Abstract
Most early human immunodeficiency virus type 1 (HIV-1) strains are macrophage (M)-tropic HIV variants and use the chemokine receptor CCR5 for infection. Neuronal loss and dementia are less severe among individuals infected with M-tropic strains. However, after several years, the T cell (T)-tropic HIV strain, which uses CXCR4 variant, can emerge in conjunction with brain abnormalities, suggesting strain-specific differences in neuropathogenicity. The molecular and cellular mechanisms of such diversity remain under investigation. We have previously demonstrated that HIV envelope protein gp120IIIB, which binds to CXCR4, causes neuronal apoptosis in rodents. Thus, we have used a similar experimental model to examine the neurotoxic effects of M-tropic gp120BaL. Gp120BaL was microinjected in the rat striatum and neuronal apoptosis was examined in the striatum, as well as in anatomically connected areas, such as the somatosensory cortex and the substantia nigra. Gp120BaL promoted neuronal apoptosis and tissue loss that were confined to the striatum. Apoptosis was associated with microglial activation and increased levels of interleukin-1β. Intriguingly, gp120BaL increased brain-derived neurotrophic factor in the striatum. Overall, our data show that gp120BaL demonstrates a different neuropathological profile than gp120IIIB. A better understanding of the pathogenic mechanisms mediating HIV neurotoxicity is vital for developing effective neuroprotective therapies against Acquired Immune Deficiency Syndrome-associated Dementia Complex.
Keywords: BDNF, gp120BaL, CXCR4, CCR5, microglia
Introduction
The chemokine receptors CXCR4 and CCR5 are necessary for the fusion of human immunodeficiency virus type 1 (HIV) strains with target cell membranes and consequently, for viral entry (Berger et al., 1999). Macrophage-tropic HIV primarily uses CCR5 as a co-receptor, whereas T-cell line-tropic (T-tropic) viruses use CXCR4 (Pierson et al., 2004). Both CCR5- and CXCR4-using viruses are cytotoxic, although their pathogenic effects might be distinct (Grivel & Margolis, 1999; Harouse et al., 1999). HIV also enters the central nervous system during the early phase of infection and infects primarily macrophages and microglia (He et al., 1997; Bissel & Wiley, 2004). Nevertheless, it has been established that HIV, in the late phase of infection, promotes loss of neurons (Adle-Biassette et al., 1995; Gelbard & Epstein, 1995; Power et al., 1998; Petito et al., 2001) and other brain abnormalities defined by the term HIV-associated dementia (HAD), a neurological disease characterized by cognitive and motor impairments (Price et al., 1988; Masliah et al., 1996; Price, 1996).
Complex mechanisms have been suggested to explain why HIV causes neurological diseases such as HAD. Two prominent hypotheses have been proposed, namely that HIV causes neuronal apoptosis and white matter atrophy by either promoting neuroinflammatory events, such as the release of pro-apoptotic cytokines (Mastroianni et al., 1992; Glass et al., 1993; Persidsky et al., 1997), excitatory amino acids (Heyes et al., 1991; Bezzi et al., 2001), or nitric oxide (Adamson et al., 1996; Bagetta et al., 1996); or by directly affecting neuronal viability via releasing viral proteins such as the envelope protein gp120. Gp120 has been shown to produce neuronal apoptosis by acting on CXCR4 (Hesselgesser et al., 1998; Meucci et al., 1998; Zheng et al., 1999; Kaul et al., 2001) or CCR5 (Kaul & Lipton, 1999; Bachis & Mocchetti, 2005). Both chemokine receptors are expressed in neurons as well as in non–neuronal cells (Albright et al., 1999; van der Meer et al., 2000; Westmoreland et al., 2002). Thus, one may predict that gp120 from M- or T- tropic strains could be equally neurotoxic. However, the relative potency of CCR5-selective gp120 versus CXCR4-specific gp120 has not yet been fully defined. In vitro studies have shown that activation of CCR5 by two natural ligands, CCL5 (or RANTES), or macrophage inflammatory proteins, protects neurons from gp120-induced apoptosis (Meucci et al., 1998; Kaul & Lipton, 1999; Bachis & Mocchetti, 2005). Neuroprotection by CCL5 has also been confirmed by studies showing that cortical cultures from CCR5 knock-out mice are more sensitive to the neurotoxic effect of T-tropic gp120 (Kaul et al., 2007). On the other hand, stromal cell-derived factor 1 alpha or CXCL12, the natural ligand of CXCR4, promotes neuronal apoptosis (Hesselgesser et al., 1998; Kaul & Lipton, 1999; Zheng et al., 1999; Bezzi et al., 2001). In addition, it has been shown that HIV-infected individuals with higher cerebrospinal fluid concentrations of macrophage inflammatory proteins and CCL5 perform better on neuropsychological measures than those with low or undetectable levels (Sozzani et al., 1997; Letendre et al., 1999). Moreover, higher levels of CCL5 correlate with lower plasma HIV (Zanussi et al., 1996; Saha et al., 1998; Paxton et al., 2001). These findings suggest that CCL5 might slow down the progression of HAD.
We have previously reported that gp120IIIB, a strain that binds selectively to CXCR4, causes a time-dependent neuronal apoptosis in the rodent brain both at the site of injection as well as in distal areas (Bachis et al., 2006; Nosheny et al., 2007). In the current study, we have used a similar experimental design to examine the neurotoxic property of gp120BaL, a CCR5 specific strain (McDyer et al., 1999), as well as to dissect the role of the chemokine receptor CCR5 in M-tropic gp120-induced neurotoxicity. We report that gp120BaL exhibits a different profile than gp120IIIB.
Materials and Methods
Animal treatment
All surgical procedures on rats were performed in strict accordance with the Laboratory Animal Welfare Act, with National Institutes of Health Guide for the Care and Use of Laboratory Animals, and after approval from the Georgetown University Animal Care and Use Committee. Coordinates for injecting gp120 strain BaL (NIH AIDS Research Reference Reagent Program) in 0.1% bovine serum albumin or vehicle control (VEH, boiled gp120 in 0.1% bovine serum albumin) into the rat striatum (anteroposterior +0.7 mm; mediolateral ±3.0 mm; dorsoventral −6.0 mm from bregma), were according to Paxinos & Watson (Paxinos & Watson, 1998). Details of injection procedures have been described previously (Nosheny et al., 2004; Bachis et al., 2006). In brief, VEH or gp120BaL (400 ng) were delivered by a microperfusion pump (0.2 μl/min for 10 min) in anesthetized (ketamine/xylazine, 80/10 mg/kg i.p.) adult male Sprague Dawley rats (Taconic Farm, Hudson, NY). After completion of each injection, the needle was left in place for an additional 4 min to accomplish quantitative diffusion of the volume delivered. Animals were then returned to their cages. At the appropriate survival times, animals were deeply anesthetized with ketamine/xylazine (80 and 10 mg/kg, i.p.), followed by intracardiac perfusion of 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for immunohistochemical analyses, or euthanized by decapitation for the biochemical determinations.
Immunohistochemistry
Brains were removed and post-fixed in 4% paraformaldehyde, then transferred into buffered graded sucrose (10%, 20% and 30%), and serial cross sections (16 μm) were prepared. For apoptosis, sections were analyzed for in situ terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) according to the manufacturer instructions (ApopTag® Fluorescein In Situ Apoptosis Detection Kit, Cat. # S7110, Millipore, Bedford, MA) and activated caspase-3 as previously described (Nosheny et al., 2004; Bachis et al., 2006). In brief, for caspase-3 and cellular markers, after incubation with 5% normal goat serum, sections were incubated with a cleaved caspase-3 antibody (1:150 dilution; Cell Signaling, Beverly, MA; cat. # 9661) for 48 hr at 4°C, and NeuN (1:500, Millipore; cat. #MAB377) to visualize neurons. CD68 (1:500; Serotec, Raleigh, NC; cat. # MCA341R), or glia fibrillary acidic protein (GFAP, 1:200; Millipore; cat. # MAB360) were used to visualize microglia or astrocytes, respectively. After three washes, sections were incubated with Alexa-Fluor® 488 and Alexa-Fluor® 594 secondary antibodies (1:1000, Millipore; cat. # A11034 and A11032) to visualize caspase-3 and cellular markers, respectively. Control sections were incubated either with primary or secondary antibody only. A positive control for the TUNEL staining was done by incubating sections with DNase I (0.5 μg/ml, Sigma) in 30 mM Trizma base, pH 7.2, 4 mM MgCl2, 0.1 mM DTT at room temperature for 10 min. For negative control, terminal deoxynucleotidyl transferase was omitted.
For tyrosine hydroxylase (TH) and caspase-3 immunoreactivity, sections were incubated with an anti-TH antibody (1:200; Millipore; cat. # MAB5280) together with the caspase-3 antibody described above for 48 hr at 4°C. Sections were then incubated with Alexa-Fluor® 594 and Alexa-Fluor® 488 (1:1000, Millipore) secondary antibodies to visualize TH and caspase-3, respectively.
Histological analysis
Sections were analyzed with a Zeiss fluorescence microscope Axioplan2 supported with AxioVision software (Carl Zeiss Microimaging, Inc., Thornwood, NY) as previously described (Bachis et al., 2006; Nosheny et al., 2007). In brief, positive cells were counted using a 20× objective and MetaMorph® Imaging software (Universal Imaging Corp, Downingtown, PA). The number of TUNEL and/or caspase-3 and CD68 positive cells in the striatum was assessed in an area of at least 0.25 mm2 per section after excluding the sections with the needle tract (~40 μm). A total of at least 10 sections (1 section every 100 μm) rostral and caudal to the injection site per animal were used (N=5 rats per group). The entire striatum was analyzed. For the substantia nigra (SN), caspase-3 positive cells were counted along its entire length. Caspase-3 positive neurons in the somatosensory cortex were counted in a total of 15 sections (1 section every 100 μm). To ensure equal overall staining intensity between sections, total intensity of fluorescent signal in the corpus callosum was compared between sections.
For TH immunoreactivity in the SN, total intensity of fluorescent signal was measured using MetaMorph® software in a series of 20X digitized microscope images (6 sections/animal; n=5 animals/group) from the following regions of interest: −5.00 to −5.30 mm from bregma (medial SN), −5.80 to −6.30 mm from bregma (lateral SN) (Paxinos & Watson, 1998). TH values for each group were then averaged and expressed as percentage of control levels. Size and shape of the lateral and 3rd ventricles were examined to ensure that sections from identical coronal planes were used for each animal.
Enzyme-Linked ImmunoSorbent Assay (ELISA) for cytokines
Interleukin-1β (IL-1β) levels were measured in the striatum by ELISA. Tissues were homogenized using RIPA buffer (Millipore; cat. # 20-188) containing 1 μg/mL aprotinin, 1 μg/mL leupeptin, and 1 μg/mL pepstatin (all from Sigma). Samples were centrifuged for 10 min at 15,000 g and protein content was measured in the supernatant by the Bradford Comassie Blue colorimetric assay (Bio-Rad). The ELISA assay was performed according to the manufacturer instructions (Rat IL-1 beta/IL-1F2 DuoSet, cat. # DY501; R&D, Minneapolis, MN) using a 96 well plate covered with a monoclonal anti IL-1β antibody overnight at room temperature. A seven point standard curve using 2-fold serial dilutions was also prepared. The highest standard had a concentration of 2000 pg of IL-1β per 100 μl. Each sample was done in triplicate. Wells were then washed and incubated with anti-rat IL-1β polyclonal antibody for 2 hr at room temperature. After washing, 100 μl of Streptavidin-HRP (1:200 dilution, R&D) were added to each well and incubated for 20 minutes at room temperature, followed by three washes and a 20-minute incubation with a solution containing 50% H2O2 and 50% Tetramethylbenzidine (Substrate solution, R&D). The color was developed by adding 50 μl of Stop Solution (2N H2SO4, R&D). The optical density was immediately determined using a microplate reader set to 450 nm. Total IL-1β proteins for each sample were calculated by plotting sample absorbance values against the standard curve.
Cerebellar granule cells
Cerebellar granule cells were prepared from 7-day-old Sprague Dawley rat pups as described previously (Bachis et al., 2003). In brief, neurons were plated onto poly-L-lysine precoated 6 well plates at a density of 1×106 cells/ml and grown in Basal Medium Eagle (Invitrogen Corp., Grand Island, NY) containing fetal calf serum(10%), L-glutamine (0.5 mM), KCl (25 mM), gentamycin (50 μg/ml), and penicillin-streptomycin(10,000U/ml). Cells were maintained at 37°C in 5% CO2-95% air for 7 days before adding gp120s.
ELISA for neurotrophic factors
Brain-derived neurotrophic factor (BDNF), glial-cell derived neurotrophic factor (GDNF) and nerve growth factor (NGF) levels were determined using the Emax immunoassay systems from Promega, Madison, WI (Cat. # G7610, G7620 and G7630, respectively), as previously described (Marini et al., 1998; Nosheny et al., 2004). When neurotrophic factor levels were measured in brain tissue we used 100 μg of tissue homogenate prepared as described above. For the measurement of BDNF in cerebellar granule cells, we used 100 μl of the medium. Each sample was done in triplicate. Total levels of neurotrophic factors for each sample were calculated by plotting sample absorbance values against a standard curve.
Statistical analysis
Data were evaluated by ANOVA with post hoc Holm-Sidak’ test (SigmaStat(R) software, Systat Software, Inc, Point Richard, CA).
Results
Gp120BaL causes cell death at the site of injection
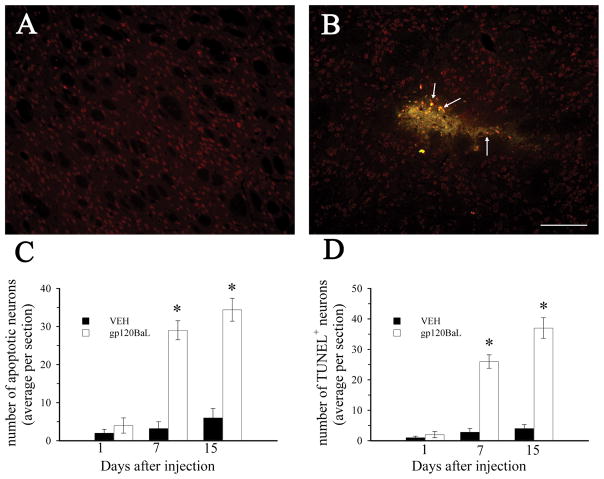
We have previously shown that gpBaL causes caspase-3-dependent neuronal cell death in vitro (Bachis & Mocchetti, 2005). To determine whether gp120BaL is neurotoxic in vivo, adult male rats received an acute injection of gp120BaL (400 ng) or vehicle control (VEH) in the striatum. Animals were sacrificed 1, 7 and 15 days after the injection. Brain sections where then analyzed for activated caspase-3 and TUNEL (Fig. 1), both markers of apoptosis, along with NeuN, a neuronal marker. The striata of VEH-injected rats exhibited few caspase-3 positive cells at all times examined (Figs. 1A and C). In gp120-injected rats there was a time dependent increase in apoptotic cells. In fact, by 7 days and up to 15 days, several sections from the striatum exhibited caspase-3 (arrows in Fig. 1B, Fig. 1C) and TUNEL positive cells (Fig. 1D) which were also NeuN positive, suggesting neurons.
Figure 1. gp120BaL promotes neuronal apoptosis.
VEH or gp120BaL was microinjected into the rat striatum. Animals were sacrificed at the indicated times after the injection. Caspase-3, TUNEL and NeuN immunoreactivity were examined by immunohistochemistry in serial sections from the striatum. Representative sections from VEH (A) and gp120BaL (B) injected rats taken 50 μm from the injection site showing caspase-3 immunoreactivity (green) in NeuN (red) positive cells 7 days after injection. Arrows indicate examples of apoptotic neurons. Bar = 200 μm. C and D, The average number of caspase-3 and TUNEL positive neurons per section was determined by MetaMorph®. Data are expressed as the mean ± SEM of four animals per group (at least 10 sections per animal). *p<0.001 versus VEH control (1C: F = 73.663 for 7 days, F = 50.855 for 15 days; 1D: F = 89.832 for 7 days, F = 80.074).
The extent of tissue damage was then determined by calculating the length and area of TUNEL and caspase-3 immunoreactivity, rostral and caudal to the injection site, as well as the area of tissue loss, as described previously (Bachis et al., 2006). By 7 days, gp120BaL caused an area of tissue damage extending several μm rostral and caudal to the injection site with multiple TUNEL- and caspase-3-positive cells (Table 1). Nevertheless, when compared to the effect of gp120IIIB previously described (Nosheny et al., 2004), the M-tropic gp120 did not cause neuronal injury as extensive as that caused by gp120IIIB.
Table 1.
Extent of tissue damage after acute parenchymal gp120BaL injection
| Groups | Length (μm) | Lesion area (μm2) | Penumbra area (μm2) |
|---|---|---|---|
| VEH | ND | 3.2±1.4 | ND |
| Gp120BaL | 860 ± 26.5 | 11.8 ± 8.3 | 220 ± 35.2 |
Striatal sections from VEH and gp120-treated rats (400 ng, 7 days after the injection) were processed for TUNEL and caspase-3 immunoreactivity. Tissue damage was determined rostrally and caudally from the injection site, by calculating the length of an area showing TUNEL and caspase-3 immunoreactivity, a lesion area (showing no tissue), and a penumbra area (area with caspase-3 positive cells surrounding the lesion area). ND= not detected. Data are the mean ± SEM of at least 15 sections from 3 rats per group. In a previous study (Nosheny et al., 2004), we have found that gp120IIIB-mediated tissue damage was: length 1800 ± 20μm, lesion area 33.8 ± 9.3 μm2 and penumbra area 366.9 ± 45.2 μm2. Thus, gp120BaL evokes a tissue damage that is significantly less than that obtained with gp120IIIB.
Gp120BaL does not affect neurons’ viability in distal areas
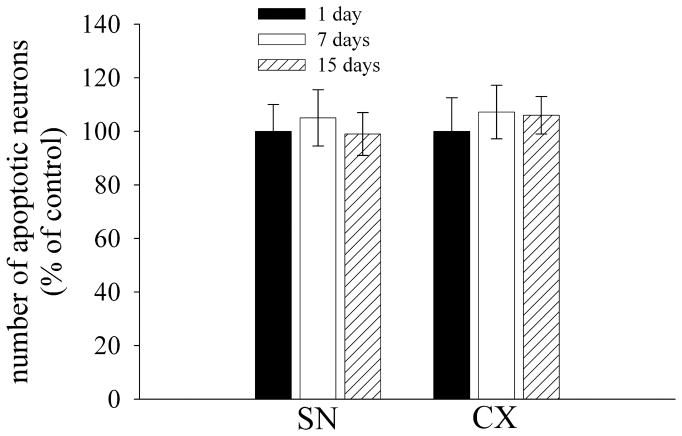
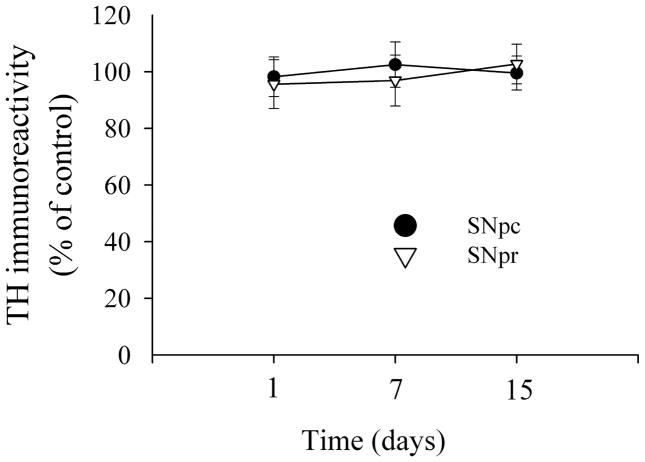
The data presented so far indicate that the pathological profile of M-tropic gp120 is different from that of T-tropic gp120. We have recently shown that intrastriatal gp120IIIB causes neuronal damage in the substantia nigra (SN) (Bachis et al., 2006; Nosheny et al., 2007). Thus, to further analyze the neurotoxic effect of gp120BaL, we determined neuronal loss in areas that send axons to the striatum, such as the SN and the somatosensory cortex (CX). Sections were double stained with an antibody against cleaved caspase-3 and an antibody against NeuN. The SN and CX of control rats exhibited an average number of caspase-3 positive neurons per section that was 0.9 ± 1 and 4±1, respectively. This number did not change through the investigation (Fig. 2). Gp120BaL failed to induce more neuronal apoptosis in these brain areas at either 1, 7 or 15 days (Fig. 2). In addition, we did not observed an effect of gp120BaL on TH immunoreactivity in the SN. Indeed, there was no difference between VEH and gp120-treated in TH immunoreactivity at any time point (Fig. 3). These data indicate that M-tropic gp120 does not cause retrograde degeneration.
Figure 2. gp120BaL does not cause apoptosis in distal areas.
Rats were microinjected with VEH or gp120BaL into the striatum and were sacrificed at the indicated days after the injection. Sections from the SN and somatosensory cortex (CX) were processed for caspase-3 and NeuN. Data are the mean ± SEM of the number of caspase-3 positive neurons determined in at least 15 sections per area from five rats per group.
Figure 3. Analysis of TH immunoreactivity.
TH immunoreactivity was analyzed in the SN of rats microinjected with VEH or gp120BaL into the striatum. TH immunoreactivity was quantified using MetaMorph® software in a series of sections from the SN pars compacta (pc) and SN pars reticulata (pr) as previously described (Nosheny et al., 2006). Data, expressed as percentage of controls, are the mean ± SEM of at least 15 sections per rat, n=5 rats per time point.
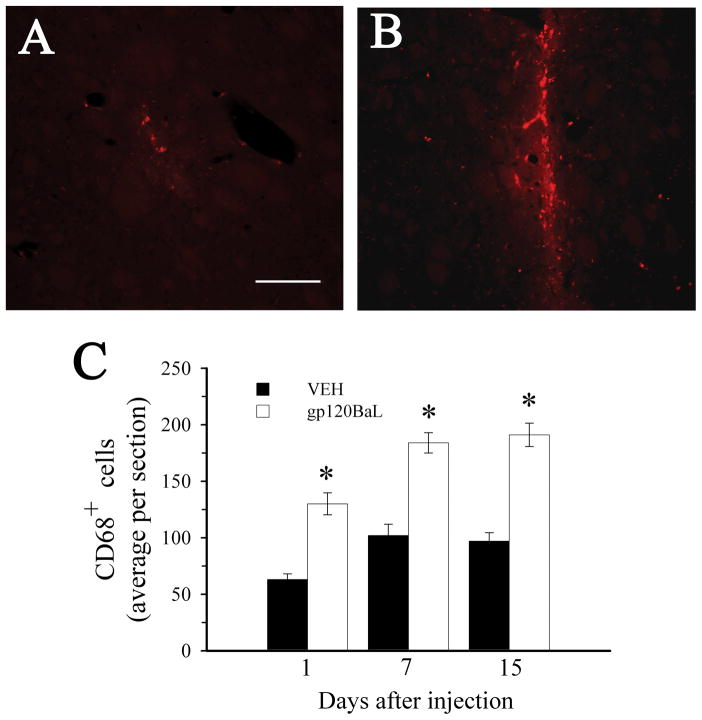
Gp120BaL promotes activation of microglia
We have previously shown that gp120IIIB causes neuronal apoptosis in vivo without promoting microglial activation (Bachis et al., 2006). To determine whether gp120BaL behaves in a similar fashion, sections from the rat striatum were stained with CD68, a marker for microglia. In control (VEH) rats we observed CD68+ cells only in an area immediately surrounding the injection site along the needle tract (Fig. 4A). In gp120-treated rats, the area containing CD68 positive cells in each section was wider than VEH (Fig. 4B). Time-course studies revealed that gp120BaL causes a time-dependent increase in CD68 immunoreactivity in several sections that did not include the injection site (Fig. 4C). Moreover, we did not find any difference in GFAP staining between VEH and gp120-treated rats (data not shown).
Figure 4. gp120BaL causes microglia activation at the site of injection.
Animals were microinjected with VEH or gp120BaL into the striatum and were sacrificed at various days after the injection. Activated microglia was detected using an antibody against CD68. A and B. Representative striatal sections of VEH and gp120-treated rats, respectively, showing CD68 immunoreactivity. Sections were taken 50 μm from the injection site. Bar= 100 μm. C. CD68 positive cells were counted in the striatum at the indicated times after gp120 injection. Data, expressed as the mean ± SEM of five animals per group (10 sections per animal), are the average of the number of CD68 positive cells in an area of 0.25 mm2 per section. *p< 0.001 vs VEH (F = 37.496 for 1 day; F = 34.535 for 7 days; F = 54.983 for 15 days).
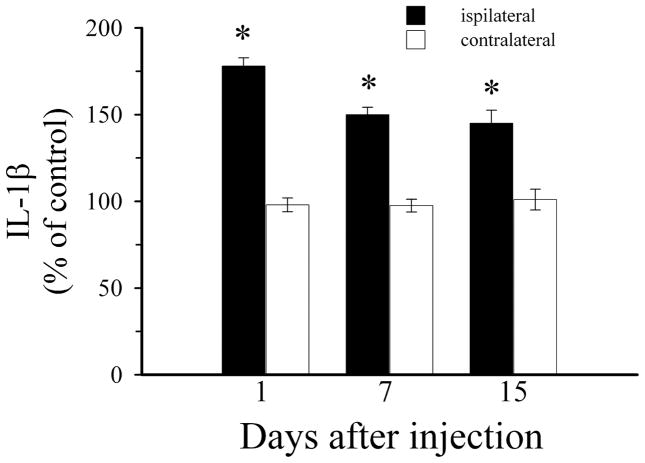
To test the hypothesis that gp120BaL promotes microglia activation and perhaps an inflammatory response we examined levels of a pro-inflammatory cytokine, IL-1β, in rat striatum 1, 7 and 15 days after injection. Gp120BaL elicited a significant increase in IL-1 β levels by 24 hr and up to 15 days (Fig. 5). Overall, these data suggest that inflammatory responses may help evoke and maintain gp120BaL-mediated neurotoxicity and that gp120BaL toxicity may also involve an indirect mechanism.
Figure 5. IL-1β is increased after gp120BaL.
Striatal levels of IL-1βwere measured by ELISA 1, 7 and 15 days after the striatal injection of VEH or gp120BaL. Data expressed as % of VEH-treated animals are the mean ± SEM of five animals per group. *p<0.001 vs VEH (F = 166.967 for 1 day; F = 80.438 for 7 days; F = 27.695 for 15 days). The amount of IL-1βin ipsilateral VEH was 41 ± 8.2 pg/100 μg proteins.
Gp120BaL increases BDNF
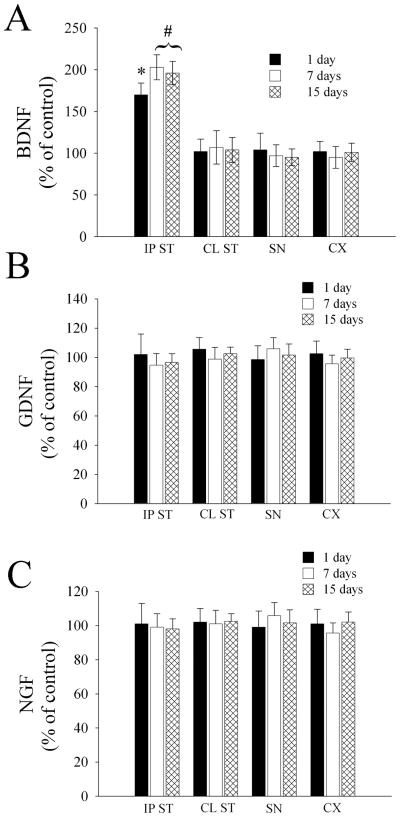
Gp120IIIB has been shown to reduce BDNF and GDNF levels in the striatum and SN, respectively (Nosheny et al., 2004; Nosheny et al., 2006), and to increase nerve growth factor (NGF) in the hippocampus (Bagetta et al., 1996). Alterations of neurotrophic support has been suggested to contribute to neuronal loss in chronic neurodegenerative diseases (Mocchetti & Brown, 2008). Thus, gp120BaL toxicity may involve a reduction of neurotrophic factors. To test this hypothesis, rats received gp120BaL or VEH control in the striatum, and were sacrificed 1, 7 and 15 days later. The levels of BDNF, GDNF and NGF were measured in the ipsilateral and contralateral striatum, SN and cortex by ELISA. No difference was observed in the levels of GDNF (Fig. 6B) and NGF (Fig. 6C) between VEH and gp120-injected animals in all tissues examined at all time points. Interestingly, gp120BaL increased the levels of BDNF in the ipsilateral striatum by one day, and up to 15 days (Fig. 6A). No changes in BDNF were observed in the contralateral striatum, SN or cortex (Fig. 6A).
Figure 6. gp120BaL increases BDNF.
The levels of BDNF, GDNF and NGF were determined in the indicated brain areas 1, 7 and 15 days after gp120BaL injection into the striatum (IP ST=ipsilateral striatum, CL ST=contralateral striatum). Data are the mean ± SEM of five animals per group. *p< 0.05 vs VEH (F = 20.361); #p< 0.001 (F = 45.475 for 7 days; F = 38.738 for 15 days). BDNF, GDNF and NGF levels in the IP striatum of VEH-treated rats were (in pg/100 μg proteins): 400 ± 21; 22.5 ± 4.2; 18.5 ± 2.0, respectively.
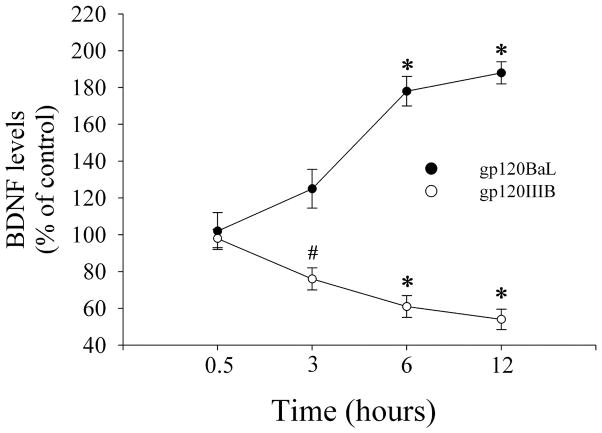
Striatal BDNF is mostly produced by cortical neurons (Altar et al., 1997). Thus, the apparent discrepancy between the increase in striatal BDNF and the lack of effect of gp120BaL on cortical BDNF may be attributed to the fact that gp120Bal affects only the release of BDNF. To examine this mechanism, we used cerebellar granule cells in culture which express both CXCR4 and CCR5 receptors (Bachis et al., 2003) and release BDNF upon appropriate stimuli (Marini et al., 1998). Neurons were exposed to gp120s (5 nM, each) or VEH and the medium was collected at different time points to determine released BDNF. We found that BDNF accumulation in the medium varies depending upon the strain of gp120 used. In fact, gp120BaL evoked a time-dependent increase in BDNF levels (Fig. 7). The opposite was obtained by gp120IIIB (Fig. 7). These data help support the notion that gp120BaL exhibits a different cellular mechanism than gp120IIIB.
Figure 7. BDNF release is differentially regulated by gp120s.
Cerebellar granule cells were exposed to gp120BaL or gp120IIIB (5 nM, each) and medium was collected at the indicated time points. BDNF was determined in 100 μl fraction of the medium by ELISA. BDNF levels in the medium of control cells were 75 ± 10 pg/ml. Data are the mean ± SEM of three separate samples each group. #p<0.05 vs VEH (F = 9.914); *p<0.001 vs VEH (gp120BaL: F = 28.743 for 6 hr; F = 42.202 for 12 hr. gp120IIIB: F = 87.432 for 6 hr; F = 174.988 for 12 hr).
Discussion
Several studies have shown that gp120 activation of CXCR4 is directly involved in HIV-associated neuronal damage (Hesselgesser et al., 1998; Meucci et al., 1998; Kaul & Lipton, 1999). Activation of CCR5 paradoxically can result in neuronal injury or protection depending upon the ligand used. Indeed, gp120 binding to CCR5 causes neuronal apoptosis whereas the natural CCR5 ligands RANTES (Kaul & Lipton, 1999; Bachis & Mocchetti, 2005) or MIP-1b (Meucci et al., 1998) confer neuroprotection against gp120. In this work, we have used gp120BaL, a M-tropic envelop protein, in an attempt to provide information as to whether this strain is as toxic as gp120IIIB. We have found similarities as well as differences. Gp120BaL, similar to gp20IIIB, induced caspase-3 dependent neuronal apoptosis in the rat striatum. However, the extent of neuronal damage after the M-strain differed from that of gp120IIIB both anatomically and quantitatively. Indeed, gp120BaL-mediated apoptosis was seen only in the striatum and was restricted to an area smaller than 250 μm2 per section. Gp120IIIB-mediated apoptosis, instead, has been seen in distal areas from the injection site, such as the SN, and covered an area greater than 250 μm2 per section (Nosheny et al., 2004; Bachis et al., 2006). Thus, it appears that gp120BaL, unlike gp120IIIB, does not cause retrograde neuronal cell death. Furthermore, the apoptotic effect of M-tropic gp120 appears also to involve activated microglia, suggesting that gp120BaL may use cellular mechanisms that are different from that utilized by gp120IIIB.
The role of CXCR4 receptor in the neurotoxic effect of T-tropic strain of gp120 has been established (Hesselgesser et al., 1998; Meucci et al., 1998; Zheng et al., 1999). Consequently, it is not surprising that an intrastriatal injection of gp120IIIB evokes apoptosis only in CXCR4 positive neurons (Bachis et al., 2006; Nosheny et al., 2006). We have previously shown that gp120BaL or HIV-1BaL reduces the survival of neuronal cultures in a CCR5 dependent manner (Bachis & Mocchetti, 2005; Bachis et al., 2009). Thus, we were expecting a similar result in vivo. Instead, the toxic effect of gp120BaL was quantitatively weaker than that of gp120IIIB previously observed (Bachis et al., 2006; Nosheny et al., 2007). Both CXCR4 and CCR5 are expressed in the adult rat striatum (Banisadr et al., 2002; Bachis et al., 2006; Ahmed et al., 2008; Trecki et al., 2009). However, previous data have shown that CXCR4 expression in various brain areas is wider than that of CCR5 (van der Meer et al., 2000). This difference could account for the widespread neurotoxicity of gp120IIIB but, it is unlikely that the abundance of CCR5 in the striatum can solely account for gp120BaL weaker effect. On the other hand, CCR5 is also expressed by astrocytes and microglia (Rottman et al., 1997; van der Meer et al., 2000). The latter, when activated, can cause an inflammatory response that ultimately leads to neuronal injury. Such mechanism could account for gp120BaL neurotoxicity. We have observed that gp120BaL, unlike gp120IIIB (Bachis et al., 2006), promoted proliferation of microglial cells. Activated microglia is known to induce the release of pro-inflammatory molecules such as cytokines (Floyd, 1999; Raivich et al., 1999), and nitric oxide (Perry et al., 1993; Ogura et al., 1994), potent stimuli that trigger neuronal cell death. We have seen an increase in IL-1β in gp120BaL-treated rats. Levels of IL-1 β are elevated in the cerebrospinal fluid of AIDS patients (Gallo et al., 1989) and in postmortem brains (Tyor et al., 1992). IL-1 β and other cytokines can act on a variety of neuronal populations independently from the presence of chemokine receptors. Collectively, these results suggest that gp120BaL may activate CCR5 on non-neuronal cells that may have been recruited to inflammatory sites, which, in turn, induce the synthesis of pro-inflammatory cytokines. Overall, while we cannot underscore enough the role of CCR5 in gp120BaL-mediated neuronal apoptosis, our data point at a multi-factorial effect of gp120BaL which may include the ability of this strain to increase microglia activation/proliferation.
Intrastriatal gp120IIIB has been shown to cause a decrease in the levels of BDNF in the striatum (Nosheny et al., 2004) and GDNF in the SN (Nosheny et al., 2006). Other studies have shown that gp120IIIB increases NGF in the hippocampus (Bagetta et al., 1996). While NGF has little or no trophic activity on central dopamine neurons (Hagg, 1998), BDNF and GDNF are two of the most potent neurotrophic factors for the survival of dopaminergic neurons of the nigro-striatal system (Hyman et al., 1991; Altar et al., 1992; Beck et al., 1995; Choi-Lundberg et al., 1997). Indeed, dysregulation of BDNF and GDNF synthesis has been proposed to promote neurodegenerative diseases such as Parkinson’s disease (Nagatsu et al., 2000). Moreover, BDNF heterozygous mice are more sensitive to the neurotoxic action of gp120IIIB (Nosheny et al., 2004). A number of HIV infected individuals exhibits Parkinsonian-like motor abnormalities (Berger & Arendt, 2000; Koutsilieri et al., 2002; Nath & Berger, 2004) which are consistent with a nigro-striatal dysfunction. Thus, the dopaminergic loss observed in HAD may be caused by a lack of neurotrophic support. We have determined the overall neurotrophic factor environment after gp120BaL in an attempt to characterize its similarities with gp120IIIB. Our data show that gp120BaL increases BDNF and does not change GDNF levels in vivo whereas gp120IIIB decreases both (Nosheny et al., 2004; Nosheny et al., 2006). This diverse profile was also observed in vitro. In fact, gp120BaL increased whereas gp120IIIB decreased BDNF release. This and other evidence suggest that gp120BaL and gp120IIIB exhibit a different neuropathological profile.
An attractive albeit speculative suggestion that emerges from our studies is that gp120BaL may promote, in addition to a neurotoxic insult, a neuroprotective response via the increase in BDNF. The mechanism(s) whereby BDNF participates in a neuroprotective response evoked by gp120BaL is still a matter of speculation. BDNF is a strong neuroprotective agent against various toxins that are believed to be released by gp120. These include nitric oxide (Adamson et al., 1996; Bagetta et al., 1996; Kume et al., 1997) and glutamate (Lindholm et al., 1993; Marini et al., 1998; Bezzi et al., 2001). In addition, HIV/gp120-mediated apoptosis has been linked to the expression and trafficking of N-methyl-D-aspartate receptors (Eugenin et al., 2003; Viviani et al., 2006). BDNF has also been shown to down-regulate this receptor (Brandoli et al., 1998). Thus, BDNF may modulate negatively toxic responses that are activated by gp120s. Because these mechanisms may work synergistically, modulating one may lead to reduced toxicity. More studies are needed to characterize which of these mechanisms are most likely crucial for the limited (when compared to gp120IIIB) toxic effect of gp120BaL.
Collectively, our data suggest a speculative hypothesis that M-tropic gp120 has a weaker toxic property than T-tropic. M-tropic and CCR5-preferring HIV has been shown to be present in the early phase of infection whereas CXCR4-preferring strains occur in the late stage when a subset of individuals develops HAD. Moreover, dual-tropic viruses can use both co-receptors and appear to be specialized in replicating within the CNS (Gray et al., 2009). Our results might help explain the suggestion that M-tropic strains of HIV that infect the CNS are not sufficient to cause dementia or encephalitis (Power et al., 1994). Additional experiments with other M-tropic gp120s and/or M-tropic HIV are needed to fully support this hypothesis.
Acknowledgments
Special thanks to NIH AIDS Research Reference Reagent Program for gp120BaL and Diane M Leader for critical reading of the manuscript. This work was supported by NIH grants NS040670, NS059323 and DA026174.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- CX
somatosensory cortex
- ELISA
enzyme-linked immunosorbent assay
- GDNF
glial-cell derived neurotrophic factor
- GFAP
glia fibrillary acidic protein
- HAD
HIV-associated dementia
- HIV
human immunodeficiency virus type 1
- IL-1β
interleukin-1β
- NGF
nerve growth factor
- SN
substantia nigra
- TH
tyrosine hydroxylase
- TUNEL
in situ terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling
- VEH
vehicle control
References
- Adamson DC, Wildemann B, Sasaki M, Glass JD, McArthur JC, Christov VI, Dawson TM, Dawson VL. Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science. 1996;274:1917–1921. doi: 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]
- Adle-Biassette H, Levy Y, Colombel M, Poron F, Natchev S, Keohane C, Gray F. Neuronal apoptosis in HIV infection in adults. Neuropathol Appl Neurobiol. 1995;21:218–227. doi: 10.1111/j.1365-2990.1995.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Ahmed F, Tessarollo L, Thiele C, Mocchetti I. Brain-derived neurotrophic factor modulates expression of chemokine receptors in the brain. Brain Res. 2008;1227:1–11. doi: 10.1016/j.brainres.2008.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright AV, Shieh JT, Itoh T, Lee B, Pleasure D, O’Connor MJ, Doms RW, Gonzalez-Scarano F. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol. 1999;73:205–213. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Boylan CB, Jackson C, Hershenson S, Miller J, Wiegand SJ, Lindsay RM, Hyman C. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci U S A. 1992;89:11347–11351. doi: 10.1073/pnas.89.23.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I. Axonal transport of Human Immunodeficiency Virus Type 1 envelope glycoprotein 120 is found in association with neuronal apoptosis. J Neurosci. 2006;26:6771–6780. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Biggio F, Major EO, Mocchetti I. M- and T-tropic HIVs promote apoptosis in rat neurons. J Neuroimmune Pharmacol. 2009;4:150–160. doi: 10.1007/s11481-008-9141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23:5715–5722. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Mocchetti I. Brain-derived neurotrophic factor is neuroprotective against human immunodeficiency virus-1 envelope proteins. Ann NY Acad Sci. 2005;1053:247–257. doi: 10.1196/annals.1344.022. [DOI] [PubMed] [Google Scholar]
- Bagetta G, Corasaniti MT, Aloe L, Berliocchi L, Costa N, Finazzi-Agro A, Nistico G. Intracerebral injection of human immunodeficiency virus type 1 coat protein gp120 differentially affects the expression of nerve growth factor and nitric oxide synthase in the hippocampus of rat. Proc Natl Acad Sci U S A. 1996;93:928–933. doi: 10.1073/pnas.93.2.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Parsadaniantz SM. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2002;16:1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, Rosenthal A, Hefti F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;273:289–290. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Berger JR, Arendt G. HIV dementia: The role of the basal ganglia and dopaminergic systems. J Psychopharmacol. 2000;14:214–221. doi: 10.1177/026988110001400304. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Bissel SJ, Wiley CA. Human immunodeficiency virus infection of the brain: pitfalls in evaluating infected/affected cell populations. Brain Pathol. 2004;14:97–108. doi: 10.1111/j.1750-3639.2004.tb00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandoli C, Sanna A, De Bernardi MA, Follesa P, Brooker G, Mocchetti I. Brain-derived neurotrophic factor and basic fibroblast growth factor downregulate NMDA receptor function in cerebellar granule cells. J Neurosci. 1998;18:7953–7961. doi: 10.1523/JNEUROSCI.18-19-07953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- Gallo P, Frei K, Rordorf C, Lazdins J, Tavolato B, Fontana A. Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system: an evaluation of cytokines in cerebrospinal fluid. J Neuroimmunol. 1989;23:109–116. doi: 10.1016/0165-5728(89)90029-5. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, Epstein LG. HIV-1 encephalopathy in children. Curr Opin Pediatr. 1995;7:655–662. doi: 10.1097/00008480-199512000-00005. [DOI] [PubMed] [Google Scholar]
- Glass JD, Wesselingh SL, Selnes OA, McArthur JC. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology. 1993;43:2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- Gray L, Roche M, Churchill MJ, Sterjovski J, Ellett A, Poumbourios P, Sherieff S, Wang B, Saksena N, Purcell DF, Wesselingh S, Cunningham AL, Brew BJ, Gabuzda D, Gorry PR. Tissue-specific sequence alterations in the human immunodeficiency virus type 1 envelope favoring CCR5 usage contribute to persistence of dual-tropic virus in the brain. J Virol. 2009;83:5430–5441. doi: 10.1128/JVI.02648-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivel JC, Margolis LB. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat Med. 1999;5:344–346. doi: 10.1038/6565. [DOI] [PubMed] [Google Scholar]
- Hagg T. Neurotrophins prevent death and differentially affect tyrosine hydroxylase of adult rat nigrostriatal neurons in vivo. Exp Neurol. 1998;149:183–192. doi: 10.1006/exnr.1997.6684. [DOI] [PubMed] [Google Scholar]
- Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay CR, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Brew BJ, Martin A, Price RW, Salazar AM, Sidtis JJ, Yergey JA, Mouradian MM, Sadler AE, Keilp J, et al. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991;29:202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA. HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ. 2007;14:296–305. doi: 10.1038/sj.cdd.4402006. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Sopper S, Scheller C, ter Meulen V, Riederer P. Parkinsonism in HIV dementia. J Neural Transm. 2002;109:767–775. doi: 10.1007/s007020200063. [DOI] [PubMed] [Google Scholar]
- Kume T, Kouchiyama H, Kaneko S, Maeda T, Akaike A, Shimohama S, Kihara T, Kimura J, Wada K, Koizumi S. BDNF prevents NO mediated glutamate cytotoxicity in cultured cortical neurons. Brain Res. 1997;756:200–204. doi: 10.1016/s0006-8993(97)00195-9. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Lanier ER, McCutchan JA. Cerebrospinal fluid beta chemokine concentrations in neurocognitively impaired individuals infected with human immunodeficiency virus type 1. J Infect Dis. 1999;180:310–319. doi: 10.1086/314866. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Dechant G, Heisenberg CP, Thoenen H. Brain-derived neurotrophic factor is a survival factor for cultured rat cerebellar granule neurons and protects them against glutamate-induced neurotoxicity. Eur J Neurosci. 1993;5:1455–1464. doi: 10.1111/j.1460-9568.1993.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Marini AM, Rabin SJ, Lipsky RH, Mocchetti I. Activity-dependent release of brain-derived neurotrophic factor underlies the neuroprotective effect of N-methyl-D-aspartate. J Biol Chem. 1998;273:29394–29399. doi: 10.1074/jbc.273.45.29394. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Mucke L. Pathogenesis of HIV-1 associated neurodegeneration. Crit Rev Neurobiol. 1996;10:57–67. doi: 10.1615/critrevneurobiol.v10.i1.30. [DOI] [PubMed] [Google Scholar]
- Mastroianni CM, Paoletti F, Valenti C, Vullo V, Jirillo E, Delia S. Tumour necrosis factor (TNF-alpha) and neurological disorders in HIV infection. J Neurol Neurosurg Psychiatry. 1992;55:219–221. doi: 10.1136/jnnp.55.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDyer JF, Dybul M, Goletz TJ, Kinter AL, Thomas EK, Berzofsky JA, Fauci AS, Seder RA. Differential Effects of CD40 Ligand/Trimer Stimulation on the Ability of Dendritic Cells to Replicate and Transmit HIV Infection: Evidence for CC-Chemokine-Dependent and -Independent Mechanisms. J Immunol. 1999;162:3711–3717. [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I, Brown M. Targeting neurotrophin receptors in the central nervous system. CNS & neurological disorders drug targets. 2008;7:71–82. doi: 10.2174/187152708783885138. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm Suppl. 2000:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- Nath A, Berger J. HIV Dementia. Curr Treat Options Neurol. 2004;6:139–151. doi: 10.1007/s11940-004-0023-6. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Amhed F, Yakovlev AG, Meyer EM, Ren K, Tessarollo L, Mocchetti I. Brain-derived neurotrophic factor prevents the nigrostriatal degeneration induced by human immunodeficiency virus-1 glycoprotein 120 in vivo. Eur J Neurosci. 2007;25:2275–2284. doi: 10.1111/j.1460-9568.2007.05506.x. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Acquas E, Mocchetti I. Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci. 2004;20:2857–2864. doi: 10.1111/j.1460-9568.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Aden SA, De Bernardi MA, Mocchetti I. Intrastriatal administration of human immunodeficiency virus-1 glycoprotein 120 reduces glial cell-line derived neurotrophic factor levels and causes apoptosis in the substantia nigra. J Neurobiol. 2006;66:1311–1321. doi: 10.1002/neu.20288. [DOI] [PubMed] [Google Scholar]
- Ogura K, Ogawa M, Yoshida M. Effects of ageing on microglia in the normal rat brain: immunohistochemical observations. Neuroreport. 1994;5:1224–1226. doi: 10.1097/00001756-199406020-00016. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Paxton WA, Neumann AU, Kang S, Deutch L, Brown RC, Koup RA, Wolinsky SM. RANTES Production from CD4+ Lymphocytes Correlates with Host Genotype and Rates of Human Immunodeficiency Virus Type 1 Disease Progression. J Infect Dis. 2001;183:1678–1681. doi: 10.1086/320701. [DOI] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Buttini M, Limoges J, Bock P, Gendelman HE. An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis. J Neurovirol. 1997;3:401–416. doi: 10.3109/13550289709031186. [DOI] [PubMed] [Google Scholar]
- Petito CK, Roberts B, Cantando JD, Rabinstein A, Duncan R. Hippocampal injury and alterations in neuronal chemokine co-receptor expression in patients with AIDS. J Neuropathol Exp Neurol. 2001;60:377–385. doi: 10.1093/jnen/60.4.377. [DOI] [PubMed] [Google Scholar]
- Pierson TC, Doms RW, Pohlmann S. Prospects of HIV-1 entry inhibitors as novel therapeutics. Rev Med Virol. 2004;14:255–270. doi: 10.1002/rmv.435. [DOI] [PubMed] [Google Scholar]
- Power C, McArthur JC, Johnson RT, Griffin DE, Glass JD, Perryman S, Chesebro B. Demented and nondemented patients with AIDS differ in brain-derived human immunodeficiency virus type 1 envelope sequences. J Virol. 1994;68:4643–4649. doi: 10.1128/jvi.68.7.4643-4649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, McArthur JC, Nath A, Wehrly K, Mayne M, Nishio J, Langelier T, Johnson RT, Chesebro B. Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients. J Virol. 1998;72:9045–9053. doi: 10.1128/jvi.72.11.9045-9053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RW. Neurological complications of HIV infection. Lancet. 1996;348:445–452. doi: 10.1016/S0140-6736(95)11035-6. [DOI] [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Raivich G, Jones LL, Werner A, Bluthmann H, Doetschmann T, Kreutzberg GW. Molecular signals for glial activation: pro- and anti-inflammatory cytokines in the injured brain. Acta Neurochir Suppl. 1999;73:21–30. doi: 10.1007/978-3-7091-6391-7_4. [DOI] [PubMed] [Google Scholar]
- Rottman JB, Ganley KP, Williams K, Wu L, Mackay CR, Ringler DJ. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am J Pathol. 1997;151:1341–1351. [PMC free article] [PubMed] [Google Scholar]
- Saha K, Bentsman G, Chess L, Volsky DJ. Endogenous production of beta-chemokines by CD4+, but not CD8+, T-cell clones correlates with the clinical state of human immunodeficiency virus type 1 (HIV-1)-infected individuals and may be responsible for blocking infection with non-syncytium-inducing HIV-1 in vitro. J Virol. 1998;72:876–881. doi: 10.1128/jvi.72.1.876-881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzani S, Introna M, Bernasconi S, Polentarutti N, Cinque P, Poli G, Sica A, Mantovani A. MCP-1 and CCR2 in HIV infection: regulation of agonist and receptor expression. J Leukoc Biol. 1997;62:30–33. doi: 10.1002/jlb.62.1.30. [DOI] [PubMed] [Google Scholar]
- Trecki J, Brailoiu GC, Unterwald EM. Localization of CXCR4 in the forebrain of the adult rat. Brain Res. 2009 doi: 10.1016/j.brainres.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- van der Meer P, Ulrich AM, Gonzalez-Scarano F, Lavi E. Immunohistochemical analysis of CCR2, CCR3, CCR5, and CXCR4 in the human brain: potential mechanisms for HIV dementia. Exp Mol Pathol. 2000;69:192–201. doi: 10.1006/exmp.2000.2336. [DOI] [PubMed] [Google Scholar]
- Viviani B, Gardoni F, Bartesaghi S, Corsini E, Facchi A, Galli CL, Di Luca M, Marinovich M. Interleukin-1 beta released by gp120 drives neural death through tyrosine phosphorylation and trafficking of NMDA receptors. J Biol Chem. 2006;281:30212–30222. doi: 10.1074/jbc.M602156200. [DOI] [PubMed] [Google Scholar]
- Westmoreland SV, Alvarez X, deBakker C, Aye P, Wilson ML, Williams KC, Lackner AA. Developmental expression patterns of CCR5 and CXCR4 in the rhesus macaque brain. J Neuroimmunol. 2002;122:146–158. doi: 10.1016/s0165-5728(01)00457-x. [DOI] [PubMed] [Google Scholar]
- Zanussi S, D’Andrea M, Simonelli C, Tirelli U, De Paoli P. Serum levels of RANTES and MIP-1 alpha in HIV-positive long-term survivors and progressor patients. AIDS. 1996;10:1431–1432. doi: 10.1097/00002030-199610000-00018. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]