0.25) and exhibits only negligible anti-bacterial activity (Nakagawa et al., 1994). A first step in determining if clavulanic acid should be explored as an alternative to direct ceftriaxone therapy for managing CNS disorders is to compare its efficacy with that of ceftriaxone in an animal model. Prior work indicates ceftriaxone displays anti-seizure properties in mice (Jelenkovic et al., 2008). The goal of the present study was to investigate and compare the activities of β-lactamase inhibitors (clavulanic acid and tazobactam), β-lactam antibiotics (ceftriaxone), and non-β-lactam antibiotics (vancomyocin) in an assay designed to screen for anti-seizure activity (Rawls et al., 2009). In addition, the effect of a GLT-1 transporter inhibitor, dihydrokainate, on glutamate- and cocaine-induced seizure like activity was investigated.
Experimental Procedures
Planarians (Dugesia dorotocephala) were purchased from Carolina Biological Supply and tested within 3 days of receipt. Solutions of L-glutamic acid, cocaine, ceftriaxone, clavulanic acid, and tazobactam were prepared daily in distilled water. Planarian seizure-like activity (pSLA) was quantified as the number of C-like hyperkinesias during a 5-min drug exposure (Rawls et al., 2009). C-like hyperkinesias have been observed previously upon exposure to cholinergic, opioid and dopaminergic agents, as well as upon cocaine exposure (Buttarelli et al., 2000; Palladini et al., 1996; Passarelli et al., 1999; Pagan et al., 2008). Drug combinations tested in each experiment are described in Table 1. Comparisons of group means (± S.E.M.) were evaluated by one-way ANOVA followed by a Dunnett’s post-hoc analysis. p < 0.05 was considered statistically significant.
Table 1.
Experimental design. Each planarian was placed into a clear plastic petri dish containing a drug or drug combination and tested individually for pSLA for 5 min.
| Experiment | Drug(s) |
|---|---|
| 1A | Ceftriaxone (0.01 – 25 mM) or water + Glutamate (3 mM) |
| 1B | Ceftriaxone (0.01 – 25 mM) or water + Cocaine (3 mM) |
| 2A | Clavulanic Acid (0.1 – 5 mM) or water + Glutamate (3 mM) |
| 2B | Clavulanic Acid (0.0001 – 1 mM) or water + Cocaine (3 mM) |
| 3A | Tazobactam (0.005 – 0.1 mM) or water + Glutamate (3 mM) |
| 3B | Tazobactam (0.005 – 0.1 mM) or water + Cocaine (3 mM) |
| 4A | DHK (0.01 – 1 mM) or water + Glutamate (3 mM) |
| 4B | DHK (0.01 – 1 mM) or water + Cocaine (3 mM) |
| 5A | Vancomyocin (1 – 10 mM) or water + Glutamate (3 mM) |
| 5B | Vancomyocin (1 – 10 mM) or water + Cocaine (3 mM) |
Results
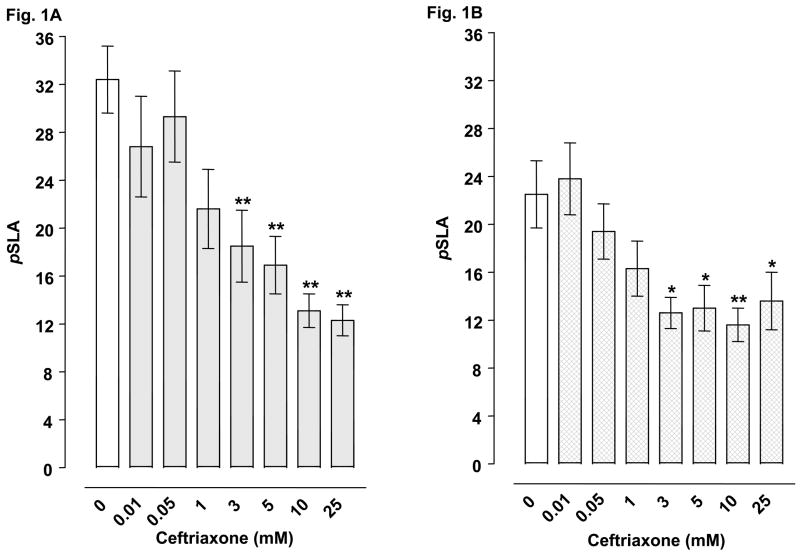
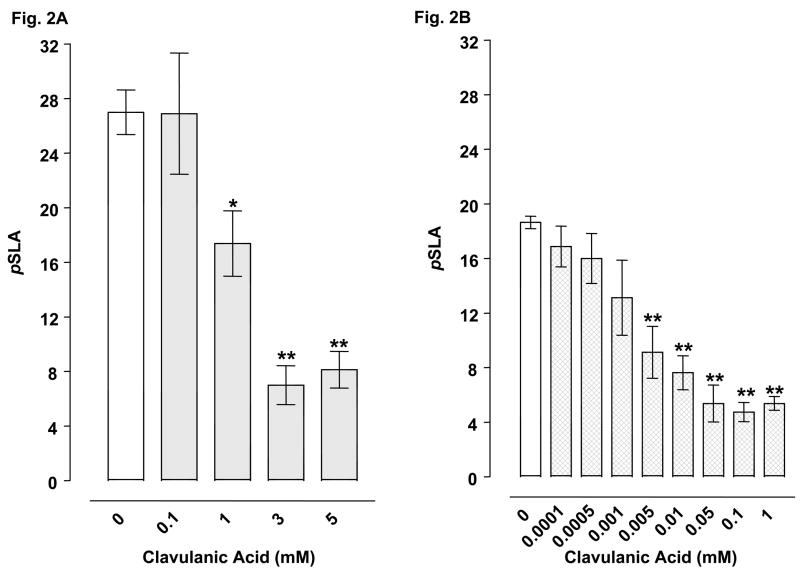
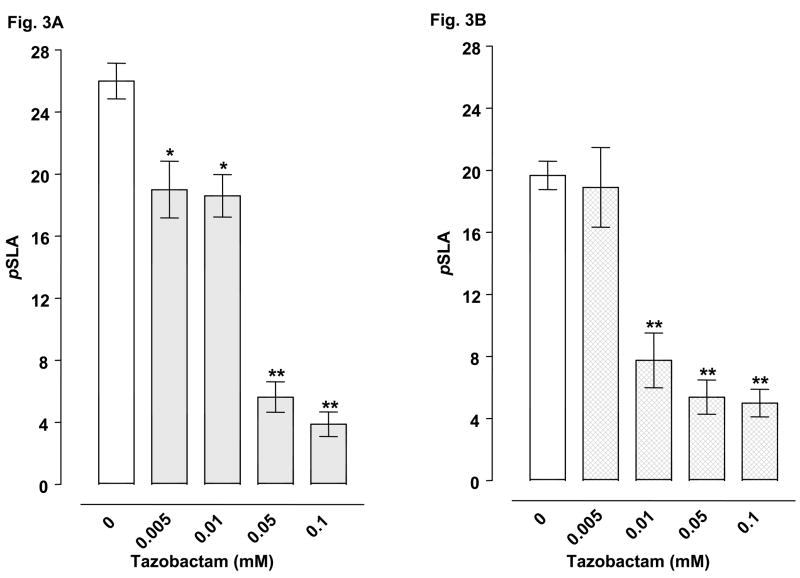
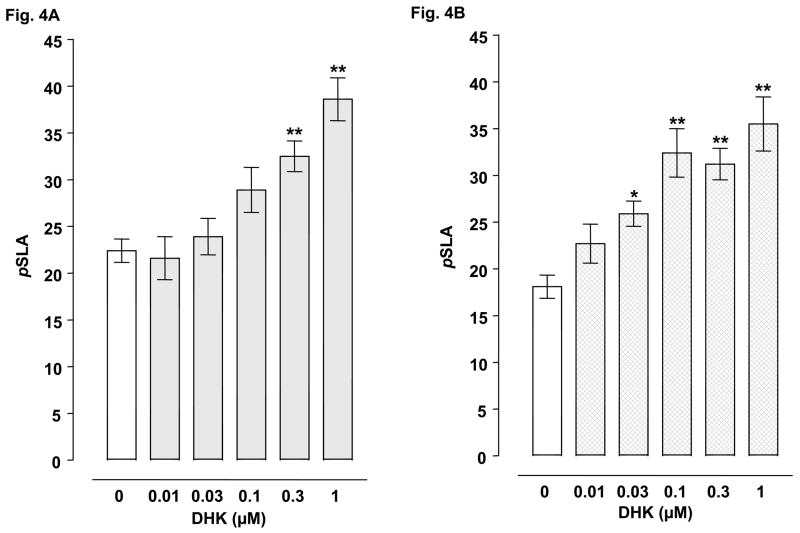
Planarians exposed to water did not display pSLA. However, they did display concentration-related pSLA following an acute 5-min exposure to glutamate or cocaine (Table 2). Concentrations of 3 mM glutamate and 3 mM cocaine were selected for drug combination experiments. For combined administration, ceftriaxone (3, 5, 10, 25 mM) significantly inhibited glutamate- or cocaine-induced pSLA (Fig 1A-B). Clavulanic acid (1, 3, 5 mM) attenuated glutamate-induced pSLA (Fig 1C) (Fig. 2A), and a similar inhibition of cocaine-induced pSLA was detected, but at even lower clavulanic acid concentrations (0.005, 0.01, 0.05, 0.1, 1 mM) (Fig. 2B). Tazobactam (0.01, 0.05, 1 mM) also produced significant inhibition of pSLA induced by glutamate or cocaine exposure (Fig 3A-B). Half-maximal inhibitory concentrations (IC50) of the β-lactam compounds were (glutamate, cocaine): ceftriaxone (5.8, 24.5 mM); clavulanic acid (1.44, 0.010 mM); and tazobactam (0.018, 0.019 mM). Ceftriaxone administered by itself at concentrations of 0.01- 25 mM did not produce pSLA, but it did cause pSLA at a concentration of 50 mM (8.5 ± 1.56 pSLA/5 min). Clavulanic acid or tazobactam, when given alone, did not produce pSLA at the concentrations used here. Dihydrokainate (0.3, 1 μM) significantly inhibited glutamate-induced pSLA (Fig 4A), and a similar inhibition of cocaine-induced pSLA was observed for dihydrokainate (0.03, 0.1, 0.3, 1 μM) (Fig. 4B). Dihydrokainate (0.01- 1 μM) did not produce pSLA at the concentrations used in combination experiments, but it did induce pSLA at higher concentrations (10 μM, 3.2 ± 0.56 pSLA/5 min; 30 μM, 6.7 ± 1.22 pSLA/5 min; and 100 μM, 12.5 ± 2.68 pSLA/5 min). The non-β-lactam antibiotic vancomyocin (1, 3, 10 mM) did not significantly affect pSLA produced by glutamate [F(3, 32) = 1.958, P > 0.05] or cocaine [F(3, 24) = 0.697, P > 0.05] (data not shown).
Table 2.
Planarians exposed to either glutamate or cocaine for 5 min display concentration-related pSLA. Control planarians exposed to drug-free water did not display pSLA. N = 6–8 planarians per group.
| Drug Concentration (mM) | pSLA (Glutamate) | pSLA (Cocaine) |
|---|---|---|
| 0.01 | 3.4 ± 0.5 | 0.7 ± 0.9 |
| 0.1 | 11.4 ± 1.5 | 2.5 ± 1.2 |
| 1 | 18.5 ± 0.6** | 7.3± 0.7 |
| 3 | 26.3 ± 2.4** | 23.4± 1.2** |
| 10 | 34.5 ± 3.9** | 29.8± 0.8** |
p < 0.01 compared to 0.01 mM group.
Fig. 1.
Effects of ceftriaxone on pSLA induced by 3 mM glutamate (1A) or 3 mM cocaine (1B). Planarians were treated for 5 min with glutamate or cocaine by itself or in combination with ceftriaoxne. Data are expressed as mean pSLA ± S.E.M. versus concentration of ceftriaxone. Main effects are indicated on the panels. **p < 0.01 and *p < 0.05 compared to the glutamate or cocaine alone group (i.e., ceftriaxone concentration of 0 mM) following identification of significant main effects for the ceftriaxone/glutamate [F(7, 56) = 6.488, p < 0.0001] and ceftriaxone/cocaine [F(7, 56) = 7.592, p = 0.0005] data sets. N = 8 planarians per group.
Fig. 2.
Effects of clavulanic acid on pSLA induced by 3 mM glutamate (2A) or 3 mM cocaine (2B). Planarians were treated for 5 min with glutamate or cocaine by itself or in combination with clavulanic acid. Data are expressed as mean pSLA ± S.E.M. versus concentration of clavulanic acid. Main effects are indicated on the panels. **p < 0.01 and *p < 0.05 compared to the glutamate or cocaine alone group (i.e., clavulanic acid concentration of 0 mM) following identification of significant main effects for the clavulanic acid/glutamate [F(4, 35) = 13.58, p < 0.0001] and clavulanic acid/cocaine [F(8, 64) = 12.77, p < 0.0001] data sets. N = 8 planarians per group.
Fig. 3.
Effects of tazobactam on pSLA induced by 3 mM glutamate (3A) or 3 mM cocaine (3B). Planarians were treated for 5 min with glutamate or cocaine by itself or in combination with tazobactam. Data are expressed as mean pSLA ± S.E.M. versus concentration of tazobactam. Main effects are indicated on the panels. **p < 0.01, *p < 0.05 compared to the glutamate or cocaine alone group (i.e., tazobactam concentration of 0 mM) following identification of significant main effects for the tazobactam/glutamate [F(4, 35) = 30.52, p < 0.0001] and tazobactam/cocaine [F(4, 35) = 21.46, p < 0.0001] data sets. N = 8 planarians per group.
Fig. 4.
Effects of dihydrokainate (DHK) on pSLA induced by 3 mM glutamate (4A) or 3 mM cocaine (4B). Planarians were treated for 5 min with glutamate or cocaine by itself or in combination with DHK. Data are expressed as mean pSLA ± S.E.M. versus concentration of DHK. **p < 0.01 and *p < 0.05 compared to the glutamate or cocaine alone group (i.e., ceftriaxone concentration of 0 mM) following identification of significant main effects for the DHK/glutamate [F(5, 42) = 10.89, p < 0.0001] and DHK/cocaine [F(5, 42) = 10.06, p < 0.0001] data sets. N = 8 planarians per group.
Discussion
The demonstration that clavulanic acid and tazobactam, as well as ceftriaxone, display activity against glutamate- and cocaine-induced seizure-like activity in invertebrates confirms the documented anti-seizure properties of ceftriaxone and indicates β-lactamase inhibitors may be capable of mimicking the efficacy of β-lactam antibiotics in animal models of CNS diseases caused by excessive glutamate activity (Jelenkovic et al., 2008). Prior work suggests the behavioral assay used here is predictive for anti-seizure activity (Rawls et al., 2009). For example, pro-convulsants (e.g., AMPA, NMDA, glutamate, cocaine) produce quantifiable seizure-like activity (pSLA) that is inhibited by clinically approved anti-epileptic agents (e.g., topiramate, valproate), but not by control drugs (e.g., propranolol) (Rawls et al., 2009). It is already documented that ceftriaxone inhibits seizures induced by the GABA receptor antagonist penteleyneterazole in mice (Jelenkovic et al., 2008). The present data indicate ceftriaxone efficacy extends to an invertebrate model of seizure. More importantly, anti-seizure activity was dependent on the presence of a β-lactam ring but not anti-bacterial activity. Three β-lactam-containing compounds ceftriaxone, clavulanic acid, and tazobactam were active in the assay whereas vancomyocin, an antibiotic lacking the β-lactam ring, was inactive. There is one structural difference in the β-lactam core between these compounds. The β-lactam ring in clavulanic acid and tazobactam lacks the primary amine that is present on the β-lactam ring in ceftriaxone. Thus, it can be predicted that the anti-seizure activity of the β-lactam compounds observed in the present assay is not dependent on the presence of a primary amine in the β-lactam ring, a finding that may prove to be useful in the future synthesis of CNS-active β-lactam derivatives possessing the desirable characteristics of enhanced brain penetrability and minimal anti-bacterial activity.
It is not possible from the results of the present assay to determine if the β-lactam compounds produced anti-seizure effects by enhancing glutamate uptake. It is interesting to note that the GLT-1 transporter inhibitor dihydrokainate enhanced glutamate- and cocaine-induced seizure-like activity, an effect that was opposite to that produced by the β-lactam compounds.
The effect of dihydrokainate also raises the possibility that increased glutamate transmission caused by a glutamate uptake block is capable of triggering, or at least augmenting, seizure-like activity in planarians. Planarians do synthesize glutamate and GABA and display abstinence-induced withdrawal that is highly dependent on increased glutamate transmission (Eriksson and Panula, 1994; Rawls et al., 2006, 2007). Seizure is associated with excessive glutamate transmission and failures of inhibitory control (Sheldon and Robinson, 2007; Campbell and Hablitz, 2008; Guo et al., 2010), and it is well documented that ceftriaxone displays anti-glutamate effects through enhancement of cellular glutamate uptake (Rothstein et al., 2005; Chu et al., 2007; Rawls et al., 2010a, b; Miller et al., 2008; Knackstedt et al., 2010; Sari et al., 2009). Future studies will be directed toward determining if the anti-seizure properties of clavulanic acid are also detectable in a standard rodent model of seizure and if β-lactamase inhibitors, akin to β-lactam antibiotics, display anti-glutamate properties through GLT-1 transporter activation.
Acknowledgments
This study was funded by National Institute on Drug Abuse grants R15DA025314 (SMR) and RC1 DA028153 (SMR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bolton GC, Allen GD, Davies BE, Filer CW, Jeffery DJ. The disposition of clavulanic acid in man. Xenobiotica. 1986;16:853–863. doi: 10.3109/00498258609038967. [DOI] [PubMed] [Google Scholar]
- Buttarelli FR, Pontieri FE, Margotta V, Palladini G. Acetylcholine/dopamine interaction in planaria. Comp Biochem Physiol C Toxicol Pharmacol. 2000;125:225–231. doi: 10.1016/s0742-8413(99)00111-5. [DOI] [PubMed] [Google Scholar]
- Campbell SL, Hablitz JJ. Decreased glutamate transport enhances excitability in a rat model of cortical dysplasia. Neurobiol Dis. 2008;32:254–261. doi: 10.1016/j.nbd.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, Kim SJ, Park DK, Jung KH, Song EC, Lee SK, Kim M, Roh JK. Pharmacological Induction of Ischemic Tolerance by Glutamate Transporter-1 (EAAT2) Upregulation. Stroke. 2007;38:177–182. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- Eriksson KS, Panula P. gamma-Aminobutyric acid in the nervous system of a planarian. J Comp Neurol. 1994;345:528–536. doi: 10.1002/cne.903450405. [DOI] [PubMed] [Google Scholar]
- Guo F, Sun F, Yu JL, Wang QH, Tu DY, Mao XY, Liu R, Wu KC, Xie N, Hao LY, Cai JQ. Abnormal expressions of glutamate transporters and metabotropic glutamate receptor 1 in the spontaneously epileptic rat hippocampus. Brain Res Bull. 2010;81:510–516. doi: 10.1016/j.brainresbull.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Jelenkovic AV, Jovanovic MD, Stanimirovic DD, Bokonjic DD, Ocic GG, Boskovic BS. Beneficial effects of ceftriaxone against pentylenetetrazole-evoked convulsions. Exp Biol Med. 2008;233:1389–1394. doi: 10.3181/0803-RM-83. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Wan CK, Bai JZ, Pi R, Li D, Donnelly D. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience. 2007;146:617–629. doi: 10.1016/j.neuroscience.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 2008;153:329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Yamada M, Tokiyoshi K, Miyawaki Y, Kanayama T. Penetration of potassium clavulanate/ticarcillin sodium into cerebrospinal fluid in neurosurgical patients. Jpn J Antibiot. 1994;47:93–101. [PubMed] [Google Scholar]
- Pagán OR, Rowlands AL, Azam M, Urban KR, Bidja AH, Roy DM, Feeney RB, Afshari LK. Reversal of cocaine-induced planarian behavior by parthenolide and related sesquiterpene lactones. Pharmacol Biochem Behav. 2008;89:160–170. doi: 10.1016/j.pbb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Palladini G, Ruggeri S, Stocchi F, De Pandis MF, Venturini G, Margotta V. A pharmacological study of cocaine activity in planaria. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;115:41–45. doi: 10.1016/s0742-8413(96)00053-9. [DOI] [PubMed] [Google Scholar]
- Passarelli F, Merante A, Pontieri FE, Margotta V, Venturini G, Palladini G. Opioid-dopamine interaction in planaria: a behavioral study. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;124:51–55. doi: 10.1016/s0742-8413(99)00048-1. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Gomez T, Stagliano GW, Raffa RB. Measurement of glutamate and aspartate in Planaria. J Pharmacol Toxicol Methods. 2006;53:291–295. doi: 10.1016/j.vascn.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Gomez T, Raffa RB. An NMDA antagonist (LY 235959) attenuates abstinence-induced withdrawal of planarians following acute exposure to a cannabinoid agonist (WIN 55212–2) Pharmacol Biochem Behav. 2007;86:499–504. doi: 10.1016/j.pbb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Thomas T, Adeola M, Patil T, Raymondi N, Poles A, Loo M, Raffa RB. Topiramate antagonizes NMDA- and AMPA-induced seizure-like activity in planarians. Pharmacol Biochem Behav. 2009;93:363–367. doi: 10.1016/j.pbb.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Zielinski M, Patel H, Sacavage S, Baron DA, Patel D. Beta-lactam antibiotic reduces morphine analgesic tolerance in rats through GLT-1 transporter activation. Drug Alcohol Depend. 2010a doi: 10.1016/j.drugalcdep.2009.10.010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Baron DA, Kim J. β-lactam antibiotic inhibits development of morphine physical dependence in rats. Behav Pharmacol. 2010b doi: 10.1097/FBP.0b013e328337be10. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT-1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int. 2007;51:333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]