Abstract
Objective
Factors released by perivascular adipose tissue (PVAT) disrupt coronary endothelial function via phosphorylation of eNOS by PKC-β. However, our understanding of how PVAT potentially contributes to coronary disease as a complication of obesity/metabolic syndrome (MetS) remains limited. The current study investigated whether PVAT derived leptin impairs coronary vascular function via PKC-β in MetS.
Methods and Results
Coronary arteries with and without PVAT were collected from lean or MetS Ossabaw miniature swine for isometric tension studies. Endothelial-dependent vasodilation to bradykinin was significantly reduced in MetS. PVAT did not affect bradykinin-mediated dilation in arteries from lean swine, but significantly exacerbated endothelial dysfunction in arteries from MetS swine. PVAT-induced impairment was reversed by inhibition of either PKC-β with ruboxistaurin or leptin receptor signaling with a recombinant, pegylated leptin antagonist. Western and immunohistochemical analysis demonstrated increased PVAT-derived leptin and coronary leptin receptor (ObR) density with MetS. Coronary PKC-β activity was increased in both MetS arteries exposed to PVAT and lean arteries exposed to leptin. Finally, leptin-induced endothelial dysfunction was reversed by ruboxistaurin.
Conclusions
Increases in epicardial PVAT leptin exacerbate coronary endothelial dysfunction in MetS via a PKC-β-dependent pathway. These findings implicate PVAT-derived leptin as a potential contributor to coronary atherogenesis in MetS.
Keywords: epicardial perivascular adipose tissue, obesity, coronary artery disease, endothelium
Introduction
Adipose tissue is widely accepted to be an active endocrine and paracrine organ. As a signaling organ, the production of adipose-derived cytokines (adipokines) has been well documented to influence many physiologic and pathophysiologic conditions.1 Importantly, adipokine production has been shown to influence key pathogenic mediators of atherogenesis1 including chemotaxis,2 inflammation3 and endothelial function.4-6 While adipokines have been proposed to be a molecular link between obesity and cardiovascular disease,1 the exact relationship remains uncertain.
Recent studies implicate adipose tissue that normally surrounds large coronary arteries, i.e. perivascular/periadventitial adipose tissue (PVAT), as a local source of adipokines that contribute to the initiation of vascular dysfunction and atherosclerotic disease.7-11 This contention is supported by data indicating that coronary atherosclerotic plaques primarily occur in the larger epicardial arteries encased by PVAT10 as well as other findings illustrating a positive association between epicardial PVAT volume and the severity of coronary artery disease.9;12 A recent investigation from Greif et al. documented that PVAT volume, hypoadiponectinemia, and inflammation represent the strongest risk factors for the presence of coronary atherosclerosis.9 Importantly, our laboratory has demonstrated that factors released by PVAT in normal lean animals significantly impair coronary endothelial-dependent vasodilation and nitric oxide (NO) production via protein kinase C (PKC)-β dependent phosphorylation of endothelial NO synthase (eNOS) at the inhibitory amino acid residue Thr495.13;14 These findings establish a mechanistic link between local cardiac PVAT and coronary endothelial function, which is widely accepted to be the inciting event in the pathogenesis of atherosclerosis.15;16 However, the degree to which alterations in local PVAT adipokine signaling influences coronary vascular dysfunction/disease (i.e. endothelial dysfunction) in the metabolic syndrome (MetS) has yet to be examined.
The purpose of the present investigation was to test the hypothesis that augmented PVAT-derived leptin exacerbates underlying coronary endothelial dysfunction in the MetS via a PKC-β dependent pathway. We tested this hypothesis directly in vitro by manipulation of PVAT leptin signaling in the absence of systemic leptin. This hypothesis is supported by recent evidence that epicardial PVAT from patients with MetS contain activated macrophages and expresses significantly higher levels of potentially atherogenic adipokines, including an approximate 7-fold increase in leptin expression.7 In addition, our laboratory has demonstrated that hyperleptinemia alone markedly impairs coronary endothelial function,5 which we propose occurs via a PKC-β dependent pathway.14 Findings from this investigation offer novel insight into the potential role of PVAT-derived leptin in the increased prevalence and severity of coronary disease in the setting of the MetS.17
Methods
Ossabaw Miniature Swine Model of Metabolic Syndrome
Lean Ossabaw swine (total n = 11 animals, males = 8, female = 3) were fed standard chow (5L80, Purina, Richmond, IN; ~2200 kcal/day) containing (in % kcal) 18% protein, 71% complex carbohydrates, 11% fat and 0% cholesterol. MetS Ossabaw swine (total n = 15, males = 8, females = 7) were fed ~8000 kcal/day with modified chow (5B4L, Purina) containing (in % kcal) 17% protein, 20% complex carbohydrates, 20% fructose, and 43% fat (lard and hydrogenated soybean and coconut oils). MetS diet was supplemented with 2% cholesterol and 0.7% sodium cholate by weight.18 The duration of the diet period was 20 weeks for both lean and MetS swine. No differences in vascular responses were noted between male and female swine. Protocols for plasma parameters and quantification of the degree of coronary atherosclerosis were conducted as previously described.19;20
Functional Assessment of Isolated Epicardial Coronary Rings
Isolated coronary artery studies were performed for both experimental groups as previously described.13;14 Briefly, left anterior descending (LAD) coronary arteries were dissected out with naturally surrounding PVAT (average weight of PVAT arteries was 0.45 ± 0.03 g per ring for both lean and MetS swine; Figure 1). Arteries were then cut into 3 mm rings and the PVAT was either left intact or carefully dissected. Clean and PVAT containing arteries from lean and MetS swine were then mounted in organ baths for isometric tension studies. Arteries were taken to optimal length (3.9 ± 0.4 g tension on average) and pre-contracted with the thromboxane A2 mimetic U46619 (1 μM). Vascular function was assessed by the addition of graded concentrations of the bradykinin (0.1 nM - 10 μM, n = 7 lean; n = 9 MetS) or sodium nitroprusside (1.0 nM–0.1 mM, n = 4 lean; n = 6 MetS) to the tissue bath. Additional bradykinin concentration responses were conducted in MetS coronary arteries with and without PVAT in the presence of a recombinant, pegylated leptin receptor antagonist (1 μM, n = 4, Protein Laboratories, Rehovot, Israel)21 or the PKC-β specific inhibitor ruboxistaurin (1 μM, n = 6). The leptin receptor antagonist is a mutant leptin analogue that functions as a competitive inhibitor. Further proof of principle studies were performed with leptin (30 ng/ml) ± ruboxistaurin (1 μM) in coronary arteries from lean swine without PVAT (n = 4). Data are reported as the percentage of relaxation for arterial rings from individual animals. 100 percent relaxation was defined as the resulting tension following the administration of nitroglycerin (20 μM); i.e. equivalent to the loss of all active tension developed in response to U46619.
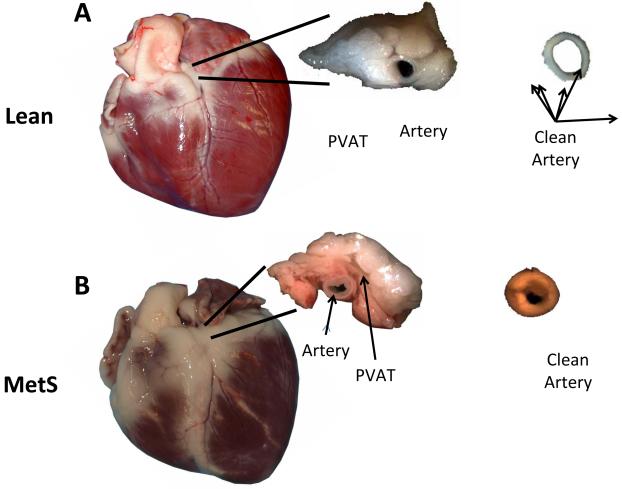
Figure 1. Representative pictures of coronary arteries with and without PVAT from lean and MetS swine.
LAD arteries from lean (A) and MetS (B) swine hearts were cut into 3 mm ring segments and the PVAT was either left intact or carefully dissected away. Clean and PVAT containing arteries from lean and MetS swine were then mounted in organ baths for isometric tension studies. Note visible neointimal formation in arteries from MetS swine (B). n = 11 lean and 15 MetS animals for all experiments.
Western Blot and RT-PCR Analyses
Western blotting was performed as previously described.14 Briefly, tissues were isolated, frozen in liquid N2 and stored at −80°C. PVAT was homogenized, centrifuged, and the resulting supernatants were collected for analysis. Equivalent amounts of protein were loaded onto 15% acrylamide gels for electrophoresis and blotting with a primary antibody against leptin (1:1000, Abcam). Immunoreactivity was visualized using an ECL western blotting detection kit (GE Healthcare) and quantified by scanning densitometry (Bio-Rad Quantity One 1-D Analysis Software). Real-time PCR was performed using total RNA isolated from PVAT of both lean and MetS swine as previously described22. Primers specific for human leptin were used (SA Biosciences), and expression was normalized by threshold cycle (CT) number for each group.
Immunohistochemistry
Fixed artery segments were embedded in paraffin and cross-sectioned (Zymed Laboratories, Inc.). Tissue sections were rehydrated, and antigen retrieval (0.1M citrate buffer) was performed prior to blocking. Tissue sections were then incubated overnight at 4°C with polyclonal antibodies directed against leptin receptor (ObR) (1:100; Abcam). Sections were then rinsed and incubated with an anti-rabbit biotinylated antibody followed by a tertiary streptavidin peroxidase conjugate (Zymed Laboratories, Inc.). Tissue sections were developed (five minute exposure to 3-amino-9-ethyl-carbazole) and counter stained with hemotoxylin. Photomicrographs were obtained using standard light microscopy (Nikon Spot camera system).
PKC Activity Assay
PKC-β enzymatic activity from coronary arteries was assessed using a PKC activity assay (Assay Designs). Experimental groups included PVAT exposed MetS arteries with and without ruboxistaurin (1μM) pretreatment and lean arteries pretreated with exogenous leptin (30 ng/mL; n = 3 animals for each group). Crude protein extracts were then prepared and measured at equal loading concentrations. PKC activity was measured by spectroscopy as indicated by the manufacturer.
Statistical Analyses
Data are presented as mean ± standard error for n number of animals. For isometric tension studies, a two-way ANOVA was used to test the effects of the perivascular adipose (Factor A) and various drugs (Factor B) on coronary dilator responsiveness. A t-test was used in specific cases (e.g. lean vs. MetS) to compare phenotypic data, half maximal effective concentration (EC50) values and data from Western analyses (Sigma Stat 3.0). When statistical differences were found with ANOVA a Student-Newman-Keuls multiple comparison test was performed. The criterion for statistical significance was P < 0.05 in all tests.
Results
Phenotype of Lean and MetS Ossabaw Miniature Swine
Compared to their lean counterparts, MetS swine exhibited a 49% increase in body weight (kg), 90% increase in fasting glucose, 662% increase in total cholesterol, 140% increase in triglyceride levels, and a 17% increase in blood pressure and heart rate. Importantly, MetS swine also exhibited greater atherosclerotic arterial wall coverage and percent luminal stenosis (Table 1). Figure 1 illustrates representative coronary arteries with and without PVAT isolated from lean (A) and MetS (B) swine. The volume of coronary PVAT was typically larger in hearts from MetS vs. lean swine, however PVAT volume was not experimentally quantified.
Table 1.
Phenotypic characteristics of lean and metabolic syndrome Ossabaw swine
| Phenotype | Lean | MetS |
|---|---|---|
| Body Weight (kg) | 45 ± 4 | 67 ± 3* |
| Mean arterial pressure (mmHg) | 95 ± 7 | 111 ± 4* |
| Heart rate (bpm) | 79 ± 3 | 92 ± 5* |
| Fasting glucose (mg/dl) | 68 ± 4 | 129 ± 4* |
| Total cholesterol (mg/dl) | 66 ± 4 | 503 ± 41* |
| Triglycerides (mg/dl) | 20 ± 4 | 48 ± 5* |
| % Arterial Wall Coverage | 20 ± 10 | 71 ± 18* |
| % Luminal Stenosis | 6 ± 1 | 11 ± 2* |
Values are mean ± SE for lean (n = 6) and MetS (n = 6) swine.
P < 0.05 vs. Lean.
Epicardial PVAT and Coronary Endothelial Function in MetS
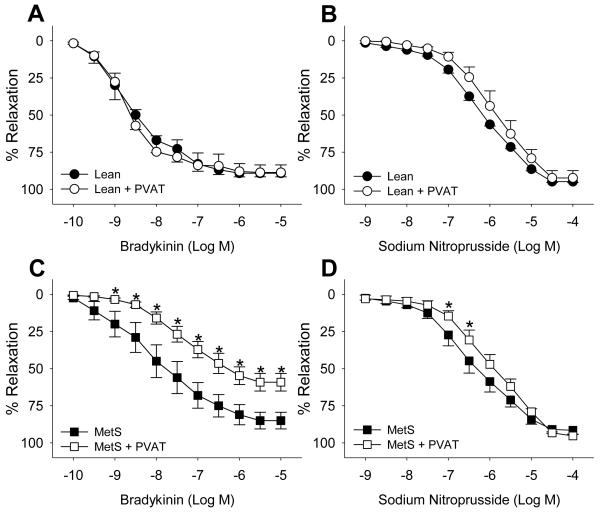
Baseline pre-tension averaged ~3.9 ± 0.4 g for coronary artery rings from lean and MetS swine with and without PVAT (P = 0.44). Active tension developed in response to 1 μM U46619 was significantly decreased from 10.5 ± 0.8 g in lean to 6.9 ± 0.7 g in MetS arteries without PVAT (P = 0.004). Active tension development was unaffected by the presence of PVAT in coronary arteries from both lean and MetS swine (P = 0.41 for MetS and 0.90 for Lean). The presence of PVAT had no significant effect on endothelial-dependent vasodilation to bradykinin (Figure 2A) or endothelial-independent vasodilation to sodium nitroprusside (Figure 2B) in coronary arteries obtained from lean swine. In contrast, induction of the MetS alone resulted in significant endothelial dysfunction that was markedly exacerbated by the presence of PVAT (Figure 2A and C). In particular, the EC50 value for bradykinin-induced relaxation in MetS coronary arteries without PVAT was 9.6 ± 4.3 nM compared to 1.3 ± 0.5 nM for lean (P < 0.05). PVAT-derived factors markedly exacerbated underlying endothelial dysfunction in MetS arteries as evidenced by the approximate log-order rightward shift in bradykinin EC50 (from 9.6 ± 4.3 nM to 92.3 ± 32.8 nM, P < 0.05). In addition, bradykinin-mediated vasodilation was significantly attenuated in the concentration range of 1 nM to 10 μM (Figure 2C, P < 0.001). Importantly, the maximal dilator response to bradykinin (10 μM) was also significantly attenuated by MetS PVAT (from 87 ± 7% to 59± 6%, P < 0.001). MetS PVAT modestly attenuated of endothelial-independent relaxation to sodium nitroprusside in the concentration range of 100 to 320 nM (P < 0.01) however, the maximal response and EC50 were unaffected (Figure 2D).
Figure 2. Epicardial PVAT exacerbates coronary endothelial dysfunction in MetS swine.
PVAT failed to attenuate bradykinin or sodium nitroprusside-induced vasodilation in arteries from lean-control animals (A, n = 7 and B, n = 4). In contrast, arteries from MetS swine displayed significant endothelial dysfunction that was markedly exacerbated by PVAT (C, n = 9). PVAT modestly reduced endothelial-independent vasodilation in MetS swine (D, n = 6). * P < 0.01 vs. MetS.
Epicardial PVAT-Derived Leptin and Coronary Endothelial Dysfunction in MetS
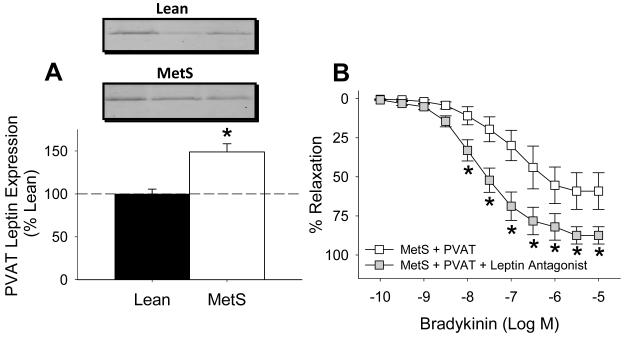
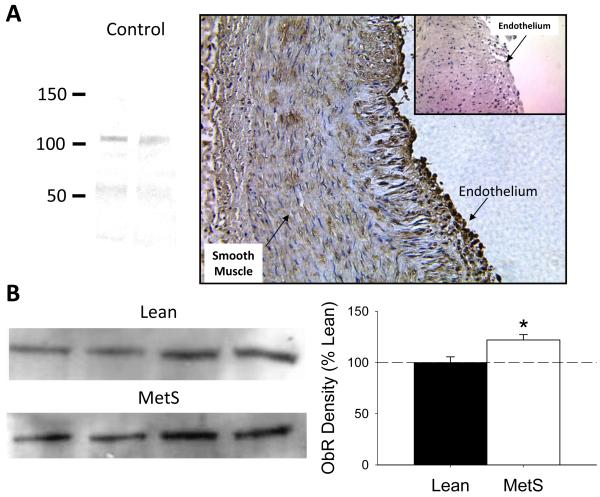
Western blot analysis demonstrated that induction of the MetS increased epicardial PVAT leptin protein ~50 ± 10% relative to PVAT from lean swine (Figure 3A, P < 0.05, n = 4 lean and MetS animals each). β-actin protein expression was not different in PVAT from lean vs. MetS swine (gel not shown, P = 0.60). RT-PCR revealed no significant difference in leptin gene expression between lean (Ct = 42.4) and MetS (Ct = 42.5) swine (P = 0.9). Importantly, administration of a recombinant, pegylated leptin receptor antagonist (200 ng/ml) significantly improved endothelial-dependent dilation to bradykinin (from 10 nM to 10 μM) in MetS coronary arteries with PVAT (Figure 3B, P < 0.001). Inhibition of leptin signaling also augmented the maximal dilator response to bradykinin (from 59 ± 12% to 88 ± 6%, P < 0.001), and tended to improve bradykinin EC50 from 153 ± 60 nM to 29 ± 14 nM (P < 0.09). Additional immunohistochemistry studies confirmed expression of the leptin receptor (ObR) in coronary arteries from lean and Mets swine. Coronary ObR expression was located predominantly in the coronary endothelium (Figure 4A). Selectivity of the ObR antibody (Abcam) was confirmed by Western analysis. Additional Western analyses also showed that coronary ObR expression was significantly elevated in coronary arteries obtained from MetS swine (n = 4, Figure 4B).
Figure 3. Augmented coronary PVAT-derived leptin exacerbates endothelial dysfunction in MetS swine.
Western blot analysis demonstrated that induction of MetS increased epicardial PVAT leptin expression ~ 50 ± 10% relative to PVAT from lean swine (A, n = 4 each group). * P < 0.05 vs. lean in panel A. Inhibition of leptin receptor signaling with a recombinant, pegylated leptin antagonist (200 ng/ml) significantly improved endothelial-dependent dilation to bradykinin in coronary arteries from MetS swine with PVAT (B, n = 4). * P < 0.001 vs. MetS + PVAT in panel B.
Figure 4. MetS augments expression of signaling competent leptin receptor in coronary arteries.
Representative immunohistochemistry slide confirming the prominent expression of the leptin receptor (ObR) in MetS coronary endothelium (A); (inset) negative control with secondary antibody. Selectivity of primary ObR antibody was confirmed by Western blot (A). Additional Western blot data demonstrated a ~25% increase in ObR expression in MetS vs. lean coronary arteries. * P < 0.05 vs. lean (n = 4 each group).
Leptin and MetS PVAT induce Coronary Endothelial Dysfunction via a PKC-β
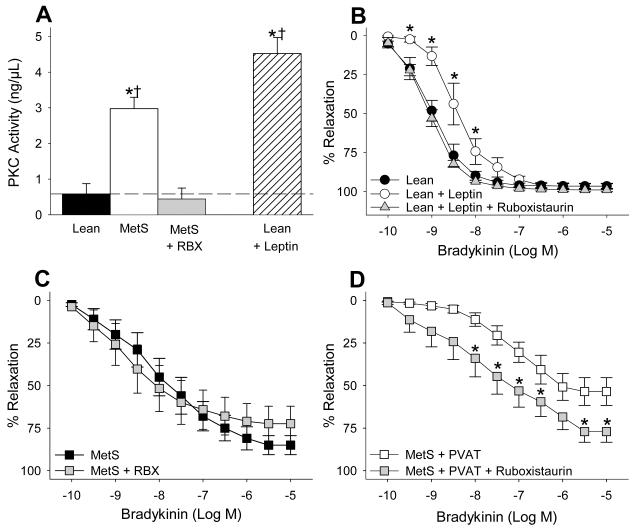
We found that PKC activity was markedly increased in MetS coronary arteries exposed to PVAT relative to PVAT exposed arteries from lean swine (Figure 5A). This increase in activity was reversed by pre-treatment with the PKC-β inhibitor ruboxistaurin (1 μM), and was similar to the elevation of PKC activity observed following the administration of exogenous leptin (30 ng/ml) to lean coronary arteries without PVAT. Additional “proof of principle” studies determined that acute administration of leptin (30 ng/ml) attenuated endothelial-dependent dilation to bradykinin (from 0.3 nM to 10 nM, P < 0.001), and shifted EC50 response from 1.3 ± 0.5 nM to 5.2 ± 1.8 nM (P < 0.05) in coronary arteries without PVAT from lean swine (Figure 5B). Leptin administration also significantly diminished coronary endothelial-dependent vasodilation in MetS coronary arteries without PVAT as evidenced by the increase in EC50 from 3.7 ± 2.0 nM in untreated MetS arteries to 16.9 ± 7.8 nM following treatment with 30 ng/ml leptin. This ~ 4.5-fold increase in the EC50 of MetS arteries is similar to the 4-fold increase in EC50 observed when lean arteries were treated with the same dose of leptin. Although inhibition of PKC-β with ruboxistaurin (1 μM) had little effect in MetS coronary arteries without PVAT (Figure 5C, P = 0.13 vs. untreated MetS arteries), ruboxistaurin significantly improved endothelial-dependent dilation to bradykinin (range 10 nM to 10 μM) in MetS coronary arteries with PVAT (Figure 5D, P < 0.001). This effect was evidenced by a decrease in the EC50 from 139.4 ± 48.8 nM to 22.1± 11.9 nM (P < 0.05) and increase in the maximal vasodilatory response from 54 ± 8% to 77 ± 6% (P < 0.05). Other studies also demonstrated that ruboxistaurin completely restored leptin-induced coronary endothelial dysfunction in coronary arteries without PVAT from lean swine (Figure 5B).
Figure 5. Leptin and MetS PVAT induce coronary endothelial dysfunction via PKC-β dependent pathway.
PVAT markedly increased PKC activity in coronary arteries from MetS swine (A, n = 3 each group). This increased activity is similar to that of clean-lean arteries exposed to leptin (30 ng/mL). Leptin also impaired bradykinin-mediated dilation in clean coronary arteries from lean swine (B, n = 4 each group). These effects of leptin were reversed by the PKC-β inhibitor ruboxistaurin (RBX). In addition, RBX reversed PVAT-induced endothelial dysfunction (D) without altering baseline endothelial response in MetS arteries (C, n = 6).
Discussion
Recent investigations have implicated PVAT in the initiation and development of atherosclerotic disease.7-11;13;14 However, the degree to which alterations in local PVAT adipokine expression influences coronary endothelial dysfunction (i.e. a precursor of atherosclerosis) in the MetS has not been established. Accordingly, this investigation was designed to examine the effects of MetS epicardial PVAT on coronary endothelial function and elucidate the primary PVAT-derived adipokine and signaling pathway involved. The novel findings of this study include: 1) epicardial PVAT from lean swine has little effect on coronary endothelial-dependent or independent dilation; 2) coronary arteries from MetS swine display significant endothelial dysfunction that is markedly exacerbated by PVAT; 3) MetS increases expression of coronary ObR and is associated with elevated epicardial PVAT leptin protein, while leptin gene expression is unaltered; 4) administration of a recombinant, pegylated leptin antagonist essentially reverses the effect of MetS PVAT on coronary endothelial-dependent dilation; 5) PKC activity is elevated in MetS coronary arteries exposed to PVAT and this effect is reversed by ruboxistaurin; 6) acute administration of leptin alone impairs coronary endothelial function and increases PKC-β activity; and 7) inhibition of PKC-β improves coronary endothelial-dependent dilation in the presence of MetS PVAT. Taken together, these results suggest that epicardial PVAT-derived leptin exacerbates coronary endothelial dysfunction in MetS primarily via a PKC-β dependent signaling pathway.
Results from the present study are the first to identify PVAT-derived leptin and PKC-β signaling in MetS-induced coronary endothelial dysfunction, which is recognized to be an inciting event in the initiation and development of atherosclerotic disease.15;16 Our findings are consistent with recent clinical studies documenting increased epicardial PVAT volume as a risk factor for coronary atherosclerosis9;12 and implicate the local production and paracrine release of potentially atherogenic adipokines from PVAT in MetS-induced coronary artery disease. The effect of MetS PVAT on coronary endothelial-dependent and independent dilation is consistent with recent data from our laboratory which demonstrated that PVAT-derived factors significantly diminish endothelial NO production via PKC-β-dependent, site-specific phosphorylation of eNOS at the inhibitory Thr495 residue.13;14 However, these earlier findings, which were documented in coronary arteries from lean, healthy dogs are in contrast to the present data from lean Ossabaw swine in which PVAT had no effect on coronary endothelial function (Figure 2A). We propose that these disparate findings are largely related to differences in adipokine expression between canines and swine. While we have currently been unable to directly quantify coronary PVAT leptin concentration in Ossabaw swine, previous studies have documented a 3-fold difference in plasma leptin concentrations in lean canines (~6 ng/ml)23 vs. lean Ossabaw swine (~2 ng/ml). Importantly, our present Western blot analysis suggests that increased trafficking/secretion of leptin from adipocytes resulted in elevated leptin protein in MetS PVAT (Figure 3); which is consistent with the increase in epicardial adipose leptin expression observed in patients with coronary artery disease.7 The observation that inhibition of leptin signaling essentially mitigates the effect of MetS PVAT on coronary endothelial-dependent dilation (Figure 3B) supports the functional relevance of the increase in PVAT leptin expression. Furthermore, additional data demonstrating that exogenous leptin administration (30 ng/ml; Figure 5B) impairs coronary endothelial function further supports the potential role of leptin in the pathogenesis of MetS-induced coronary disease. Importantly, we found that exogenous leptin reduced endothelial-dependent dilation in clean coronary arteries from both lean and MetS swine. Thus, we found no evidence of coronary “leptin resistance” in this study, which is in contrast to our previous study in chronically high-fat fed dogs.23 Clearly future studies are needed to more fully characterize the production and release of leptin from coronary PVAT and to establish a direct causal role for local PVAT-derived adipokines in coronary atherogenesis.
Leptin-induced activation of PKC-β-dependent signaling is supported by the ruboxistaurin-sensitive increase in PKC activity in MetS coronary arteries exposed to PVAT and in clean coronary arteries from lean swine exposed to leptin (Figure 5A). We propose that the increase in PKC activity in MetS coronary arteries is mediated by elevated local production of leptin in PVAT (Figure 3) as well as augmented coronary ObR density (Figure 4). The functional coupling of leptin and PKC is significant, as PKC-β has been suggested to play a critical role in obesity-induced endothelial dysfunction.{Bohlen, 2004 1860 /id;Mehta, 2009 1858 /id} Results from the Bohlen laboratory demonstrated that inhibition of PKC-β substantially augmented endothelial NO production in obese Zucker rats.24 Although our data directly implicate PKC-β as an essential component of coronary endothelial leptin signaling, characterization of the intermediate signaling cascade remains to be completed. Of note, ObR signaling is associated with both JAK2-dependent and -independent pathways, including the STAT3, PI 3-kinase, MAPK, AMPK, and mTOR pathways. These pathways act coordinately to form a network that fully mediates leptin response26;27. However, recent studies also indicate that phosphoinositide 3-kinases (PI3K)28 and Ras homolog gene family, member A (RhoA)29 are involved with leptin-induced activation of PKC-β as well as the pro-atherogenic/inflammatory nature of leptin. Further studies are needed to elucidate the precise mediators and pathways involved in this cascade.
The current findings highlight local PVAT-derived leptin as a novel potential contributor to the initiation of coronary atherogenesis in the setting of the MetS. Furthermore, our data are among the first to demonstrate functional coupling between PVAT and PKC-β signaling that supports the “outside to inside” signaling paradigm for PVAT-derived factors in the pathogenesis of coronary artery disease.30 Future studies are needed to address the overall pathophysiologic relevance of PVAT-derived factors, in particular leptin, to MetS-induced coronary vascular dysfunction/disease which, at present is limited by our understanding of how periadventitial factors effectively communicate with the endothelium.
Condensed Abstract.
The current study investigated mechanisms by which perivascular adipose-derived factors impair coronary endothelial function in metabolic syndrome. Results indicate that paracrine leptin expression exacerbates endothelial dysfunction in metabolic syndrome via a PKC-β-dependent pathway. These findings, importantly implicate perivascular adipose-derived leptin as a mediator of coronary disease in obesity.
Acknowledgements
Support: AHA grant 0810048Z (LB), NIH grants HL092245 (JDT), RR13223 (MS), HL62552 (MS), and the Fortune-Fry Ultrasound Research Fund of the Department of Cellular and Integrative Physiology, IU School of Medicine.
Footnotes
Disclosure: The authors have no conflict(s) of interest to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am. J. Physiol Heart Circ. Physiol. 2005;288:H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 2.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler. Thromb. Vasc. Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 3.Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: a regulator of inflammation. Best. Pract. Res. Clin. Endocrinol. Metab. 2005;19:547–566. doi: 10.1016/j.beem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Dick GM, Katz PS, Farias M, III, Morris M, James J, Knudson JD, Tune JD. Resistin impairs endothelium-dependent dilation to bradykinin, but not acetylcholine, in the coronary circulation. Am. J. Physiol Heart Circ. Physiol. 2006;291:H2997–H3002. doi: 10.1152/ajpheart.01035.2005. [DOI] [PubMed] [Google Scholar]
- 5.Knudson JD, Dincer UD, Zhang C, Swafford AN, Jr, Koshida R, Picchi A, Focardi M, Dick GM, Tune JD. Leptin Receptors are Expressed in Coronary Arteries and Hyperleptinemia Causes Significant Coronary Endothelial Dysfunction. Am. J. Physiol Heart Circ. Physiol. 2005;289:H48–56. doi: 10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- 6.Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ. Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 7.Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, Chiu CC, Voon WC, Sheu SH, Lai WT. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int. J. Obes. (Lond) 2008;32:268–274. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 8.Gorter PM, van Lindert AS, de Vos AM, Meijs MF, van der GY, Doevendans PA, Prokop M, Visseren FL. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197:896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Greif M, Becker A, von ZF, Lebherz C, Lehrke M, Broedl UC, Tittus J, Parhofer K, Becker C, Reiser M, Knez A, Leber AW. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009;29:781–786. doi: 10.1161/ATVBAHA.108.180653. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa Y, Ishii T, Asuwa N, Masuda S. Absence of atherosclerosis evolution in the coronary arterial segment covered by myocardial tissue in cholesterol-fed rabbits. Virchows Arch. 1997;430:163–171. doi: 10.1007/BF01008038. [DOI] [PubMed] [Google Scholar]
- 11.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 12.Jeong JW, Jeong MH, Yun KH, Oh SK, Park EM, Kim YK, Rhee SJ, Lee EM, Lee J, Yoo NJ, Kim NH, Park JC. Echocardiographic epicardial fat thickness and coronary artery disease. Circ. J. 2007;71:536–539. doi: 10.1253/circj.71.536. [DOI] [PubMed] [Google Scholar]
- 13.Payne GA, Borbouse L, Bratz IN, Roell WC, Bohlen HG, Dick GM, Tune JD. Endogenous adipose-derived factors diminish coronary endothelial function via inhibition of nitric oxide synthase. Microcirculation. 2008;15:417–426. doi: 10.1080/10739680701858447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD. Periadventitial adipose tissue impairs coronary endothelial function via PKC-beta-dependent phosphorylation of nitric oxide synthase. Am. J. Physiol Heart Circ. Physiol. 2009;297:H460–H465. doi: 10.1152/ajpheart.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 17.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 18.Sturek M, Alloosh M, Wenzel J, Byrd JP, Edwards JM, Lloyd PG, Tune JD, March KL, Miller MA, Mokelke EA, Brisbin IL. Ossabaw island miniature swine: cardiometabolic syndrome assessment. In: Swindle MM, editor. Swine in the Laboratory: Surgery, Anesthesia, Imaging and Experimental Techniques. CRC Press; Boca Raton: 2007. [Google Scholar]
- 19.Borbouse L, Dick GM, Payne GA, Payne BD, Svendsen MC, Neeb ZP, Alloosh M, Bratz IN, Sturek M, Tune JD. Contribution of BKCa channels to local metabolic coronary vasodilation: effects of metabolic syndrome. Am J. Physiol Heart Circ. Physiol. 2010;298:H966–H973. doi: 10.1152/ajpheart.00876.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, Sturek M. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am. J. Physiol Heart Circ. Physiol. 2008;294:H2489–H2496. doi: 10.1152/ajpheart.01191.2007. [DOI] [PubMed] [Google Scholar]
- 21.Elinav E, Niv-Spector L, Katz M, Price TO, Ali M, Yacobovitz M, Solomon G, Reicher S, Lynch JL, Halpern Z, Banks WA, Gertler A. Pegylated leptin antagonist is a potent orexigenic agent: preparation and mechanism of activity. Endocrinology. 2009;150:3083–3091. doi: 10.1210/en.2008-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, Rogers PA, Tune JD. Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. Am J. Physiol Heart Circ. Physiol. 2008;294:H2371–H2381. doi: 10.1152/ajpheart.01279.2007. [DOI] [PubMed] [Google Scholar]
- 23.Knudson JD, Dincer UD, Dick GM, Shibata H, Akahane R, Saito M, Tune JD. Leptin resistance extends to the coronary vasculature in prediabetic dogs and provides a protective adaptation against endothelial dysfunction. Am. J. Physiol Heart Circ. Physiol. 2005;289:H1038–H1046. doi: 10.1152/ajpheart.00244.2005. [DOI] [PubMed] [Google Scholar]
- 24.Bohlen HG. Protein kinase betaII in Zucker obese rats compromises oxygen and flow-mediated regulation of nitric oxide formation. Am. J. Physiol Heart Circ. Physiol. 2004;286:H492–H497. doi: 10.1152/ajpheart.00818.2003. [DOI] [PubMed] [Google Scholar]
- 25.Mehta NN, Sheetz M, Price K, Comiskey L, Amrutia S, Iqbal N, Mohler ER, Reilly MP. Selective PKC Beta Inhibition with Ruboxistaurin and Endothelial Function in Type-2 Diabetes Mellitus. Cardiovasc. Drugs Ther. 2009;23:17–24. doi: 10.1007/s10557-008-6144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem. J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J. Physiol Endocrinol. Metab. 2009;297:E1247–E1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konstantinidis D, Paletas K, Koliakos G, Kaloyianni M. Signaling Components Involved in Leptin-Induced Amplification of the Atherosclerosis-Related Properties of Human Monocytes. J. Vasc. Res. 2008;46:199–208. doi: 10.1159/000161234. [DOI] [PubMed] [Google Scholar]
- 29.De RS, Cirillo P, Pacileo M, Di, P V, Paglia A, Chiariello M. Leptin stimulated C-reactive protein production by human coronary artery endothelial cells. J. Vasc. Res. 2009;46:609–617. doi: 10.1159/000226229. [DOI] [PubMed] [Google Scholar]
- 30.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc. Res. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]