Abstract
Since Helicobacter pylori persist for decades in the human stomach, the aim of this study was to examine the long-term course in H. pylori-specific serum IgG responses with respect to subclass and antigenic target. We studied paired serum samples obtained in 1973 and in 1994 in Vammala, Finland from 64 healthy H. pylori-positive adults and from other healthy controls. H. pylori serum IgA, IgG, and IgG subclass responses were determined by antigen-specific ELISAs. H. pylori-specific IgG1 and IgG4 subtype responses from 47 subjects were similar in 1973 and 1994, but not when compared to unrelated persons. H. pylori-specific IgG1/IgG4 ratios amongst the participants varied > 1000-fold; however, 89.4% had an IgG1/IgG4 ratio >1.0, consistent with a predominant IgG1 (Th1) response. Furthermore, ratios in individual hosts were stable over the 21-year period (r=0.56, p< 0.001). The immune response to heat shock protein HspA was unchanged in 49 (77%) of the 64 subjects tested; of the 15 who changed serostatus, all seroconverted and were significantly younger than those who did not change status. These findings indicate that H. pylori-specific antibody responses are host-specific with IgG1/IgG4 ratios stable over 21 years, IgG1 responses predominating, and HspA seroconversion with aging.
INTRODUCTION
Helicobacter pylori colonizes the gastric mucous layer of much of the world's population (1, 2). H. pylori colonization is clinically inapparent, despite histological evidence of host responses, termed chronic gastritis, (3), but is a risk factor for the development of peptic ulceration, gastric MALT-lymphoma, and non-cardia gastric cancer (3–5) yet may protect against other illnesses (6). H. pylori persists for decades, and often for life (1, 2). The ability to persist occur through a long-standing dynamic equilibrium between microbe and host (6), influenced by many factors, including the adaptive immune responses (7). However, after an initial transience, bacterial persistence implies that the immune responses are ineffective at microbe elimination, resulting in the establishment of host colonization (8, 9).
H. pylori strains may carry the cag genomic island, which encodes a type IV secretion system, including the CagA protein (10). Persons carrying such strains produce serum IgG antibodies to the CagA protein (11). Patients colonized with H. pylori develop strong serologic responses to H. pylori-specific antigens (12). In contrast, only about 30–40% of colonized persons develop antibodies to a highly conserved 13kDa H. pylori heat shock protein (HspA) (13), response that appears age-associated (14, 15).
The adaptive immune responses in H. pylori-colonized persons involve both T and B lymphocytes (12), and may be considered as being polarized by the commitment of naïve T cells to undergo Th1 or Th2 responses. Th1-dominated responses affect local immune effectors including IL-2, IFN-γ, and TNF-α (16, 17), whereas Th2 responses involve IL-4, IL-5, and IL-6 (7, 18). Host B cells are activated by both Th1 and Th2 cells via specific cytokines, and responses involving IgG1, IgG2, IgG3, and IgG4 (19) predominate systemically. IgG1 and IgG4 are considered as markers of Th1 and Th2 responses, respectively (20, 21). The influence of environmental factors such as parasitic infections (22), host factors including aging, and presence of H. pylori in the differentiation and regulation of T-cell responses remains uncertain (23).
We studied antigen-specific serum immunoglobulin levels over a 21-year period in a group of healthy adults. Our goal was to assess HspA immune responses and the stability of H. pylori-specific IgG1 and IgG4 over decades of colonization.
MATERIALS AND METHODS
Subjects
Based on a population of 16,000 inhabitants, 408 healthy subjects in Vammala were selected at random from Finland's National Population Register during both 1973 and 1994 (24). Individuals who had participated in the initial 1973 study and were still alive in 1994 were invited to re-participate; a total of 224 (54.9%) persons agreed.
We selected 64 adults who were persistently H. pylori-seropositive in 1973 and 1994, as previous determined (24, 25) and from whom paired serum samples were available. The subjects included 29 males and 35 females. We also selected 25 subjects known to be H. pylori-seronegative in both 1973 and 1994 to serve as negative controls for assay standardization and determine background levels of immune responses to specific antigens. In total, 89 (39.7%) of the 224 original subjects sampled at both times were included in these studies. We studied 47 of the 64 subjects since a sufficient serum sample was available from each tube to perform all of the assays for IgG subclasses in duplicate. Subjects were classified as younger (n= 22; age 16.6–28 years, median 24.7) or older (n=25; age 31.3–58.2 years, median 46.4); we chose these two groups to examine whether antibody kinetics differed in younger or older adults. As controls, we used serum from 1994 from 37 H. pylori-seropositive subjects (median age 53.6 years); a subset of 24 of these subjects who were seropositive in all three of the IgG and IgA H. pylori and IgG CagA assays were used as controls in particular studies.
The selection of subjects who were consistently positive was necessary to determine the HspA status, which only is possible in H. pylori-positive subjects, and to determine variations in IgG subclasses that only are possible in persons with detectable H.pylori-specific IgG responses at both time points. Among the overall population of 221 subjects assessed in 1973 and 1994, only 12 (5.4%) changed H. pylori status, as previously reported (25). As such, the effect of our selection is minimal.
Serum antibodies to H. pylori antigens
Antibody responses were determined by antigen-specific enzyme-linked immunosorbent assays (ELISAs) including acid-glycine extract (24, 26), H. pylori CagA antigen (11, 25), and H. pylori HspA (13, 14). CagA-positivity was defined as optical density ratio (ODR) > 0.35 as described (25).
IgG subclass determinations
IgG1 and IgG4 subclasses ELISAs to H. pylori antigens were performed in 47 subjects from whom sufficient sera were available for complete determinations in duplicate (27).
Statistical analysis
Student's T-test analysis assuming equal variance was performed; p<0.05 was considered significant in all comparisons using SPSS software (SPSS Inc., Chicago IL). The Chi-square analysis also was performed for dichotomous variables using EPI-INFO 2000 software. Correlation analysis between results in 1973 and 1994 was performed, and linear regression coefficients and p values calculated using SPSS.
RESULTS
Inter-host variation in humoral responses to H. pylori antigens
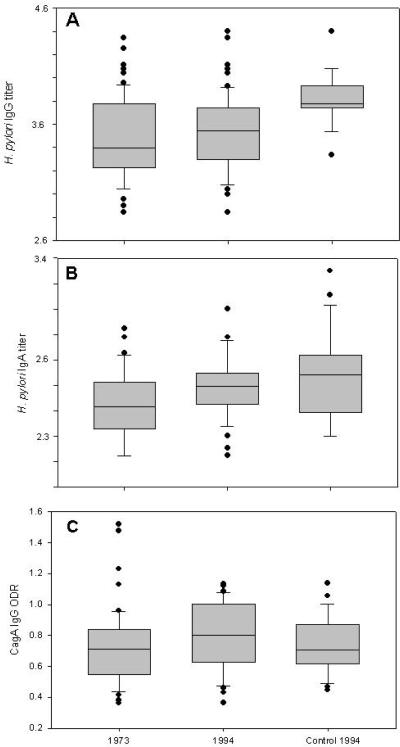
In our population of 64 H. pylori-seropositive subjects, all had IgG responses to H. pylori in both 1973 and 1994, by definition. Persistent IgA- and CagA-positivity was observed in 42 (65.6%) and in 55 (85.9%) of these subjects, respectively. There was no difference in age or gender among the 22 IgA-negative subjects, but the 22 were significantly lower in CagA ODR (0.46) and IgG titer (2450) in 1973 than the 42 persistently positive (0.71 and 5460, respectively) (p <0.005 for both comparisons). The positive responses of hosts ranged from reciprocal titers of 700-25000 for IgG and from 70–1000 for IgA, and OD ratios of 0.36–1.52 for CagA (Figure 1). The ranges observed were similar in 1973 and 1994, and in 24 unrelated persons used as a control group. These data indicate considerable interhost variation in serological responses in all three assays, even among adults who were persistently positive.
Figure 1. Stability of H. pylori− and CagA-specific immune responses over 21 years.
Panel A. Log10 H. pylori-specific reciprocal IgG titers in 64 subjects in 1973 and 1994, and comparison with 1994 values in 24 controls. Panel B: Log10 H. pylori-specific reciprocal IgA titers in 42 subjects and 24 controls. Panel C: CagA IgG in 55 subjects and 24 controls. Data are shown for Median+ InterQuartile Range (IQR); circles represent values > 90th or < 10th percentile. In each panel, the number of subjects reflects those positive in that assay in both 1973 and 1994; 24 subjects were positive in all three assays in both years, and were matched with 24 similarly-aged controls in 1994.
Stability of IgG and IgA responses to H. pylori antigens in individual hosts
We assessed stability of the serological responses in individual persons over a 21-year time period. Among the 64 subjects who were H. pylori-seropositive in both 1973 and 1994 (24, 25), the IgG responses to H. pylori whole cell (WC) antigens were extremely stable (mean log10 variation in titer 0.23± 0.15) during the 21-year period (Table 1). As a control, we compared the 1973 levels with the 1994 levels in unrelated positive persons, which showed significantly greater (0.41+0.27 log10) variation) p<0.001. Of the 42 persistently IgA-positive subjects, there also was a low level (0.20+0.17) of variation in the titers from 1973 to 1994, in comparison to the variation with unrelated persons (Table 1). Over the 21-year period, anti-CagA antibodies also were relatively stable, varying only by 24.1%+15.7%. For the paired samples over the 21-year period, the IgA and IgG responses to the H. pylori WC and CagA antigens in individual hosts were highly correlated (r=0. 59, 0.68, and 0.70, respectively; all p<0.001). In contrast, immune responses to the same antigens compared to the test group of 24 unrelated individuals in 1994 showed little relationship, as expected. From these data, we conclude that among individuals with paired samples, immune responses to H. pylori antigens were significantly stable and host-specific over the 21-year observation period.
Table 1.
Variation in Helicobacter pylori- and CagA-specific immune responses in study subjects, over a 21-year period.
| Category | Paired Samplesb | Control subjectsc (n=24) | |||
|---|---|---|---|---|---|
| Assay | Number of subjects studieda | Absolute log 10 variation in titerf or % variationg | p value | Absolute log 10 variation in titerf or % variationg | p value |
| H. pylori IgG d | 64 | 0.23+0.15f | NS | 0.41+0.27f | 0.008 |
| H. pylori IgA d | 42 | 0.20+0.17f | 0.0008 | 0.38+0.30f | 0.02 |
| CagA IgG e | 55 | 24.1+15g | 0.002 | 47.1+40g | <0.0001 |
Includes only those subjects who were seropositive in the indicated assay in both 1973 and 1994.
p values determined using Student's t-test; NS, p>0.05
Single 1994 sample from an unrelated H. pylori-positive subject; p-values compare with samples from 24 subjects in 1973
Measured as reciprocal titer
Measured as OD value.
Variation measured as absolute difference between the two studied samples, and shown as Mean ± SD log10
Variation measured as absolute percent difference between the two studied samples, and shown as Mean+SD.
IgG subclass-specific responses to H. pylori antigens
A subset of 47 persistently IgG seropositive subjects, was used for analysis of IgG subclass responses. Of these subjects, 34 (72.5%) also were persistently positive for α-H. pylori IgA and 46 (97.9%) α-CagA antibodies. Serum IgG1 α-H. pylori concentrations ranged from 0.09 to 101.7 ng/ml in 1973 and from 2.2 to 129.6 ng/ml in 1994 (Table 2 and Supplemental Figure 1). Thus, there was substantial (>3 log10) interindividual variation, but most values were within a narrower range (Table 2 and Supplemental Figure 1). IgG4 values ranged from 0.1 to 73.0 in 1973 and from 0.2 to 73.3 ng/ml in 1994, also, nearly 3 log10 range (Table 2). However, despite these wide ranges, more than 50% of the subjects varied within less than a 1 log10 range (Table 2), and across the population, the median IgG1/IgG4 ratio approximated 2.0. However, the log10 IgG1/IgG4 ratio of individual test subjects ranged from −1.98 to 1.79 in 1973, and from −0.66 to 1.38 in 1994, showing >2 log10 interindividual variation (Table 2). The 37 control subjects obtained in 1994 showed comparable levels of variation in subclass responses to e H. pylori antigens. Thus, the IgG1 and IgG4 subclass responses to H. pylori WC antigens also showed extensive inter-individual variation.
Table 2.
Helicobacter pylori-specific IgG subclass concentrations, over a 21-year period.
| Subjects | 1973 | 1994 | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | Number of subjects | Age in 1994 (years) | Median [IQR] | Median [IQR] | ||||
| ng/ml | Ratio | ng/ml | Ratio | |||||
| IgG1 | IgG4 | IgG1/IgG4 | IgG1 | IgG4 | IgG1/IgG4 | |||
| All (paired) | 47 | 56.3+12.5 | 17.4 [9.0–32.1] | 9.5 [2.4–14.7] | 2.0 [1.0–6.4] | 13.5 [7.4–28.6] | 7.9 [2.8–16.3] | 2.1 [1.0–.4.8] |
| Young subjects | 22 | 44.9+3.7 | 15.8 [8.8–35.3] | 9.7 [2.3–15.1] | 2.0 [1.0–6.8] | 11.6 [6.7–25.6] | 9.6 [4.4–15.55] | 2.0 [0.87–4.4] |
| Old subjects | 25 | 66.2+8.2 | 17.5 [8.9–28.6] | 9.5 [2.6–15.1] | 2.5 [1.1–5.9] | 16.1 [8.7–31.2] | 6.3 [2.4–16.5] | 2.7 [1.05–6.8] |
| Controla subjects | 24 | 51.4+5.2 | NSb | NAc | NA | 17.5 [6.5–46.1] | 16.4 [7.4–20.5] | 1.5 [0.5–4.3] |
Single 1994 sample from an unrelated H. pylori-positive subject
NS, no specimen analyzed
NA, not applicable
Stability of serum IgG subtypes over 21 years
We asked whether age of subjects correlated with the immune response levels observed. There were no significant differences in IgG1, IgG4 levels, or in the IgG1/IgG4 ratio for the 1973 and 1994 sera from the young (mean age 23.9+3.7y) or the older (mean age 45.2+8.2 years) subject groups (Table 2). We then addressed whether in individual hosts, the specific IgG1, IgG4, and ratio values were stable over the 21-year period. For the 47 subjects tested, H. pylori-specific IgG1 and IgG4 levels in 1973 and 1994 were highly similar (Table 2 and Supplemental Figure 1). However, great variation in the magnitude of the IgG1/IgG4 ratios among the 47 H. pylori positive subjects was observed. Nine subjects had extremely high or low ratios (>11.0 or <0.1) on at least one of the 2 time points. In each of these 9 cases, values did not change direction of dominance between 1973 and 1994. Eliminating the data for these 9 subjects with low values [IgG1 (n=8) and IgG4 (n=1)] confirmed a stable and host-specific response over the 21-year observation period (Table 2). Even with their inclusion, median IgG1/IgG4 ratios were uniform within the groups. We observed higher IgG4 values in the controls, with a consequent decrease in the IgG1/IgG4 ratio; however, these were not significantly different from the paired samples. Stability of serum IgG subtypes over the 21-year period was examined by regression analysis for the paired 1973 and 1994 samples. Our H.priori hypothesis is that there would be correlation, indicative of a stable immunologic response, and we found highly significant R values (Supplemental Table 1). As controls, we compared the 1973 serum (serum 1) with a 1994 serum from an unrelated person (unpaired sera). As expected, the IgG subtype responses for the unpaired samples showed no correlation. From the strong correlation in the paired serum (but not in unpaired specimens), we concluded that there is indeed substantial stability. The IgG1 and the IgG4 specific responses to the H. pylori WC antigens in individual hosts also were weakly (r=0.24) but significantly (p=0.02) correlated. These results indicate that the IgG1 and IgG4 levels and their ratios are host-specific and remain relatively stable over 21 years in H. pylori-positive adult subjects.
Polarity of the Th immune responses
In 1973, a Th1-dominant immune response (level of IgG1>IgG4) was observed in 34 (80.9%), (Mean+SD age 35.9±12.8), of the 42 subjects with clear-cut responses (Table 3). Th2 was dominant in 8 (19%) (Mean+SD age 37.0±13.3), and 5 others had essentially equivalent (borderline) levels (Table 3). Over the next 21 years, 31 (73.2%) of the 42 with clear-cut responses continued to be in the same direction, 8 (19.0%) changed direction, and 3 (7.1%) became borderline. Of the 13 subjects who were Th2 or borderline in 1973, 6 (46.1%) converted to Th1, (in 1973, 3 were borderline and 3 were Th2-dominant). Thus, by 1994, 32 (74.4%) (Mean age 55.6±12.4) of 43 definable persons had Th1–predominant responses, 11 had Th2-dominant responses (Mean age 52.8±12.4), and four were borderline. Three (37.5%) of eight subjects who had Th2-dominant responses in 1973 changed the direction of the response in 1994, vs. only 5 (14.7%) of 34 persons who had Th1-dominant responses in 1973 (p=0.01, FET). Nevertheless, the proportions of Th1 and Th2 immune responses were about the same at the two time points, 21 years apart. The magnitude of the Th1 and Th2 ratios in 1973 and 1994 (Table 3) show clear distinctions for the majority of hosts.
Table 3.
Magnitude of Th1 and Th2 ratios among 47 H. pylori-positive asymptomatic adults
| Categorya | Ratio Range | 1973 | 1994 | ||
|---|---|---|---|---|---|
| Number of subjects | Median/(IQR) | Number of subjects | Median/(IQR) | ||
| Th1-dominant | 1.1–413.5 | 34 | 3.74 (1.84–6.90) | 32 | 3.57 (2.20–6.70) |
| Th2-dominant | 0.01–0.89 | 8 | 0.48 (0.30–0.60) | 11 | 0.42 (0.22–0.67) |
| Borderlineb | 0.9–1.1 | 5 | 0.99 (0.94–1.06) | 4 | 1.05 (0.99–1.08) |
Th1 dominance defined as level (ng/ml) of H. pylori-specific IgG1>IgG4, and for Th2 dominance, IgG4>IgG1.
Borderline was defined as IgG1/IgG4 ratios between 0.9 and 1.1
Stability of IgG-specific responses to HspA antigens
We next examined host responses to H. pylori HspA, a conserved antigen to which only a fraction of H. pylori-positive subjects respond (15–17). Over the 21-year period, 49 (76.6%) of the 64 study subjects had stable HspA immune response status, either persistently HspA positive (n=29, 45.3%) or negative (n=20, 31.3%) (Table 4). Over the 21 years, there were 15 seroconversions (neg→pos), and no sero-reversions (p<0.001). In 1973, the 29 sero-positive subjects were older (62.5+12.2) than the 35 seronegatives (52.0+11.1) (p=0.005). The mean age of the persons who seroconverted (52.5+9.9 in 1994) was almost identical to that of the persons who remained seronegative (51.6+12.2) (Table 4). In 1994, the 44 sero-positive subjects were significantly (p=0.03) older (59.1+12.3) than the 20 who remained seronegative (51.6+12.2). Among the 37 control subjects, the 25 HspA-seropositive subjects also were significantly older in 1994 than the 12 seronegative subjects, results that confirm the association of HspA seropositivity with host age (14, 15). Thus, with adult aging, there is a strong tendency toward increasing HspA sero-responsiveness, and no sero-reversion observed.
Table 4.
H. pylori HspA serostatus in relation to subject age
| Group | HspA Statusa | Number of subjects | Age (years) Mean±SDb | |
|---|---|---|---|---|
| Paired groups | 1973 | 1974 | ||
| Serofast-positive | + | + | 29 | 62.5 + 12.2c,d |
| Serofast-negative | − | − | 20 | 51.6 + 12.2c,d |
| Seroconversion | − | + | 15 | 52.5 + 9.9d |
| Controls | NAf | + | 25 | 54.1 + 4.8e |
| − | 12 | 48.8 + 8.5e | ||
HspA status was defined as indicated in the Materials and Methods section.
Age of subjects in 1994
p-values, based on Student's T-testfor two-samples, assuming equal variances
0.003
0.009
0.02
NA; Not applicable
Association of HspA status and immune responses to other H. pylori antigens
Since responses to HspA are acquired over the course of H. pylori colonization, we asked whether there was any relationship between a subject's seropositivity for HspA and their IgG and IgA immune responses against either H. pylori WC or CagA antigens. No associations between subject HspA status and IgG responses to CagA or IgA responses to H. pylori WC antigens were found. However, the persistently HspA-negative (serofast-negative) subjects had significantly lower IgG immune responses to H. pylori WC antigens than did the subjects who were HspA seroconverters and those with stable positive responses (serofast-positive) to HspA (Data not shown). There were not significant differences in the Th1/Th2 ratios in the hosts according to their HspA serostatus in 1973 or in 1994 (Data not shown).
DISCUSSION
The equilibrium between host and microbe that allows H. pylori to persist in the human gastrointestinal system for several decades must depend on a variety of host, bacterial and environmental factors (28); however the bacterium's ability to evade eradication by the host immune response is central to this capacity (7, 29). We sought to examine subjects who were unequivocally H. pylori-positive for a long period of time, without having to factor in any losses of H. pylori due to specific treatment. Thus, the present study focused on H. pylori-colonized adults from a community-wide survey who had not been treated with antibiotics to eradicate H. pylori and who were stably positive for H. pylori-specific serum antibodies over a 21-year interval. With this well-characterized (24–26) group of subjects representing a sample of the normal population, we could ascertain the stability and broad characteristics of their immune responses.
Overall, we found stability in the IgG values and IgG1 and IgG4 subclass values over the study period. We also observed that despite evidence of heterogeneity in CagA proteins (30), the immunological responses to CagA also were largely stable during the 21-year interval (25). Cross-sectional and longitudinal studies that examined H. pylori seroprevalence across age groups have indicated the stability of positivity (24, 31), but the exact relationships had not been dissected. Other studies have documented CagA as a strongly immunogenic protein, and showed a significant relationship between IgG1 and CagA antibody levels in patients with gastric cancer (32).
Characterization of adaptive immune responses to H. pylori in humans is essential to permit determining whether such responses contribute to the development of disease or its prevention (6), as well as identifying the bacterial constituents that evoke the responses. The immune responses to H. pylori in most individuals have been considered to be skewed toward Th1 (3, 12, 33–34), and the predominant responses have been in IgG1 and IgG2 (32, 35), as in other chronic infections (36). Studying the immune responses to H. pylori in asymptomatic persons is a better way to understand the natural history of the interactions than focusing on symptomatic persons with altered gastric physiology (37), who present for endoscopy (27, 38).
IgG1, the predominant IgG subclass in humans (19), is considered a serologic marker of Th1 responses (20, 39). An IgG1 (Th1) predominance of the H. pylori immune response has been observed in children (40). We confirmed that IgG1 production is predominantly stimulated, and that the immunoglobulin subclass levels were relatively stable during a 21-year interval. IgG4, part of B-cell responses to chronic infections (41), does not form large immune complexes and therefore less often induces inflammation (41). Host immune responses with non-complement-fixing IgG isotypes, such as IgG4 in humans (39), are considered as markers for Th2 responses (21, 39). That levels of H. pylori-specific IgG4 in the studied population were stable during the 21-year interval, independent of age, provides evidence for ongoing Th2 responses in adults. As in the chronic Mycobacterial infections, tuberculosis and leprosy, persistent IgG1 and IgG4 responses often are both present (36).
We also showed that H. pylori− antigen-specific IgG subclass responses occur in different proportions to those observed for total IgG subclasses, in which the IgG1/IgG4 ratio usually varies between 10:1 to 20:1 (42). In contrast, in Schistosomiasis, specific IgG1/IgG4 ratios are 2:1 or 5:1 (43), and in patients with tuberculosis, the ratio of IgG1/IgG4 responses to the Hsp65kDa heat shock protein is essentially at unity (44). These examples indicate that specific IgG1/IgG4 ratios are not fixed, but reflect the nature of the immunologic response to the microbial antigen in question.
Overall, examination of a 21-year window of H. pylori colonization shows that, there are both Th1 and Th2 host responses with Th1 dominance in most adult hosts, but Th2 predominance in a small (~10%) proportion. The data also suggest that by the time in adulthood that adaptive immune responses have become established, the direction of adaptive immunity (Th1- or Th2-dominance) to this organism is largely but not completely stable.
Prior cross-sectional studies have shown increasing serologic recognition of HspA in older H. pylori-positive hosts (14, 15). In this longitudinal study, we showed that HspA status is generally stable but that there is a unidirectional trend toward sero-conversion with aging. Thus, of 35 H. pylori+ HspA-seronegative healthy subjects in their early 30's in 1973, 15 (42.8%) seroconverted over the 21-year period. This phenomenon indicates that there is a maturing of the immune response to H. pylori with age, even in persons who presumably already had been colonized by the organism for decades. This contrasts with the general stability of responses to H. pylori WC and CagA antigens and should be explored further to understand its immunological and possible clinical significance. The HspA protein is produced by a well-conserved and probably essential H. pylori gene hspA (13). Whether the age-related increased responses represent increases in bacterial expression of the protein or loss of tolerance to a conserved antigen is not known. However, the association of seroconversion with lower immune responses to other H. pylori antigens, at baseline suggests that the phenomenon is mediated primarily at the host level rather than by changing bacterial antigenic expression. An age-related increase in the number of responders and in titers for H. pylori specific IgA has been reported by Salomaa-Rasanen et al. (45).
This study is limited by several factors. The population studied is from a small community in a developed country in which the prevalence of H. pylori colonization now is relatively low (24). However, when subjects acquired their H. pylori in the early to mid-20th century, H. pylori was highly prevalent in Finland (24, 46). In addition, only adults were studied, and only three antigens were tested. However, the antigens are representative of broad categories. We examined IgG and IgA responses to H. pylori WC antigens, and IgG responses to a strain-specific antigen (CagA), and to a conserved but host-specific antigen (HspA) (13). Nevertheless, considering a relatively homogeneous population, as in this Finnish village, aids analysis, and H. pylori prevalence results are consistent with studies of other western adult populations (47, 48). Although the sample was moderate in size, the study design was able to demonstrate stability of antibodies over a 21-year period, as we have documented using other measurements in another study of this same population (25). In addition, in this population, nearly all (85.9%) subjects also had IgG responses to CagA, whereas fewer (65.6%) had IgA responses to WC antigen, consistent with the notion that IgG responses to H. pylori antigens are more universal than IgA responses (49). That among those who were positive in 1973 in the IgG CagA assay or the IgA H. pylori assay, nearly all remained so, also indicates the stability of the responses. Finally, while immune responses in adults may be relevant to the development of H. pylori-associated diseases such as peptic ulceration or gastric cancer, important questions need to be resolved about the development of α-H.pylori responses in childhood, which may be critical to understanding gastric physiology (50). The methodologies reported in studies of adults might be useful for parallel longitudinal studies of children.
Supplementary Material
Acknowledgments
This study was supported, in part by RO1GM63270 from the NIH and by the Diane Belfer Program for Human Microbial Ecology
Footnotes
Conflict of interest: No conflicts of interest are reported for all the authors.
References
- 1.Perez Perez GI, Rothenbacher D, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter. 2004;9(suppl):1–6. doi: 10.1111/j.1083-4389.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 2.Malaty HM. Epidemiology of Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21:2005–2014. doi: 10.1016/j.bpg.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Sommer F, Faller G, Konturek P, Kirchner T, Hahn E-G, Zeus J, Rollinghoff M, Lohoff M. Antrum and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect Immun. 1998;66:5543–5546. doi: 10.1128/iai.66.11.5543-5546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner H, Rothenbacher D, Bode G, Adler G. The individual and joint contributions of Helicobacter pylori infection and family history to the risk for peptic ulcer disease. J Infect Dis. 1998;177:1124–1127. doi: 10.1086/517410. [DOI] [PubMed] [Google Scholar]
- 5.Helicobacter and Cancer collaborative group Gastric cancer and Helicobacter pylori: a combined analysis of 12 case-control studies nested within prospective cohorts. Gut. 2001;49:347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atherton JC, Blaser MJ. Co-adaptation of Helicobacter pylori and humans, ancient history moderm implications. J Clin Invest. 2009;119:2475–2487. doi: 10.1172/JCI38605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson K, Loughlin MF, Potter R, Jenks PJ. Host adaptation and immune modulation are mediated by homologous recombination in Helicobacter pylori. J Infect Dis. 2005;191:579–587. doi: 10.1086/427657. [DOI] [PubMed] [Google Scholar]
- 8.Perez Perez GI, Sack RB, Reid R, Santosham M, Croll J, Blaser MJ. Transient and persistent Helicobacter pylori colonization in Native American children. J Clin Microbiol. 2003;41:2401–2407. doi: 10.1128/JCM.41.6.2401-2407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois A, Berg DE, Incecik ET, Fiala N, Heman-Ackah LM, Perez Perez GI, Blaser MJ. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect immune. 1996;64:2885–2891. doi: 10.1128/iai.64.8.2885-2891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cellular Microbiology. 2008;10:1573–1581. doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 11.Blaser MJ, Perez Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmerman GH, Nomura A. Infection with H. pylori strains possessing CagA associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Research. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 12.Suarez G, Reyes VE, Beswick EJ. Immune response to H. pylori. World J Gastroenterol. 2006;12:5593–5598. doi: 10.3748/wjg.v12.i35.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suerbaum S, Thiberge JM, Kansau I, Ferrero RL, Labigne A. Helicobacter pylori hspA–hspB heat shock gene cluster: nucleotide sequence, expression, putative function, and immunogenicity. Mol Microbiol. 1994;14:959–974. doi: 10.1111/j.1365-2958.1994.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 14.Perez Perez GI, Thiberge JM, Labigne A, Blaser MJ. Relationship of the immune response to heat-shock protein and characteristics of Helicobacter pylori infected patients. J Infect Dis. 1996;174:1046–1050. doi: 10.1093/infdis/174.5.1046. [DOI] [PubMed] [Google Scholar]
- 15.Ng EK, Thompson SA, Perez Perez GI, Kansau I, van der Ende A, Labigne A, Sung JJ, Chung SC, Blaser MJ. Helicobacter pylori heat shock protein A: serologic responses and genetic diversity. Clin Diagn Lab Immunol. 1999;6:377–382. doi: 10.1128/cdli.6.3.377-382.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundgren C, Trollmo C, Edebo A, Svennerholm AM, Lundin BS. Helicobacter pylori-specific CD4(+) T cells home to and accumulate in the human Helicobacter pylori-infected gastric mucosa. Infect Immun. 2005;73:5612–5619. doi: 10.1128/IAI.73.9.5612-5619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto T, Kita M, Ohno T, Iwakura Y, Sekikawa K, Inanishi J. Role of tumor necrosis factor-alpha and interferon-gamma in Helicobacter pylori infection. Microbiol Immunol. 2004;48:647–654. doi: 10.1111/j.1348-0421.2004.tb03474.x. [DOI] [PubMed] [Google Scholar]
- 18.Mossmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 19.Maguire GA, Kumararatne DS, Joyce HJ. Are there any clinical indications for measuring IgG subclasses. Ann Clin Biochem. 2002;39:374–377. doi: 10.1258/000456302760042678. [DOI] [PubMed] [Google Scholar]
- 20.Toellner KM, Luther SA, Sze DMY, Choy RK, Taylor DR, Maclennan IC, Acha-Orbea H. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med. 1998;187:1193–1204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aalberse RC, Van der Gaag R, Van Leeuwen J. Serologic aspect of IgG4 antibodies. I Prolonged immunization results in an IgG4 restricted response. J Immunol. 1983;130:722–726. [PubMed] [Google Scholar]
- 22.Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric heliminth infection modulates inflammation and gastric immune response and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–552. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 23.Robinson K, Argent RH, Atherton JC. The inflammatory and immune response to Helicobacter pylori infection. Best Practice & Res Clin Gastroenterol. 2007;21:237–259. doi: 10.1016/j.bpg.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Kosunen TU, Aromaa A, Knekt P, Salomaa A, Rautelin H, Lohi P, Heinonen OP. Helicobacter antibodies in 1973 and 1994 in the adult population of Vammala Finland. Epidemiol Infect. 1997;119:29–34. doi: 10.1017/s0950268897007565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez Perez GI, Salomaa A, Kosunen TU, Daverman B, Rautelin H, Aromaa A, Knekt P, Blaser MJ. Evidence that cagA+ Helicobacter pylori strains are disappearing more rapidly than cagA− strains. Gut. 2002;50:295–298. doi: 10.1136/gut.50.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosunen TU, Seppala K, Sarna S, Sipponen P. Diagnostic value of decreasing IgG, IgA, and IgM antibody titers after eradication of Helicobacter pylori. The Lancet. 1992;339:893–895. doi: 10.1016/0140-6736(92)90929-w. [DOI] [PubMed] [Google Scholar]
- 27.Romero Gallo J, Perez Perez GI, Novick RP, Kamath P, Norbu T, Blaser MJ. Responses of endoscopy patients in Ladakh India to Helicobacter pylori whole-cell and CagA antigens. Clin Diagn Lab Immunol. 2002;9:1313–1317. doi: 10.1128/CDLI.9.6.1313-1317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blaser MJ, Kirschner D. The equilibria that allow bacterial persistence in human hosts. Nature. 2007;449:843–849. doi: 10.1038/nature06198. [DOI] [PubMed] [Google Scholar]
- 29.Suerbaum S, Josenhans C. Helicobacter pylori evolution and phenotypic diversification in a changing host. Nat Rev Microbiol. 2007;5:441–452. doi: 10.1038/nrmicro1658. [DOI] [PubMed] [Google Scholar]
- 30.van Doorn LJ, Figueiredo C, Sanna R, Blaser MJ, Quint WG. Distinct variants of Helicobacter pylori cagA are associated with vacA subtypes. J Clin Microbiol. 1999;37:2306–2311. doi: 10.1128/jcm.37.7.2306-2311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rehnberg-Laiho L, Rautelin H, Valle N, Kosunen TU. Persisting Helicobacter antibodies in Finish children and adolescent between two and twenty years of age. Pediatr Infect Dis. 1998;17:796–799. doi: 10.1097/00006454-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Vorobjova T, Ren Z, Dunkley M, Clancy R, Maaroos HI, Labotkin R, Kull K, Uibo R. Response of IgG1 and IgG2 subclasses to Helicobacter pylori in subjects with chronic inflammation of the gastric mucosa, atrophy, and gastric cancer in a country with high Helicobacter pylori infection prevalence. APMIS. 2006;114:372–380. doi: 10.1111/j.1600-0463.2006.apm_392.x. [DOI] [PubMed] [Google Scholar]
- 33.D'Elios MM, Manghetti M, De Carli M, Costa F, Baldari CT, Burroni D, Telford JL, Romagnani S, Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 34.Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, Brooks EG, Graham DY, Reyes VE, Ernst PB. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 35.Bontkes HJ, Veenendaal RA, Peña AS, Goedhard JG, Van Duij W, Kuiper J, Meijer JL, Lamers CB. IgG subclass response to H. pylori in patients with chronic active gastritis and duodenal ulcer. Scan J Gastroenterol. 1992;27:129–133. doi: 10.3109/00365529209165432. [DOI] [PubMed] [Google Scholar]
- 36.Sousa AO, Henry S, Maroja FM, Lee FK, Brum L, Singh M, Lagrange PH, Aucouturier P. IgG subclass distribution of antibody responses to protein and polysaccharide mycobacterial antigens in leprosy and tuberculosis patients. Clin Ex Immunol. 1998;111:48–55. doi: 10.1046/j.1365-2249.1998.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology. 2008;134:1842–1860. doi: 10.1053/j.gastro.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Pérez GI, Brown WR, Dunn BD, Cover TL, Cao P, Blaser MJ. Correlation between gastric mucosal inflammation and serum immune responses in Helicobacter pylori infection. Clin Diagn Lab Immunol. 1994;1:325–329. doi: 10.1128/cdli.1.3.325-329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 40.Dzierzanowska-Fangrat K, Raeiszadeh M, Dzierzanowska D, Gladkowska-Dura M, Celinska-Cedro D, Crabtree JE. IgG subclass response to Helicobacter pylori and CagA antigens in children. Clin Exp Immunol. 2003;134:442–446. doi: 10.1111/j.1365-2249.2003.02304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aalberse RC, Schuurman J. IgG4 breaking the rule. Immunology. 2002;105:9–19. doi: 10.1046/j.0019-2805.2001.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Djurup R, Mansa B, Sondergaard I, Weeke B. IgG subclass concentrations in sera from 200 normal adults and IgG subclass determination of 106 myeloma proteins. An interlaboratory study. Scand J Clin Invest. 1998;48:77–83. doi: 10.3109/00365518809085397. [DOI] [PubMed] [Google Scholar]
- 43.Boctor FN, Peter JB. IgG subclass in human chronic schstosomiasis: overproduction of schistosome-specific and non-specific IgG4. Clin Exp Immunol. 1990;82:574–578. doi: 10.1111/j.1365-2249.1990.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai N-S, LAn J-L, Yu C-L, Lin R-H. Antibody to Mycobacterium tuberculosis 65kDa heat shock protein in patients with rheumatoid arthritis. A survey of antigen-specific antibody isotypes and subclasses in an endemic are of a previous tuberculosis infection. Ann Rheum Dis. 1995;54:225–228. doi: 10.1136/ard.54.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salomaa-Rasanen A, Kosunen TU, Karjalainen J, Aromaa A, Sarna S, Rautelin H. IgA antibodies in persisting Helicobacter pylori infection in Finnish adults. Clin Microbiol Infect. 2006;12:236–240. doi: 10.1111/j.1469-0691.2005.01357.x. [DOI] [PubMed] [Google Scholar]
- 46.Rehnberg-Laiho L, Rautelin H, Koskela P, Sarna S, Pukkala E, Aromaa A, Knekt P, Kosunen TU. Decreasing prevalence of Helicobacter pylori antibodies in Finland, with reference to the decreasing incidence of gastric cancer. Epidemiol Infect. 2001;126:37–42. [PMC free article] [PubMed] [Google Scholar]
- 47.Sorberg M, Nyren O, Granstrom M. Unexpected decrease with age of Helicobacter pylori sero-prevalence among Swedish blood donors. J Clin Microbiol. 2003;41:4038–4032. doi: 10.1128/JCM.41.9.4038-4042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robertson MS, Cade JF, Savoia HF, Clancy RL. Helicobacter pylori infection in the Australian community: current prevalence and lack of association with ABO blood groups. Intern Med J. 2003;33:163–167. doi: 10.1046/j.1445-5994.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- 49.Andersen LP, Rosenstock SJ, Bonnevie O, Jorgensen T. Seroprevalence of immunoglobulin G, M, and A antibodies to Helicobacter pylori in an unselected Danish population. Am J Epidemiol. 1996;143:1157–1164. doi: 10.1093/oxfordjournals.aje.a008694. [DOI] [PubMed] [Google Scholar]
- 50.Newton JL. Changes in upper gastrointestinal physiology with age. Mech Ageing Dev. 2004;125:867–870. doi: 10.1016/j.mad.2004.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.