Abstract
BACKGROUND
Childhood absence epilepsy, the most common pediatric epilepsy syndrome, is usually treated with ethosuximide, valproic acid, or lamotrigine. The most efficacious and tolerable initial empirical treatment has not been defined.
METHODS
In a double-blind, randomized, controlled clinical trial, we compared the efficacy, tolerability, and neuropsychological effects of ethosuximide, valproic acid, and lamotrigine in children with newly diagnosed childhood absence epilepsy. Drug doses were incrementally increased until the child was free of seizures, the maximal allowable or highest tolerable dose was reached, or a criterion indicating treatment failure was met. The primary outcome was freedom from treatment failure after 16 weeks of therapy; the secondary outcome was attentional dysfunction. Differential drug effects were determined by means of pairwise comparisons.
RESULTS
The 453 children who were randomly assigned to treatment with ethosuximide (156), lamotrigine (149), or valproic acid (148) were similar with respect to their demographic characteristics. After 16 weeks of therapy, the freedom-from-failure rates for ethosuximide and valproic acid were similar (53% and 58%, respectively; odds ratio with valproic acid vs. ethosuximide, 1.26; 95% confidence interval [CI], 0.80 to 1.98; P = 0.35) and were higher than the rate for lamotrigine (29%; odds ratio with ethosuximide vs. lamotrigine, 2.66; 95% CI, 1.65 to 4.28; odds ratio with valproic acid vs. lamotrigine, 3.34; 95% CI, 2.06 to 5.42; P<0.001 for both comparisons). There were no significant differences among the three drugs with regard to discontinuation because of adverse events. Attentional dysfunction was more common with valproic acid than with ethosuximide (in 49% of the children vs. 33%; odds ratio, 1.95; 95% CI, 1.12 to 3.41; P = 0.03).
CONCLUSIONS
Ethosuximide and valproic acid are more effective than lamotrigine in the treatment of childhood absence epilepsy. Ethosuximide is associated with fewer adverse attentional effects. (ClinicalTrials.gov number, NCT00088452.)
Childhood absence epilepsy accounts for 10 to 17% of all cases of childhood-onset epilepsy, making it the most common form of pediatric epilepsy.1,2 The syndrome is characterized by daily frequent but brief staring spells, typically beginning at 4 to 8 years of age, in an otherwise apparently healthy child.3 The classic electroencephalogram (EEG) shows generalized spike-wave bursts (of 3 Hz) with normal background activity.3,4 Often misperceived as a benign form of epilepsy, childhood absence epilepsy is associated with variable remission rates; affected children have cognitive deficits and long-term psychosocial difficulties.5-7
Three medications are commonly used as initial monotherapy for this condition — ethosuximide, valproic acid, and lamotrigine8 — but definitive evidence of their relative efficacy is lacking.9 These medications have different side-effect and drug-interaction profiles.10,11 We performed a double-blind, randomized trial to assess the efficacy, tolerability, and neuropsychological effect of these three medications to determine the optimal initial empirical monotherapy for children with childhood absence epilepsy.
METHODS
RECRUITMENT
This trial was conducted at 32 sites across the United States. Children between 2.5 and 13 years of age were eligible to participate if they met the following criteria: had childhood absence epilepsy of new onset that was clinically diagnosed according to the International League Against Epilepsy classification of epilepsy syndromes (including frequent clinical absence seizures and reported normal development)3; had bilateral synchronous, symmetric spike waves (2.7 to 5 Hz) on a normal background with at least one electrographically recorded seizure lasting 3 seconds or more on a 1-hour, awake video EEG; weighed 10 kg or more; had a body-mass index below the 99th percentile; and had a normal complete blood count and normal levels of serum alanine aminotransferase, serum aspartate aminotransferase, and bilirubin. The girls had to be premenarchal.
Children were ineligible if they met any of the following criteria: had received antiseizure medication for more than 7 days before randomization, had a history of nonfebrile seizures other than absence seizures (e.g., afebrile generalized tonic–clonic or myoclonic seizures), had a history consistent with juvenile absence epilepsy or juvenile myoclonic epilepsy (e.g., generalized tonic–clonic or myoclonic seizures),3 had a history of a severe dermatologic reaction to any medication, or had a history of major psychiatric disease, autistic-spectrum disorder, or any clinically significant medical condition. In contrast to childhood absence epilepsy, juvenile absence epilepsy occurs in older children and is characterized by much less frequent absence seizures (often not triggered by hyperventilation); more common generalized tonic–clonic seizures; and often higher-frequency (>3 Hz), generalized spike-wave discharges on the EEG.
The study was approved by the institutional review boards of each participating site, the coordinating center, and the data and safety monitoring board appointed by the National Institutes of Health. Written informed consent was obtained from parents or guardians, and assent was obtained from the subjects when applicable.
PROTOCOL
Eligible subjects were randomly assigned to receive one of the three study medications in a 1:1:1 ratio. Treatment assignments were performed centrally according to a computer-generated random schedule in permuted blocks of three within age strata (<6 years and ≥6 years) and within study site. Baseline neuropsychological testing was performed either before or within 7 days after the start of the study medication. Tests included an age-appropriate Conners’ Continuous Performance Test (CPT-II for children ≥6 years of age, and K-CPT for children 4 to <6 years of age), which assesses attention12; standardized tests of verbal and nonverbal intelligence,13-15 vocabulary,16 memory,17-19 learning skills,17 visuomotor integration,20 executive function,21,22 and academic achievement23,24; and questionnaires on behavior25,26 and quality of life.27
Ethosuximide (Zarontin) (250-mg capsules or 250 mg per 5 ml of syrup), valproic acid (Depakote) (25-mg capsules or 125-mg dose of sprinkles), and lamotrigine (Lamictal) (5-mg and 25-mg chewable tablets or 25-mg tablets) were provided by Pfizer, Abbott Laboratories, and GlaxoSmith-Kline, respectively. These companies had no role in the design of the study, data accrual, data analysis, or manuscript preparation. The authors designed the study, analyzed the data, wrote the manuscript, and elected to submit the article for publication. Study data were gathered by the Childhood Absence Epilepsy Study Group. Blinded study medications were prepared at the central pharmacy and were shipped in prepackaged kits for dispensing; doses were increased every 1 to 2 weeks over a 16-week period until either freedom from seizures was attained or side effects limited the dose given (Table 1). The highest allowable daily doses were 60 mg per kilogram of body weight for ethosuximide, 60 mg per kilogram for valproic acid, and 12 mg per kilogram for lamotrigine (to maximum respective doses of 2000, 3000, and 600 mg per day). A single downward dose modification was allowed in the event of prespecified dose-limiting toxicity. Blinding was maintained with the use of either a double-dummy approach (for solid and liquid formulations) or overencapsulation.
Table 1.
Ethosuximide, Lamotrigine, and Valproic Acid Dosing Schedule during the Double-Blind Study.*
| Study Week | Ethosuximide | Lamotrigine | Valproic Acid |
|---|---|---|---|
| mg/kg/day† | |||
| 1 | 10 | 0.3 | 10 |
| 2 | 10 | 0.3 | 10 |
| 3 | 15 | 0.6 | 15 |
| 4 | 15 | 0.6 | 15 |
| 5 | 20 | 1.2 | 20 |
| 6 | 20 | 1.8 | 20 |
| 7 | 30 | 2.4 | 30 |
| 8 | 30 | 3.0 | 30 |
| 9 | 40 | 4.5 | 40 |
| 10 | 40 | 4.5 | 40 |
| 11 | 50 | 7.0 | 50 |
| 12 | 50 | 7.0 | 50 |
| 13 | 60 | 9.0 | 60 |
| 14 | 60 | 9.0 | 60 |
| 15 | 60 | 12.0 | 60 |
| 16 | 60 | 12.0 | 60 |
Follow-up visits were scheduled at weeks 4, 8, 12, and 16. For subjects who were still having seizures but were not receiving the highest allowable or maximally tolerated dose at the fourth visit, a single additional dose escalation was allowed, with seizure status reevaluated at a fifth visit, 4 weeks later (at week 20).
The highest allowable daily doses were 2000 mg per day for ethosuximide, 600 mg per day for lamotrigine, and 3000 mg per day for valproic acid.
Study visits occurred every 4 weeks for the first 16 weeks. If parents reported clinical seizures, upward adjustment of the dose of study drug was continued; if no seizures were reported, up to two 5-minute trials of bedside hyperventilation were performed. If bedside hyperventilation induced seizures, upward adjustment of the dose was continued; otherwise, a 1-hour video EEG was obtained. If seizures were noted on the EEG (spike-wave bursts lasting ≥3 seconds), upward adjustment was continued; if no seizures were detected, dose adjustments were discontinued, and the subject continued to receive the current dose.
At week 16 (the fourth visit), seizure status was determined by means of clinical report, bedside hyperventilation testing, and a 1-hour video EEG. At that visit, the presence of clinical or electrographic seizures in subjects who were receiving the highest allowable or maximally tolerated dose was considered a treatment failure; for subjects who were not receiving the highest allowable or maximally tolerated dose but were still having seizures, a single additional escalation of the dose was allowed, and seizure status was reevaluated at a fifth visit 4 weeks later (at 20 weeks). Data for the study’s primary outcome (freedom-from-failure rate) were based on findings at the week 16 visit unless a fifth visit took place at 20 weeks, in which case outcome data were designated as week 16 or week 20 data.
The Conners’ Continuous Performance Test was the only baseline neuropsychological test repeated before or at the time of the week 16 or week 20 visit because of the potential for substantial test–retest or practice effects with the other neuropsychological tests. Pretreatment serum samples for pharmacokinetic analyses were obtained at week 16 or week 20. Subjects without seizures at their last follow-up visit continued to receive medication in double-blind fashion for up to 2 more years.
The criteria for treatment failure included persistence of absence seizures at week 16 or week 20, a generalized tonic–clonic seizure at any time, excessive drug-related systemic toxicity (i.e., platelet count <50,000 per cubic millimeter, absolute neutrophil count <500 per cubic millimeter, alanine aminotransferase or aspartate aminotransferase level ≥10 times the upper limit of the normal range, total bilirubin level ≥5 times the upper limit of the normal range, a moderately severe rash (possibly drug-related), pancreatitis, or increase in the body-mass index (the weight in kilograms divided by the square of the height in meters) of at least 3.0 from baseline, dose-limiting toxicity after a single downward dose modification, and withdrawal initiated by the parent or physician. Treatment failure due to drug toxicity or a generalized tonic–clonic seizure could occur at any time; that due to persistence of absence seizures could occur only at or after the visit at 16 weeks. Subjects who met one of these criteria were invited to enter the open-label phase of the study. To maintain the original blinding conditions, such subjects were randomly assigned to one of the other two antiepileptic study drugs; physicians and families were told which study drug was assigned in the second phase of treatment. The coordinating center monitored the conduct of the trial on an ongoing basis.
STATISTICAL ANALYSIS
The primary outcome of the study was freedom from treatment failure at week 16 or week 20; in children 4 years of age or older, the secondary outcome was evidence of attentional dysfunction — that is, a Confidence Index of 0.60 or higher on the Conners’ Continuous Performance Test12 at the visit at 16 or 20 weeks or at an earlier visit when treatment was discontinued (as long as the discontinuation occurred 1 month or more after the baseline visit and was not due to intolerable adverse events). (A Confidence Index of 0.60 corresponds to a 60% probability that the child has clinical attention deficit disorder.)
Calculations of sample size were based on the ability to detect a 20% difference in freedom-from-failure rates (three pairwise comparisons) at 16 weeks with 80% power at a two-sided P value of 0.017 and one interim analysis, which was planned to be performed when 50% of subjects reached the primary outcome. The interim analysis was for both efficacy and futility, with the use of an O’Brien–Fleming boundary for stopping the study and adjustment with the Lan–DeMets spending function.28 The sample size of 398 was increased to 446 subjects to account for the two stratification factors and a 5% dropout rate; this sample size allowed the detection of a difference of 0.5 SD in the Confidence Index on the Conners’ Continuous Performance Test with a power exceeding 80%.
Baseline characteristics and safety variables for the three treatments were compared by means of either an exact chi-square test or a two-way analysis of variance (with treatment as one factor and age stratum as the other factor), depending on whether the characteristic being analyzed was discrete or continuous. An overall P value of 0.05 was considered to indicate statistical significance, without correction for multiple comparisons.
Outcome analyses were based on a modified intention-to-treat approach, and all analyses were prespecified. All subjects who received at least one dose of a study drug were included in the safety analyses, whereas efficacy analyses excluded five children who were deemed ineligible on central review. The primary and secondary outcomes were analyzed by means of Fisher’s exact test for the pairwise comparisons between treatments; a P value of 0.017 was considered to indicate statistical significance (accounting for a Bonferroni correction). An overall exact chi-square test was also performed, as well as an odds-ratio calculation with a 95% confidence interval. Kaplan–Meier curves were constructed to show the time to treatment failure over the 20-week study period. A log-rank test of the three pairs of study drugs was performed at week 16 or week 20. A post hoc Tukey–Kramer analysis of attentional function at the final visit incorporated baseline attentional differences. Post hoc comparisons of drug concentrations between treatment failures and successes within treatment groups were performed using a t-test. All analyses were carried out using SAS software, version 9.1 (SAS Institute), and StatXact software, version 8.0 (Cytel Software). An independent data and safety monitoring board appointed by the National Institutes of Health monitored the trial.
RESULTS
CHARACTERISTICS OF THE SUBJECTS
From July 2004 through October 2007, a total of 453 children were enrolled and were randomly assigned to one of three treatment groups (see Fig. 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). At enrollment, the median age of the cohort was 7 years 5 months; 17 children (4%) were under 4 years of age, 242 (53%) were 4 to less than 8 years of age, 184 (41%) were 8 to less than 12 years of age, and 10 (2%) were 12 to 13 years of age.
After randomization, two subjects never received a study drug. Five subjects were found to be ineligible on central review: three did not meet EEG criteria, one had an abnormal neutrophil count, and one had a BMI greater than the 99th percentile. Thus, 451 subjects were included in the safety analyses and 446 in the efficacy analyses.
There were no significant differences among the treatment groups within each age stratum or with respect to overall demographic characteristics (Table 2). Baseline testing of the cohort showed that cognition was within the normal range; however, the Confidence Index on the Conners’ Continuous Performance Test was elevated (≥0.60) in 141 of 399 subjects (35%) who could be evaluated. Both in the overall cohort and within each treatment group, there was no significant difference in the baseline Continuous Performance Test Confidence Index between subjects tested before the study drugs were started and those tested during the week after randomization.
Table 2.
Baseline Characteristics of the Subjects.*
| Characteristic | Ethosuximide (N = 155) | Lamotrigine (N = 149) | Valproic Acid (N = 147) | P Value |
|---|---|---|---|---|
| Age ≥6 yr — no. (%) | 116 (75) | 110 (74) | 113 (77) | 0.81 |
| Male sex — no. (%) | 65 (42) | 57 (38) | 71 (48) | 0.21 |
| Hispanic ethnicity — no. (%) | 36 (23) | 32 (21) | 32 (22) | 0.92 |
| Race — no. (%) | ||||
| White | 110 (71) | 117 (79) | 107 (73) | 0.47 |
| Black | 32 (21) | 26 (17) | 29 (20) | |
| Other or unknown | 13 (8) | 6 (4) | 11 (7) | |
| BMI >90th percentile — no. (%) | 43 (28) | 44 (30) | 33 (22) | 0.38 |
| Attentional difficulties (CPT Confidence Index ≥0.60) — no./total no. (%) | 48/140 (34) | 39/131 (30) | 54/128 (42) | 0.11 |
| CPT testing after randomization and before first study dose — no./total no. (%) | 94/142 (66) | 85/132 (64) | 78/132 (59) | 0.45 |
| WISC-IV full-scale IQ, composite score | 93.7±16.1 | 95.6±14.5 | 93.1±14.3 | 0.47 |
| WPPSI-III full-scale IQ, composite score | 99.1±16.6 | 92.0±14.5 | 100±14.8 | 0.07 |
Plus–minus values are means ±SD. BMI denotes body-mass index, CPT Conners’ Continuous Performance Test, WISC-IV Wechsler Intelligence Scale for Children, fourth edition, and WPPSI-III Wechsler Preschool and Primary Scale of Intelligence, third edition. The scores for both WISC-IV and WPPSI-III are scaled with a mean of 100 and a standard deviation of 15; full-scale IQ composite scores between 80 and 89 are considered low average, between 90 and 109 average, and between 110 and 119 high average. For the CPT, the Confidence Index provides a confidence level that suggests closeness of the match to a clinical or nonclinical profile of attention deficit; an index of 0.60 corresponds to a 60% probability that the child has clinically significant attention deficit disorder. Information on race was self-reported.
FREEDOM FROM TREATMENT FAILURE
Overall, 209 of the 446 children (47%) were free from treatment failure at the week 16 or week 20 visit (Table 3). Those treated with either ethosuximide or valproic acid had higher freedom-from-failure rates (53% and 58%, respectively) than those given lamotrigine (29%; odds ratio with ethosuximide vs. lamotrigine, 2.66; 95% confidence interval [CI], 1.65 to 4.28; odds ratio with valproic acid vs. lamotrigine, 3.34; 95% CI, 2.06 to 5.42; P<0.001 for both comparisons). Similar results were found on analysis of freedom-from-failure rates within each age stratum along with the log-rank test of time to treatment failure until the visit at 16 or 20 weeks (Fig. 1).
Table 3.
Outcomes and Reasons for Treatment Failure in the Three Study Groups.*
| Result | Ethosuximide | Lamotrigine | Valproic Acid | Overall P Value | Ethosuximide vs. Lamotrigine | Valproic Acid vs. Ethosuximide | Valproic Acid vs. Lamotrigine | |||
|---|---|---|---|---|---|---|---|---|---|---|
| no./total no. (%) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | ||||
| Outcome | ||||||||||
| Primary outcome: freedom from treatment failure at wk 16 or wk 20 | 81/154 (53) | 43/146 (29) | 85/146 (58) | <0.001 | <0.001 | 2.66 (1.65–4.28) | 0.35 | 1.26 (0.80–1.98) | <0.001 | 3.34 (2.06–5.42) |
| Secondary outcome: CPT confidence index ≥0.60 | 35/106 (33) | 25/104 (24) | 52/106 (49) | <0.001 | 0.17 | 1.56 (0.85–2.85) | 0.03 | 1.95 (1.12–3.41) | <0.001 | 3.04 (1.69–5.49) |
| Treatment failure | 73/154 (47) | 103/146 (71) | 61/146 (42) | <0.001 | ||||||
| Lack of seizure control | 22/154 (14) | 69/146 (47) | 18/146 (12) | <0.001 | <0.001 | 0.19 (0.11–0.32) | 0.73 | 0.84 (0.43–1.65) | <0.001 | 0.16 (0.09–0.28) |
| EEG seizures only | 10/22 (45) | 20/69 (29) | 4/18 (22) | |||||||
| Seizures either on clinical report or on bedside hyperventilation only | 6/22 (27) | 23/69 (33) | 8/18 (44) | |||||||
| Seizures on EEG and on clinical report, or seizures on EEG and on bedside hyperventilation | 6/22 (27) | 26/69 (38) | 6/18 (33) | |||||||
| Intolerable adverse effects | 37/154 (24) | 25/146 (17) | 35/146 (24) | 0.26 | 0.15 | 1.53 (0.87–2.70) | 0.99 | 1.00 (0.59–1.69) | 0.19 | 1.53 (0.86–2.71) |
| Nervous system, behavioral, or psychological effects | 12/37 (32) | 9/25 (36) | 20/35 (57) | |||||||
| Digestive disorders | 9/37 (24) | 3/25 (12) | 6/35 (17) | |||||||
| Rash† | 6/37 (16) | 5/25 (20) | 2/35 (6) | |||||||
| Fatigue | 3/37 (8) | 2/25 (8) | 5/35 (14) | |||||||
| Headache | 3/37 (8) | 2/25 (8) | 2/35 (6) | |||||||
| BMI increase that met exit criterion | 0 | 1/25 (4) | 4/35 (11) | |||||||
| Laboratory abnormality | 1/37 (3) | 2/25 (8) | 2/35 (6) | |||||||
| Other | 4/37 (11) | 4/25 (16) | 5/35 (14) | |||||||
| Withdrawal from study | 20/154 (13) | 18/146 (12) | 15/146 (10) | 0.78 | 0.99 | 1.06 (0.54–2.10) | 0.48 | 0.77 (0.38–1.56) | 0.71 | 0.81 (0.39–1.68) |
| Local investigator’s decision | 4/20 (20) | 2/18 (11) | 6/15 (40) | |||||||
| Parent or guardian’s decision | 15/20 (75) | 16/18 (89) | 11/15 (73) | |||||||
| Blinding broken | 1/20 (5) | 1/18 (6) | 1/15 (7) | |||||||
| Study drug discontinued; no reason reported | 0 | 1/146 (<1) | 0 | |||||||
BMI denotes body-mass index, CPT Conners’ Continuous Performance Test, and EEG electroencephalogram.
Rash was considered by investigators to be at least moderately severe and possibly drug-related.
Figure 1. Kaplan–Meier Curves for Freedom from Treatment Failure in the Three Study Groups.
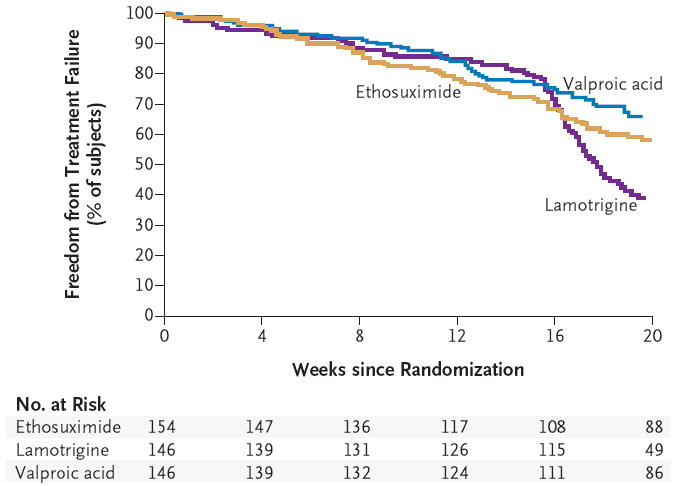
The log-rank test of time to treatment failure through the visit at 16 or 20 weeks showed significant differences according to treatment (P<0.001). The hazard ratio for treatment failure was 1.63 (95% confidence interval [CI], 1.20 to 2.20) for lamotrigine as compared with ethosuximide and 0.81 (95% CI, 0.58 to 1.14) for valproic acid as compared with ethosuximide. Patients remained in the study unless they met a criterion for treatment failure. Treatment failure due to drug toxicity or a generalized tonic–clonic seizure could occur at any time; treatment failure attributable to the persistence of absence seizures could occur only on or after the visit at week 16. The rates of freedom from treatment failure at 20 weeks were estimated to be 58% for ethosuximide (95% CI, 50 to 65), 39% for lamotrigine (95% CI, 31 to 47), and 66% for valproic acid (95% CI, 57 to 73).
The two most common reasons for treatment failure at weeks 16 and 20 were lack of seizure control (in 109 subjects [24%]) and intolerable side effects (in 97 subjects [22%]). The majority of children who had ongoing seizures were in the lamotrigine cohort. There were no significant differences among the treatment groups in the frequency of treatment failures due to either intolerable adverse events or withdrawal from the study (Table 3). In eight subjects, treatment was discontinued owing to generalized tonic–clonic seizures: three subjects in the ethosuximide group, four in the valproic acid group, and one in the lamotrigine group.
Seventeen types of adverse events were reported in 5% or more of the subjects in at least one treatment group (Table 4). By the visit at 16 or 20 weeks, eight subjects (2%) had had serious adverse events that required hospitalization: four in the ethosuximide group and two each in the lamotrigine and valproic acid groups. Reasons for hospitalization included generalized tonic–clonic seizures, in three subjects, and one subject each had nonepileptic events, longer in duration than previous absence seizures, episodes of acting out, salmonella enteritis, and pneumonia with diarrhea and vomiting. There were 13 cases of a moderately severe (possibly drug-related) rash leading to treatment failure but no cases of the Stevens–Johnson syndrome.
Table 4.
Adverse Events in 5% or More of Subjects in Any Treatment Group, in Descending Order of Overall Occurrence.
| Adverse Effect | Ethosuximide (N = 155) | Lamotrigine (N = 149) | Valproic Acid (N = 147) | Total Cohort (N = 451) |
|---|---|---|---|---|
| no. of subjects (%) | ||||
| General | ||||
| Fatigue | 15 (10) | 13 (9) | 18 (12) | 46 (10) |
| Headache | 19 (12) | 12 (8) | 12 (8) | 43 (10) |
| Sleep problem | 10 (6) | 5 (3) | 14 (10) | 29 (6) |
| Digestive or nutritional | ||||
| Nausea, vomiting, or both | 23 (15) | 2 (1) | 10 (7) | 35 (8) |
| Stomach upset | 16 (10) | 4 (3) | 8 (5) | 28 (6) |
| Increased appetite | 5 (3) | 7 (5) | 13 (9) | 25 (6) |
| Decreased appetite | 8 (5) | 5 (3) | 3 (2) | 16 (4) |
| Weight increase | 1 (1) | 3 (2) | 10 (7) | 14 (3) |
| Nervous system, behavioral, or psychological | ||||
| Hyperactivity | 14 (9) | 10 (7) | 15 (10) | 39 (9) |
| Hostility | 4 (3) | 10 (7) | 18 (12) | 32 (7) |
| Personality change | 4 (3) | 9 (6) | 16 (11) | 29 (6) |
| Decrease in concentration | 6 (4) | 5 (3) | 11 (7) | 22 (5) |
| Somnolence | 14 (9) | 3 (2) | 4 (3) | 21 (5) |
| Depression | 4 (3) | 8 (5) | 8 (5) | 20 (4) |
| Attentional difficulties | 3 (2) | 5 (3) | 10 (7) | 18 (4) |
| Dizziness | 9 (6) | 4 (3) | 2 (1) | 15 (3) |
| Memory problems | 0 | 4 (3) | 8 (5) | 12 (3) |
CONFIDENCE INDEX SCORES ON CONTINUOUS PERFORMANCE TEST
Confidence Index results from the Conners’ Continuous Performance Test were available for 316 subjects by the week 16 and week 20 visits (Table 3, and Fig. 1 in the Supplementary Appendix). At these visits, the percentage of subjects with a Confidence Index score of 0.60 or higher was greater in the valproic acid group than in the ethosuximide group (49% vs. 33%; odds ratio, 1.95; 95% CI, 1.12 to 3.41; P = 0.03) and the lamotrigine group (49% vs. 24%; odds ratio, 3.04; 95% CI, 1.69 to 5.49; P<0.001) (Table 3). On post hoc analysis (data not shown), even after adjustment for differences in the baseline Confidence Index scores, the valproic acid group had significantly worse scores at the week 16 and week 20 visits than did the ethosuximide and lamotrigine groups (P<0.001 for both comparisons), whereas there was no significant difference between the ethosuximide and lamotrigine groups (P = 0.43). Within treatment groups, there were no significant differences in these Confidence Index results between subjects with seizures and those free of seizures.
CLINICAL PHARMACOLOGY
The mean (±SD) daily dosages and steady-state pretreatment serum concentrations at weeks 16 and 20 were as follows: 33.5±15.3 mg per kilogram per day and 93 μg per milliliter (95% CI, 0 to 185) for 94 subjects in the ethosuximide group, 9.7±6.3 mg per kilogram per day and 7.8 μg per milliliter (95% CI, 0 to 15.7) for 96 subjects in the lamotrigine group, and 34.9±15.8 mg per kilogram per day and 94 μg per milliliter (95% CI, 8 to 180) for 104 subjects in the valproic acid group. Within treatment groups, there were no significant differences in the steady-state pretreatment serum concentrations between the seizure-free subjects and those who continued to have seizures (Fig. 2 in the Supplementary Appendix). By the week 16 or week 20 visit, the proportion of subjects who received the maximal dose was higher in the lamotrigine group (58.9%) than in the ethosuximide group (17.5%) or the valproic acid group (20.5%).
DISCUSSION
For children with childhood absence epilepsy, ethosuximide and valproic acid were significantly more effective than was lamotrigine in controlling seizures without intolerable side effects (primary outcome), and ethosuximide had a significantly smaller negative effect on attentional measures than did valproic acid (secondary outcome). There were no significant differences among the three groups with regard to discontinuation of treatment due to intolerable adverse events. Although certain side effects occurred more frequently among the children treated with ethosuximide or valproic acid, these side effects were generally transient and did not require discontinuation of treatment.
Drug effectiveness (the combination of efficacy and tolerability) was chosen a priori as the study’s primary outcome because of its paramount importance in the clinician’s initial selection of an antiepileptic medication. In children, cognitive side effects can be an important factor when one is selecting a drug from among medications that are equally effective.29 The prespecified secondary outcome, the short-term effect of the drugs on attention, was chosen to help clinicians differentiate between study medications having similar effectiveness. Both ethosuximide and valproic acid were more effective than was lamotrigine, but in both prespecified and post hoc analyses, ethosuximide resulted in fewer attentional effects as compared with valproic acid. The combination of primary and secondary outcomes suggests that ethosuximide is the optimal initial empirical monotherapy for childhood absence epilepsy.
The short-term effectiveness of ethosuximide and valproic acid observed in this double-blind, randomized trial was similar to that observed previously in open-label trials.30,31 However, smaller open-label studies have shown much higher efficacy rates for lamotrigine than the rates observed in our study, despite similarities in dose ranges, maximal daily doses, drug exposures, and efficacy end points.32-35 Lamotrigine’s relative lack of efficacy against absence seizures was first detected at 16 and 20 weeks, as evidenced by the disproportionately higher number of subjects who discontinued treatment at those times (Fig. 1).
In childhood absence epilepsy, attentional deficits have been identified as the most important marker of cognitive dysfunction and are associated with reduced academic performance.36 The Conners’ Continuous Performance Test Confidence Index is a measure that provides an overall indication of whether a subject’s profile best fits a clinical or nonclinical pattern of attentional problems. We used a Confidence Index of 0.60 or higher as an indicator of clinically significant difficulties with attention. Although this measure is not an indisputable basis for a diagnosis of attention deficit disorder, in clinical practice it offers strong evidence for classification.12
Both the prespecified and post hoc analyses showed that, in the short term, valproic acid negatively affected attention to a greater degree than did either lamotrigine or ethosuximide. There were no differences in the Confidence Index results between seizure-free subjects and those who continued to have seizures, confirming that attentional problems persist despite successful treatment, are not simply due to frequent absence seizures, and appear to be a core feature of the syndrome. This short-term study was not designed to detect long-term systemic or other cognitive effects of these three medications.
These results suggest that ethosuximide, one of the oldest available antiseizure medications, is a sensible choice for initial empirical monotherapy in childhood absence epilepsy. Even the best empirical therapy, however, fails in almost 50% of newly diagnosed cases. Given the increased risk of generalized tonic–clonic seizures as children with absence epilepsy grow older, and given ethosuximide’s reported lack of effectiveness in preventing such seizures,37 long-term follow-up of this study cohort is needed.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health (NS045911, NS045803, 5 U10 HD031318, 5 U10 HD037249, 1 UL1 RR026314, and P30 HD26979).
Study medications were provided free of charge by Pfizer, Abbott Laboratories, and GlaxoSmithKline.
APPENDIX
The members of the Childhood Absence Epilepsy Study Group are as follows: Executive Core: T.A. Glauser, A. Cnaan, P.C. Adamson, D.G. Hirtz, S. Shinnar, R.R. Clancy, E.V. Capparelli, G. Grabowski, J. Blumer; Medical Safety Monitor: J.M. Pellock; Pharmacogenetics Core: G. Grabowski, M. Keddache, G. Tangren, S. Srodulski; Pharmacokinetics Core: E.V. Capparelli, J. Blumer, P.C. Adamson, M.D. Reed, A.A. Vinks; EEG/Clinical Phenotyping Core: S. Shinnar, S.L. Moshé, E.M. Mizrahi, J.A. Conry, A. Berg, D. Dlugos, Y. Sogawa, J.B. Le Pichon, P. Overby, G. Von Allmen; Neuropsychology/QOL Core: S. Shinnar, D. Masur, C. O’Dell, P.M. Levisohn, J. Masur; Childhood Absence Epilepsy Coordinating Center (CAECC): A. Cnaan, C. Weiler, E. Dorsey, C. Scott, N. Thevathasan, V. Nissen, G. Nandwani, M. Gleave, J. Schneider, M. Vasconcelos, S. Evans, B. Bintliff-Janisak, K. Daniels, M. Shabbout, D. Shera, X. Luan, L. Lawrence, R. Guo, J. DiStefano-Pappas, M. Grubb, M. Taylor, G. Bernhard, J. Nevy, N. Drummond, M. Donaghue, M. Davis, N. Peccina, T. Alvarado-Taylor; Data and Safety Monitoring Board: P.R. Gilbert, B.K. Alldredge, B. Bourgeois, J.R. Buchhalter (chair), M.J. Hamberger, E.B. Roecker, J.H. Rodman (deceased); Research Pharmacy: M. Hoffman, K. Montefiore, D. LaGory; National Institute of Neurological Disorders and Stroke, Bethesda, MD: D.G. Hirtz, M. Jacobs, S. Janis, P.R. Gilbert; Children’s Healthcare of Atlanta at Scottish Rite, Atlanta: B. Philbrook, D. Schwam; Children’s Hospital of Alabama, Birmingham: P. Kankirawatana, K. Hoyle; Women and Children’s Hospital of Buffalo, Buffalo, NY: A. Weinstock, M. Elgie; Children’s Memorial Hospital, Chicago: K.R. Kelley, M. Tekateka; Cincinnati Children’s Hospital, Cincinnati: T.A. Glauser, P.O. Clark; Rainbow Babies and Children’s Hospital, Cleveland: M.S. Scher, D. Morus; Nationwide Children’s Hospital, Columbus, OH: J. Paolicchi, K. Zamel, S. Borror; Dallas Pediatric Neurology Associates, Dallas: R. Elterman, K. McEwen; Children’s Hospital, Denver: P.M. Levisohn, S. Brantz; Children’s Hospital of Michigan, Detroit: H.T. Chugani, J. Czachor; Cook Children’s Medical Center, Ft. Worth, TX: A. Hernandez, J. Kidd; Texas Children’s Hospital, Houston: A.A. Wilfong, R. Schultz, S.J. McVey; Nemours Children’s Clinic, Jacksonville, FL: W.R. Turk (deceased), H. Abram, S. Oken, T. Williams; University of Arkansas for Medical Sciences and Arkansas Children’s Hospital, Little Rock: R. Shbarou, M.L. Griebel, L. Howard, A. Riggs; Mattel Children’s Hospital at UCLA, Los Angeles: R. Sankar, S. Dewar, A. Perez; LeBonheur Children’s Medical Center, Neuroscience Institute, Memphis, TN: J. Wheless, M. Ellis; Miami Children’s Hospital, Miami: M. Duchowny, A. Halac, J. Barrera; Children’s Hospital of Wisconsin, Milwaukee: M. Zupanc, R. Werner; Department of Pediatrics, Yale University, New Haven, CT: E. Novotny, Jr., C. Cardoza; Montefiore Medical Center, New York: K. Ballaban-Gil, C. O’Dell; New York University Comprehensive Epilepsy Center, New York: D.K. Miles, M. Miceli; Children’s Hospital of the King’s Daughters, Eastern Virginia Medical School, Norfolk: L.M. Frank, T. Conklin; Children’s Hospital of Philadelphia, Philadelphia: R.R. Clancy, Y. Collins; St. Christopher’s Hospital for Children, Philadelphia: D. Khurana, A. Francis; St. Joseph’s Hospital, Phoenix, AZ: K. Chapman, J.M. Rho, A. Reese-Porter; Children’s Hospital of Pittsburgh, Pittsburgh: P.K. Crumrine, S. Williams, T. Eaton; Doernbecher Children’s Hospital, Portland, OR: C. Roberts, C. Borzy, S. Dean; Primary Children’s Medical Center, Salt Lake City: C.B. Van Orman, S. Taylor, J. Narus; University of California, San Diego, San Diego: D.A. Trauner, M. Nespeca, K. Romine; Seattle Children’s Hospital, Seattle: R.P. Saneto, M. Sotero de Menezes, L. Guidry; St. Louis Children’s Hospital, St. Louis: E. Trevathan, M. Bertrand, E. Albers, L. Grayson; Children’s National Medical Center, Washington, DC: J.A. Conry, S. Shah, D. Lowery.
Footnotes
Dr. Glauser reports receiving consulting and lecture fees from Eisai, UCB Pharma, and Johnson & Johnson; Dr. Shinnar, receiving consulting fees from Eisai, Johnson & Johnson, and King Pharmaceuticals and lecture fees from Eisai and UCB Pharma, and serving as an expert witness on seizure cases for both the plaintiff and defense; Dr. Capparelli, receiving consulting fees from Cadence Pharmaceuticals, Bristol-Myers Squibb, and Arpida Pharmaceuticals; and Dr. Adamson, receiving grant support from Abbott Laboratories. No other potential conflict of interest relevant to this article was reported.
References
- 1.Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B. How well can epilepsy syndromes be identified at diagnosis? A reassessment 2 years after initial diagnosis. Epilepsia. 2000;41:1269–75. doi: 10.1111/j.1528-1157.2000.tb04604.x. [DOI] [PubMed] [Google Scholar]
- 2.Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study. Epilepsia. 2001;42:464–75. doi: 10.1046/j.1528-1157.2001.31400.x. [DOI] [PubMed] [Google Scholar]
- 3.Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 4.Loiseau P, Duché B, Pédespan JM. Absence epilepsies. Epilepsia. 1995;36:1182–6. doi: 10.1111/j.1528-1157.1995.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 5.Bouma PA, Westendorp RG, van Dijk JG, Peters AC, Brouwer OF. The outcome of absence epilepsy: a meta-analysis. Neurology. 1996;47:802–8. doi: 10.1212/wnl.47.3.802. [DOI] [PubMed] [Google Scholar]
- 6.Wirrell EC, Camfield CS, Camfield PR, Dooley JM, Gordon KE, Smith B. Long-term psychosocial outcome in typical absence epilepsy: sometimes a wolf in sheeps’ clothing. Arch Pediatr Adolesc Med. 1997;151:152–8. doi: 10.1001/archpedi.1997.02170390042008. [DOI] [PubMed] [Google Scholar]
- 7.Pavone P, Bianchini R, Trifiletti RR, Incorpora G, Pavone A, Parano E. Neuropsychological assessment in children with absence epilepsy. Neurology. 2001;56:1047–51. doi: 10.1212/wnl.56.8.1047. [DOI] [PubMed] [Google Scholar]
- 8.Wheless JW, Clarke DF, Carpenter D. Treatment of pediatric epilepsy: expert opinion, 2005. J Child Neurol. 2005;20(Suppl 1):S1–S56. doi: 10.1177/088307380502000101. [DOI] [PubMed] [Google Scholar]
- 9.Glauser T, Ben-Menachem E, Bourgeois B, et al. ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006;47:1094–120. doi: 10.1111/j.1528-1167.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 10.French JA, Pedley TA. Initial management of epilepsy. N Engl J Med. 2008;359:166–76. doi: 10.1056/NEJMcp0801738. [DOI] [PubMed] [Google Scholar]
- 11.Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: interactions between antiepileptic drugs and other drugs. Lancet Neurol. 2003;2:473–81. doi: 10.1016/s1474-4422(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 12.Conners CK. Conners’ Continuous Performance Test II: technical guide and software manual. North Tonawanda, NY: Multi-Health Systems; 2002. [Google Scholar]
- 13.Wechsler D. Wechsler Intelligence Scale for Children. 4. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- 14.Idem. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 15.Brown L, Sherbenou RJ, Johnson SK. Test of Nonverbal Intelligence. 3. Austin, TX: Pro-Ed; 1997. [Google Scholar]
- 16.Dunn LM, Dunn LM. Peabody Picture Vocabulary Test-III. Circle Pine, MN: American Guidance Services; 1997. [Google Scholar]
- 17.Sheslow D, Adams W. Wide Range Assessment of Memory and Learning. 2. Wilmington, DE: Wide Range; 2003. [Google Scholar]
- 18.Korkman M, Kirk U, Kemp S. NEPSY-II: administration manual. San Antonio, TX: Psychological Corporation; 2005. [Google Scholar]
- 19.Idem. NEPSY: a developmental neuropsychological assessment manual. San Antonio, TX: Psychological Corporation; 1998. [Google Scholar]
- 20.Beery KE, Buktenica NA, Beery NA. Beery-Buktenica Developmental Test of Visual-Motor Integration. 5. Cleveland: Modern Curriculum Press; 1997. [Google Scholar]
- 21.Heaton KR, Chelune GJ, Talley JL, Kay GG, Curtis G. Wisconsin Card Sorting Test manual: revised and expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- 22.Heaton RK. Wisconsin Card Sorting Test manual. Odessa, FL: Psychological Assessment Resources; 1981. [Google Scholar]
- 23.Wilkinson GS. The Wide Range Achievement Test. 3. Wilmington, DE: Jastak; 1993. [Google Scholar]
- 24.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca, IL: Riverside Publishing; 2007. [Google Scholar]
- 25.Achenbach TM, Rescorla LA. Manual for ASEBA School Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth & Families; 2001. [Google Scholar]
- 26.Idem. Manual for ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth & Families; 2000. [Google Scholar]
- 27.Sabaz M, Cairns DR, Lawson JA, Nheu N, Bleasel AF, Bye AM. Validation of a new quality of life measure for children with epilepsy. Epilepsia. 2000;41:765–74. doi: 10.1111/j.1528-1157.2000.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim K, DeMets D. Design and analysis of group sequential tests based on the type I error spending rate function. Biometrika. 1987;74:149–54. [Google Scholar]
- 29.Bourgeois BF. Determining the effects of antiepileptic drugs on cognitive function in pediatric patients with epilepsy. J Child Neurol. 2004;19(Suppl 1):S15–S24. doi: 10.1177/088307380401900103. [DOI] [PubMed] [Google Scholar]
- 30.Callaghan N, O’Hare J, O’Driscoll D, O’Neill B, Daly M. Comparative study of ethosuximide and sodium valproate in the treatment of typical absence seizures (petit mal) Dev Med Child Neurol. 1982;24:830–6. doi: 10.1111/j.1469-8749.1982.tb13703.x. [DOI] [PubMed] [Google Scholar]
- 31.Martinovic Z. Comparison of ethosuximide with sodium valproate. In: Parsonage M, Grant RHE, Craig AG, Ward AW Jr, editors. Advances in epileptology: the XIVth Epilepsy International Symposium. New York: Raven Press; 1983. pp. 301–5. [Google Scholar]
- 32.Buoni S, Grosso S, Fois A. Lamotrigine in typical absence epilepsy. Brain Dev. 1999;21:303–6. doi: 10.1016/s0387-7604(99)00023-6. [DOI] [PubMed] [Google Scholar]
- 33.Frank LM, Enlow T, Holmes GL, et al. Lamictal (lamotrigine) monotherapy for typical absence seizures in children. Epilepsia. 1999;40:973–9. doi: 10.1111/j.1528-1157.1999.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 34.Holmes GL, Frank LM, Sheth RD, et al. Lamotrigine monotherapy for newly diagnosed typical absence seizures in children. Epilepsy Res. 2008;82:124–32. doi: 10.1016/j.eplepsyres.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppola G, Auricchio G, Federico R, Carotenuto M, Pascotto A. Lamotrigine versus valproic acid as first-line monotherapy in newly diagnosed typical absence seizures: an open-label, randomized, parallel-group study. Epilepsia. 2004;45:1049–53. doi: 10.1111/j.0013-9580.2004.40903.x. [DOI] [PubMed] [Google Scholar]
- 36.Masur D, Shinnar S. The neuropsychology of childhood seizure disorders. In: Segalowitz SJ, Rapin I, editors. Handbook of neuropsychology. Amsterdam: Elsevier; 1992. pp. 457–70. [Google Scholar]
- 37.Glauser TA, Morita DM. Ethosuximide. In: Wylie E, Gupta A, Lachhwani DK, editors. The treatment of epilepsy: principles and practice. 4. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


