Abstract
This study aimed to assess the influence of lobectomy and pneumonectomy on cardiac rhythm and on the dimensions and function of the right-side of the heart. Twelve dogs undergoing lobectomy and eight dogs undergoing pneumonectomy were evaluated preoperatively and one month postoperatively with electrocardiography and Doppler echocardiography at rest. Pulmonary artery systolic pressure (PASP) was estimated by the tricuspid regurgitation jet (TRJ) via the pulse wave Doppler velocity method. Systemic inflammatory response syndrome criteria (SIRS) were also evaluated based on the clinical and hematological findings in response to lobectomy and pneumonectomy. Following lobectomy and pneumonectomy, we predominantly detected atrial fibrillation and varying degrees of atrioventricular block (AVB). Dogs that died within seven days of the lobectomy (n = 2) or pneumonectomy (n = 1) had complete AVB. Preoperative right atrial, right ventricular, and pulmonary artery dimensions increased gradually during the 30 days (p<0.05) following pneumonectomy, but did not undergo significant changes during that same period after lobectomy. Mean PASP was 56.0 ± 4.5 mmHg in dogs having significant TRJ after pneumonectomy. Pneumonectomy, but not lobectomy, could lead to increases (p<0.01) in the SIRS score within the first day post-surgery. In brief, it is important to conduct pre- and postoperative cardiac evaluation of dogs undergoing lung resections because cardiac problems are a common postoperative complication after such surgeries. In particular, complete AVB should be considered a life-threatening complication after pneumonectomy and lobectomy. In addition, pneumonectomy appears to increase the likelihood of pulmonary hypertension development in dogs.
Keywords: dog, heart function, lobectomy, pneumonectomy, pulmonary hypertension
Introduction
Lobectomy is the surgical removal of parenchymal organ lobes [11]. Pneumonectomy is a lung resection technique consisting of the removal of all lung lobes in a hemithorax [11,24,34]. Lobectomy and pneumonectomy are primarily implemented to remove lung tumor metastasis in humans [37] and dogs [11,18,19,24]. Other indications for lobectomy and pneumonectomy are chronic lung collapse, chronic lung inflammation, post-traumatic parenchymal laceration [5,11,19], pneumonia [22], and lung torsion [23].
Morbidity and mortality after pneumonectomy in humans [1-3,28,34], cats and dogs [18] are extremely high due to respiratory, cardiac, and gastrointestinal problems. In particular, the mediastinal shift occurring following major lung resection can result in esophageal dysmotility in dogs [19]. Acute respiratory complications in humans include respiratory insufficiency, pneumonia, pulmonary edema, pyothorax and chylothorax [19]. Cardiac complications in humans include myocardial infarction and progression to congestive heart failure [2,28] and the most common is arrhythmia, which occurs in up to 40% of patients [3,27,28]. Most arrhythmias are supraventricular, and include supraventricular tachycardia, atrial fibrillation and flutter and premature atrial contractions. Premature ventricular contractions may also be seen [14]. While rhythm disturbances are common cardiac complications encountered after lung resection in humans, there is limited information about arrythmia in dogs [14,19]. Pulmonary hypertension and increased right ventricular systolic pressure are also reported as complications after non-cardiac thoracic surgery in humans [1,10,17,19,29,35].
The main purpose of this study was to assess the influence of lobectomy and pneumonectomy on cardiac rhythm and on the dimensions and functions of the right-side of the heart in dogs.
Materials and Methods
Study population and general procedures
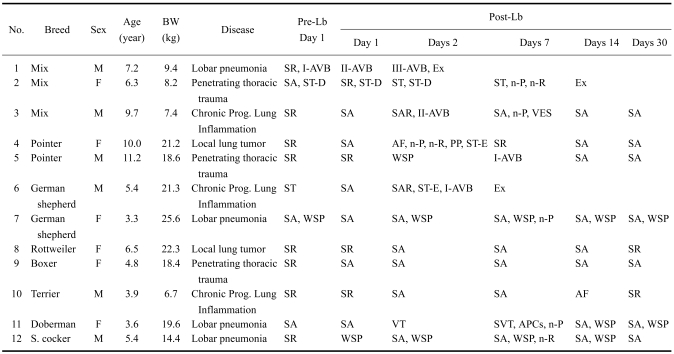
The present study was conducted at Small Animal Clinics, Faculty of Veterinary Medicine, Uludag University (Turkey). Lobectomy and pneumonectomy were performed in 20 dogs of various breeds and ages and of both sexes. The dogs presented with a variety of lung diseases prior to the surgery (Tables 1 and 2). Six dogs had secondary neoplastic disease diagnosed based on clinical, hematological, and radiological findings, together with clinical history; the neoplastic disease was confirmed histopathologically. Two of these dogs underwent lobectomy for mammary gland adenocarcinoma; the remaining four dogs underwent pneumonectomy for diffuse lung metastasis of osteosarcoma (n = 2), adenocarcinoma (n = 1) and fibrosarcoma (n = 1). In order for a dog to be considered for lobectomy or pneumonectomy, it had to present with lung tumors of at least grade III which were unresponsive to at least two chemotherapy protocols. After the radiological localization of the affected lung tissues, lobectomy and pneumonectomy were performed either to remove the metastatic lung tumor or to repair other lung pathologies (pulmonary hemorrhagic-contusion, trauma, inflammation). In the case of lobar pneumonia, lobectomy was decided upon after unsuccessful response to the antibiotic treatment of the consolidated lung lobes. Bronchoalveolar lavage samples were cultured and Staphylococcus aureus, Streptococcus spp., Pseudomonas aeruginosa, and Klebsiella pneumoniae organisms were identified.
Table 1.
Clinical characteristics and electrocardiographic assessments in dogs, before (Pre-) and after (Post-) lobectomy (Lb)
Ex: exitus lethalis. APCs: atrial premature complexes; AF: atrial fibrillation; I-, II and III-AVB: one or second or third degree atrioventricular heart block; n-P: notching (>0.05 mV/lead II) of P wave; n-R: notching (>0.1 mV/lead II) of R wave; PP: P-pulmonale (P wave > 4 mV/lead II); SA: sinus arrhythmia; SAR: sinus arrest; SR: sinus rhythm; ST-D: ST segment depression (>-0.2 mV/lead II); ST-E: ST segment elevation (>0.15 mV/lead II); VES: ventricular extrasystole; VT: ventricular tachycardia; WSP: wandering sinus pacemaker.
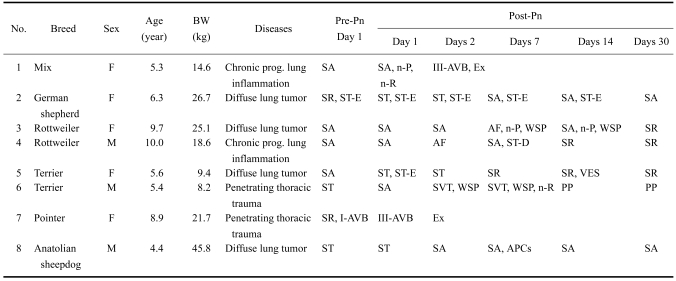
Table 2.
Clinical characteristics and electrocardiographic assessments in dogs, before (Pre-) and after (Post-) pneumonectomy (Pn)
Pneumonectomy was performed as emergencies in two dogs, for life threatening penetrating thoracic trauma involving multiple ruptures and atelectasis of lung parenchyma as revealed by clinical, radiological and surgical investigation. Meanwhile, penetrating thoracic trauma necessitated lobectomies in three dogs. These dogs had bite injuries on the thoracic wall, with some penetrating the pleural cavity; associated contusions and lacerations were found in the lung parenchyma. Chronic progressive lung inflammation was diagnosed by the presence of compatible clinical, hematological, and radiological findings. Dogs which remained unresponsive to medical treatment for two months and were subjected to pneumonectomies or lobectomies to eradicate the diseases.
The occurrence of systemic inflammatory response syndrome (SIRS) was investigated in all dogs, as reported previously [38]. For this diagnosis, we required the occurrence of two or more non-specific general and hematological variables in response to non-specific etiology. Pre- and postoperative SIRS scores were assessed according to a scale based on the alterations found in the clinical and laboratory tests: hypo- or hyperthermia (temperature lower than 37.0℃ or higher than 39.3℃, 1 point), tachycardia (heart rate higher than 140 beats/min, 1 point), tachypnea (respiratory rate higher than 30 breaths/min, 1 point), mean arterial pressure (lower than 70 mmHg or higher than 140 mmHg, 1 point), oxygen saturation (SPO2 90~100%, 0 point; 80~89%, 1 point; 60 ~79%, 2 point; and lower than 60%, 3 point), leucopenia (WBC count lower than 5,500/µL, with >5% immature form; 1 point) or leukocytosis (WBC count higher than 12,500/µL, with >5% immature form; 1 point), and thrombocytopenia (<170,000/µL, 1 point). SIRS score was estimated at all time points for each dog, and it varied between 0 and 9 points (maximum).
All dogs were evaluated pre- and postoperatively by means of clinical and hematological examinations. Non-invasive blood pressure and peripheral oxygen saturation (SPO2) measurements (BM3-Vet; Bionet, Korea) were performed in a quiet room, as per the manufacturer's instructions,. Measurements were performed with the dogs in sternal recumbency using an oscillometric technique with cuffs of different sizes for large (25 × 2.5 cm), medium (20 × 2.5 cm), or small dogs (15 × 2.5 cm), which were placed at the right antebrachium, and using an infrared sensor placed on the ear margin after clipping. During the diagnostic work-up, routine hematological analyses including leukogram, erythrogram, and thrombogram were performed using an automatic cell counter (Cell Dyne 3500; Abbott, Germany) before and 1~30 days post-operatively. WBC, neutrophil, and platelet counts were used to estimate the SIRS scores.
Tests for the presence of occult heartworms (Dirocheck, Symbiotics) were negative in all cases. All dogs included in this study had a full schedule of the vaccinations recommended in Turkey.
Electrocardiography
Electrocardiograms (ECGs, P80; Esoate, Italy) were recorded by the investigators with dogs in right lateral recumbency. Cardiac rhythms were analyzed on lead II (50 mm/sec; 10 mm/mV), according to recommended criteria [15]. The amplitudes of the ECG waves and the duration of the intervals were also measured on lead II.
Echocardiography
All echocardiograms were performed one to three days prior to surgery and one month after the operation in conscious unsedated dogs during a period of quiet respiration. Doppler echocardiography was performed using conventional clinical echocardiographic equipment (Caris Plus; Esoate, Italy) with 2.5- or 7.5-MHz phased array electronic transducers. Doppler and two-dimensional images were obtained from the right parasternal long- and short-axis, and the left apical four-chamber views. Echocardiograms were reviewed to assess the left- and right-side chamber size and cardiac function, as previously suggested [21].
Tricuspid regurgitant jet (TRJ) was identified by color flow Doppler techniques, and the maximum jet velocity was measured by continuous wave Doppler. Right ventricular systolic pressure was estimated based on the modified Bernoulli equation and was considered to be equal to the pulmonary artery systolic pressure (PASP) in the absence of right ventricular outflow obstruction [15,32,33]. The left and right end-diastolic atrial diameters were compared at the level of the atrioventricular valves in the 2D left apical 4-chamber view. The right atrium was considered to be dilated if the diameter was greater than or equal to the diameter of the left atrium. The estimated right atrial pressure was 5 mm Hg in dogs with a non-enlarged right atrium, 10 mm Hg in dogs with an enlarged right atrium and no sign of right-sided heart failure, and 15 mm Hg in dogs with either right-sided heart failure or pulmonary congestion [10]. If PASP values ≥30 mmHg were calculated on continuous wave Doppler, dogs were considered to have increased right ventricular systolic pressures [15,33]. Parameters related to the left ventricle were also measured preoperatively and 30 days after the operations. Standard M-mode measurements of the left ventricle (diastolic and systolic diameters of left ventricle, interventricular septum, and posterior wall), were performed. Fractional shortening and ejection fraction were estimated. Dimensions of the left atrium, pulmonary artery, and aorta were measured from 2-D right parasternal short axis view at aortic level, as previously suggested [21].
Anesthesia and surgical technique
Xylazine HCl (1 mg/kg, i.m.) (Alfazine 2%; Alfasan/Egevet, Turkey) was administered as premedication. Induction was carefully achieved with intravenous thiopental sodium (15 mg/kg, pentothal sodium 1 g; Abbott, UK). General anesthesia was continued with a 2% concentration of isoflurane (Furane; Abbott, UK). Respiration was ensured with mechanic ventilation (15 mL/kg tidal volume, respiration rate 15/min and 25 cm H2O airway pressure).
All operations were carried out by the same surgical team. Dogs were subjected to lateral intercostal thoracotomy on the 4th intercostal space using routine techniques. Then, either lobectomy [left (n = 7), right (n = 5)] or pneumonectomy [left (n = 3), right (n = 5)] was performed. Lobectomy and pneumonectomy were performed with the technique of individual ligation of the pulmonary arteries and pulmonary veins. Bronchi (lobectomy) or bronchus (pneumonectomy) were dissected from the surrounding pleura and then sutured manually with 2~0 suture material (Vicryl; Ethicon, UK) after excision. The vagus and heart were kept intact. Before routine thoracic closure, a 26~34 Fr chest tube (Argyle; Sherwood Medical, USA) was inserted into the affected pleural cavity. The tube was fixed to the skin with a 'Chinese finger trap' suture pattern, and a Heimlich valve was connected to the tube for continuous drainage [30].
Postoperative care
Ampicillin sulbactam (20 mg/kg, i.v.) (Combisid; Bilim Ilac, Turkey) and carprofen (2.2 mg/kg, i.v.) (Rimadyl; Pfizer, Turkey) were administered pre- and postoperatively. Lactate Ringer (Vacoliter; Baxter, Turkey) and hydroxyethyle starch (Expahes Sterile; Baxter, Turkey) solutions were infused for postoperative fluid therapy. Postoperative radiological evaluations were performed routinely. The thoracostomy tube and skin sutures were removed on days 5 and 7 after surgery, respectively.
Following the operations, digoxin (0.25 mg/tab.; Novartis, Turkey) and lidocaine (Aritmal 2%; Tems, Turkey) were used to improve arrhythmias of supraventricular (sinus tachycardia and atrial fibrillation) and ventricular origins (ventricular tachycardia), respectively [16,20].
Statistical analysis
Results were expressed as mean ± SE. Data were analyzed by one way analysis of variance for within-group changes. Percent changes from baseline were estimated for each parameter for the two groups: pneumonectomy and lobectomy. Chi-square test was also used to compare sex and age distributions of the dogs (SigmaStat; SPSS, Germany). p values smaller than 0.05 were considered significant.
Results
Study population and general variables
A total of 20 dogs were evaluated in this study. Clinical characteristics of the dogs are shown in Tables 1 and 2. The most common diseases leading to a lobectomy (Table 1) and a pneumonectomy (Table 2) were lobar pneumonia and diffuse lung tumors, respectively. There were no significant differences in sex (DF = 1, chi-square = 0.82), age (DF = 1, chi-square = 0.88; 6.5 ± 2.5 years vs. 6.9 ± 2.2 years) and body weight (16.0 ± 6.6 kg vs. 21.2 ± 12.0 kg; statistically not significant) between the two operation groups. The predominant features of the clinical history of the dogs were dyspnea with or without exercise (lobectomy, n = 3; pneumonectomy, n = 4), tachypnea (lobectomy, n = 3; pneumonectomy, n = 4), lethargy (lobectomy, n = 2; pneumonectomy, n = 3), exercise intolerance (lobectomy, n = 2; pneumonectomy, n = 4), and anorexia (lobectomy, n = 6; pneumonectomy, n = 6).
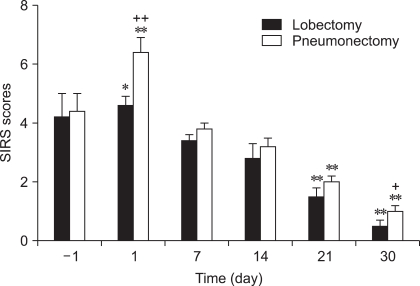
Preoperative SIRS scores of 4.2 ± 0.8 (lobectomy) and 4.6 ± 0.7 (pneumonectomy) increased to 4.6 ± 0.5 (lobectomy, p>0.05) and 6.4 ± 0.6 (pneumonectomy, p<0.001) one day after the operations. SIRS scores on postoperative days 21 and 30 were significantly lower (p<0.001) than the observed preoperative scores (Fig. 1). Also, SIRS scores estimated in dogs with pneumonectomy were statistically higher than in dogs with lobectomy on post-operative day 1 (p<0.01) and day 30 (p<0.05).
Fig. 1.
Systemic inflammatory response syndrome (SIRS) criteria, one day (-1) before and 30 days after lobectomy and pneumonectomy. *p<0.05; **p<0.001; compared to preoperative value. †p<0.05; ††p<0.01; differences between the groups on the same day.
ECG results
Pre- and post-operative rhythm analyses are shown in Tables 1 and 2. Prior to surgery, all dogs had sinus rhythm alone or together with 1st degree atriventricular block (AVB), ST segment depression, or wandering sinus pacemaker (WSP). During the observation period of 30 days, all dogs had one or more ECG abnormalities. The most common rhythm abnormalities seen after lobectomy and pneumonectomy were AVB and supraventricular tachycardia (SVT; sinus tachycardia and atrial fibrillation), respectively. The incidence of SVT was 25.0% for lobectomy, and 75.0% for pneumonectomy. SVT was first detected via ECG within two weeks of the lobectomy or pneumonectomy. The dogs that had atrial fibrillation or ventricular extrasystole required pharmacological therapy (digoxin or lidocaine, respectively) for arrhythmias. They improved within three days of the initiation of medications. Any sinus tachycardia that was observed after the operations returned to normal sinus rhythm within seven days without the need for anti-arrhythmic medications.
Pre- and post-operative morphological changes in the ECG waves were also observed (Tables 1 and 2). In one dog (No. 2, in Table 1), ST segment depression before lobectomy lasted into postoperative day 2, and then notching of the P and R waves without ST depression developed within the first postoperative week. This dog died within the second postoperative week. In another dog (No. 2, in Table 2), ST segment elevation before pneumonectomy lasted until postoperative day 14, and fully recovered by postoperative day 30. Three dogs with complete AVB died within two days of the lobectomy (n = 1) and the pneumonectomy (n = 2).
Echocardiographic results
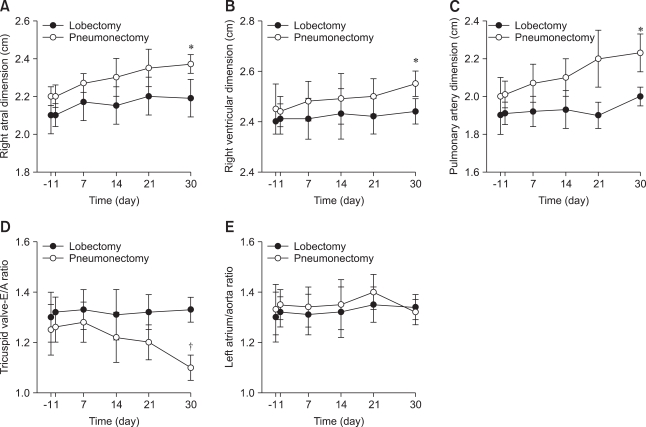
Preoperative right ventricle dimensions of 2.0 ± 0.2 cm increased gradually to 2.5 ± 0.2 cm (p<0.05) one month after pneumonectomy (Fig. 2). In contrast, preoperative right atrium and pulmonary artery dimensions hadn't changed by the end of the monitoring period (Figs. 2A-C). The E/A ratio of the tricuspid valve was between 1.22 ± 0.2 and 1.33 ± 0.3 before the surgical procedures and decreased to a nadir of 0.98 ± 0.2 (p<0.05) one month after pneumonectomy, whereas it did not change significantly after lobectomy (Fig. 2D). Prior to surgery, a significant TRJ was not detected in the study population. However, a significant TRJ, which permitted the calculation of PASP, was found one month after the operation in three dogs undergoing pneumonectomy (right pneumonectomy, n = 2; left pneumonectomy, n = 1). The mean maximal velocity of TRJ was 3.4 ± 0.3 m/sec and the mean PASP was 56.0 ± 4.50 mm Hg in three dogs with pneumonectomy. There were no significant differences between the preoperative and postoperative values of the left atrium / aorta ratio (Fig. 2E) and the morphological and functional parameters related to the left ventricle (data not shown).
Fig. 2.
Echocardiographic results. (A) Right atrial, (B) right ventricular, and (C) pulmonary artery dimensions, (D) tricuspid valve E/A ratio, and (E) left atrium to aorta ratio one day (-1) before and 30 days after lobectomy and pneumonectomy. *p<0.05 and †p<0.01; compared to preoperative value.
Discussion
These data show that atrial fibrillation was the most common rhythm abnormality found after pneumonectomy and lobectomy. Furthermore, pneumonectomy, but not lobectomy, could lead to rapid changes in right heart morphology and function in dogs.
In agreement with previous reports, systemic inflammatory reaction was characterized by the presence of compatible clinical signs and hematological findings in response to pulmonary neoplasia, lobar pneumonia, progressive lung inflammation, and penetrating thoracic trauma in dogs at time of admission to hospital [12,19,23,24,38]. Our findings that pneumonectomy, but not lobectomy, enhanced the SIRS score on postoperative day 1 may result from the magnitude of pulmonary resection [11,12,19]. The decrease in SIRS scores on days 21-30 post-surgery supports major surgical excision as the only potentially curative option for lung diseases unresponsive to medical treatments [12,18].
It remains to be established whether pulmonary diseases can cause cardiac rhythm disturbances in dogs. Our findings of cardiac arrhythmia in dogs with lung diseases were consistent with the study of Liptak et al. [19]. SVT arrhythmias are the most common cardiac complication after pneumonectomy [3,6,10,26,28]. In this study, SVT and AVBs were predominantly detected after both operations, and the incidence of SVT in dogs with pneumonectomy was much higher than that of human studies [6,8,28,39]. The rate of atrial fibrillation observed after pneumonectomy was higher than that observed after lobectomy. This result contrasts with the study of Curtis et al. [7] in humans, which found that development of atrial fibrillation was not dependent on the magnitude of pulmonary resection. Patients with pneumonectomy have been reported to require more pharmacological therapy for SVT compared with patients with lesser resection [7]. In this study, SVT, ventricular extrasystole, and ventricular tachycardia after lobectomy and pneumonectomy returned to normal sinus rhythm with or without anti-arrhythmic medications. This finding suggests that following lung resection surgery, atrial and ventricular rhythm disturbances might be spontaneously reversible or, if needed, can be easily treated with digoxin or lidocaine, respectively. In addition, results from our study suggest that complete AVB may be an important risk factor for death after lobectomy and pneumonectomy in dogs. Because ST segment changes and notching of P and R waves were reversible within 30 days of the operations, morphological changes in the ECG waves did not seem to be closely related with clinical outcome.
The reason for SVT being more common after pneumonectomy is not clear, but it is likely to be closely related to the abnormalities of hemodynamic and nervous regulation of the cardiac rhythm [14,27,28]. Arrhythmias are also reported to be associated with hypoxia, ventilation/perfusion mismatching, decreased pulmonary vascular reserve, decreased lung tidal volume, vagal irritation, adrenergic hyperactivity, myocarditis, pulmonary hypertension, and right heart dysfunction [3,19,27,28].
Right heart dysfunction and PASP are well-known and important complications of several human cardiopulmonary diseases [9,13,17,29,35,36]. Studies in humans on the late effects of pneumonectomy on the right heart function and the mean PASP at rest detected a moderate increase in mean PASP [1,3,28]. In this study, following pneumonectomy, we observed increases in the right atrium, right ventricle, and pulmonary artery dimensions, suggesting that pneumonectomy, rather than lobectomy, may have the potential to induce right heart remodeling (dilatation and dysfunction) [25]. Also, pneumonectomy seemed to be related to postoperative elevation of PASP and right ventricular dilatation, an observation consistent with Foroulis et al. [10]. A significant association between the occurrence of SVT and a postoperative increase in TRJ velocity has been previously reported in humans [2]. Similarly, in our study, sinus tachycardia and atrial fibrillation may be responsible for the observed increases in TRJ velocity in dogs. Right heart remodeling may also be attributable to a considerable decrease of the pulmonary vascular bed, with subsequent increase in the perfusion to the remaining lung and in the right ventricular afterload [25-27,39]. The presence of right ventricular dysfunction may be supported by the tricuspid inflow pattern with decreased ratio of early (E) to late (A) diastolic peak flow velocities, indicating abnormal relaxation of the right ventricle [25]. Decreased tricuspid flow E/A ratio may also result from differences in heart and respiratory rates, and changes in loading condition and contractility, in response to lung surgery [25,26,29,39]. Morphological and functional parameters of the left ventricle and the left atrium to aorta ratio did not vary for the duration of our study, suggesting that right ventricular dysfunction is unlikely to occur together with left ventricular dysfunction after major lung surgery in dogs.
These data show that it is very important to conduct pre- and post-operative cardiac evaluation of dogs undergoing lung resections because cardiac problems are a common postoperative complication after lung surgery. In particular, complete AVB should be considered as a life-threatening complication after pneumonectomy and lobectomy in dogs. Furthermore, pneumonectomy may increase the risk of marked pulmonary hypertension in dogs. Additional studies in a larger, more diverse patient population are needed to verify and assess clinical outcome indicators. Additionally, a more thorough understanding of lung resection and its influence on the pulmonary and cardiovascular systems in the dog is essential to avoid the serious complications found in this study.
References
- 1.Amar D, Burt ME, Roistacher N, Reinsel RA, Ginsberg RJ, Wilson RS. Value of perioperative Doppler echocardiography in patients undergoing major lung resection. Ann Thorac Surg. 1996;61:516–520. doi: 10.1016/0003-4975(95)00939-6. [DOI] [PubMed] [Google Scholar]
- 2.Amar D, Roistacher N, Burt M, Reinsel RA, Ginsberg RJ, Wilson RS. Clinical and echocardiographic correlates of symptomatic tachydysrhythmias after noncardiac thoracic surgery. Chest. 1995;108:349–354. doi: 10.1378/chest.108.2.349. [DOI] [PubMed] [Google Scholar]
- 3.Amar D, Roistacher N, Burt ME, Rusch VW, Bains MS, Leung DH, Downey RJ, Ginsberg RJ. Effects of diltiazem versus digoxin on dysrhythmias and cardiac function after pneumonectomy. Ann Thorac Surg. 1997;63:1374–1381. [PubMed] [Google Scholar]
- 4.Bach DS, Curtis JL, Christensen PJ, Iannettoni MD, Whyte RI, Kazerooni EA, Armstrong W, Martinez FJ. Preoperative echocardiographic evaluation of patients referred for lung volume reduction surgery. Chest. 1998;114:972–980. doi: 10.1378/chest.114.4.972. [DOI] [PubMed] [Google Scholar]
- 5.Bellenger CR, Hunt GB, Goldsmid SE, Pearson MR. Outcomes of thoracic surgery in dogs and cats. Aust Vet J. 1996;74:25–30. doi: 10.1111/j.1751-0813.1996.tb13729.x. [DOI] [PubMed] [Google Scholar]
- 6.Cagirici U, Nalbantgil S, Cakan A, Turhan K. A new algorithm for preoperative cardiac assessment in patients undergoing pulmonary resection. Tex Heart Inst J. 2005;32:159–162. [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis JJ, Parker BM, McKenney CA, Wagner-Mann CC, Walls JT, Demmy TL, Schmaltz RA. Incidence and predictors of supraventricular dysrhythmias after pulmonary resection. Ann Thorac Surg. 1998;66:1766–1771. doi: 10.1016/s0003-4975(98)00942-4. [DOI] [PubMed] [Google Scholar]
- 8.Dyszkiewicz W, Skrzypczak M. Atrial fibrillation after surgery of the lung: clinical analysis of risk factors. Eur J Cardiothorac Surg. 1998;13:625–628. doi: 10.1016/s1010-7940(98)00084-0. [DOI] [PubMed] [Google Scholar]
- 9.Fahmy Elnoamany M, Abdelraouf Dawood A. Right ventricular myocardial isovolumic relaxation time as novel method for evaluation of pulmonary hypertension: correlation with endothelin-1 levels. J Am Soc Echocardiogr. 2007;20:462–469. doi: 10.1016/j.echo.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Foroulis CN, Kotoulas CS, Kakouros S, Evangelatos G, Chassapis C, Konstantinou M, Lioulias AG. Study on the late effect of pneumonectomy on right heart pressures using Doppler echocardiography. Eur J Cardiothorac Surg. 2004;26:508–514. doi: 10.1016/j.ejcts.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 11.Fossum TW. Small Animal Surgery. St. Louis: Mosby; 2002. pp. 760–770. [Google Scholar]
- 12.Hawkins EC. Pulmonary parenchymal diseases. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat. 5th ed. Philadelphia: Saunders; 2000. pp. 1061–1088. [Google Scholar]
- 13.Huez S, Vachiéry JL, Unger P, Brimioulle S, Naeije R. Tissue Doppler imaging evaluation of cardiac adaptation to severe pulmonary hypertension. Am J Cardiol. 2007;100:1473–1478. doi: 10.1016/j.amjcard.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 14.Irino ET, Stopiglia AJ, Larsson MHMA, Guerra JL, Simoes EA, Shinkai MT, Fantoni DT, Otsuki DA, Freitas RR, Saldiva PHN, Jatene FB. Comparative study between manual and mechanical suture of bronchial stamp in dogs submited to left pneumonectomy: histopathological evaluation of right lung and electrocardiographical evaluation. Braz J Vet Res Anim Sci. 2004;41:58–66. [Google Scholar]
- 15.Johnson L, Boon J, Orton EC. Clinical characteristics of 53 dogs with Doppler-derived evidence of pulmonary hypertension: 1992-1996. J Vet Intern Med. 1999;13:440–447. doi: 10.1892/0891-6640(1999)013<0440:ccodwd>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Kittleson MD, Kienle RD. Small Animal Cardiovascular Medicine. St. Louis: Mosby; 1998. pp. 433–449. [Google Scholar]
- 17.Laaban JP, Diebold B, Zelinski R, Lafay M, Raffoul H, Rochemaure J. Noninvasive estimation of systolic pulmonary artery pressure using Doppler echocardiography in patients with chronic obstructive pulmonary disease. Chest. 1989;96:1258–1262. doi: 10.1378/chest.96.6.1258. [DOI] [PubMed] [Google Scholar]
- 18.Lansdowne JL, Monnet E, Twedt DC, Dernell WS. Thoracoscopic lung lobectomy for treatment of lung tumors in dogs. Vet Surg. 2005;34:530–535. doi: 10.1111/j.1532-950X.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- 19.Liptak JM, Monnet E, Dernell WS, Rizzo SA, Withrow SJ. Pneumonectomy: four case studies and a comparative review. J Small Anim Pract. 2004;45:441–447. doi: 10.1111/j.1748-5827.2004.tb00262.x. [DOI] [PubMed] [Google Scholar]
- 20.Miller MS, Tilley LP, Smith FWK, Fox PR. Electrocardiography. In: Fox PR, Sisson D, Moïse NS, editors. Textbook of Canine and Feline Cardiology: Principles and Clinical Practice. Philadelphia: Saunders; 1999. pp. 67–105. [Google Scholar]
- 21.Moise NS, Fox PR. Echocardiography. In: Fox PR, Sisson D, Moïse NS, editors. Textbook of Canine and Feline Cardiology: Principles and Clinical Practice. Philadelphia: Saunders; 1999. pp. 130–171. [Google Scholar]
- 22.Murphy ST, Ellison GW, Mckiernan BC, Mathews KG, Kubilis PS. Pulmonary lobectomy in the management of pneumonia in dogs: 59 cases (1972-1994) J Am Vet Med Assoc. 1997;210:235–239. [PubMed] [Google Scholar]
- 23.Neath PJ, Brockman DJ, King LG. Lung lobe torsion in dogs: 22 cases (1981-1999) J Am Vet Med Assoc. 2000;217:1041–1044. doi: 10.2460/javma.2000.217.1041. [DOI] [PubMed] [Google Scholar]
- 24.Nelson AW, Monnet E. Lungs. In: Slatter DH, editor. Textbook of Small Animal Surgery. 3rd ed. Philadelphia: Saunders; 2003. pp. 880–889. [Google Scholar]
- 25.Okada M, Ota T, Okada M, Matsuda H, Okada K, Ishii N. Right ventricular dysfunction after major pulmonary resection. J Thorac Cardiovasc Surg. 1994;108:503–511. [PubMed] [Google Scholar]
- 26.Reed CE, Dorman BH, Spinale FG. Mechanisms of right ventricular dysfunction after pulmonary resection. Ann Thorac Surg. 1996;62:225–232. doi: 10.1016/0003-4975(96)00258-5. [DOI] [PubMed] [Google Scholar]
- 27.Reed CE, Spinale FG, Crawford FA., Jr Effect of pulmonary resection on right ventricular function. Ann Thorac Surg. 1992;53:578–582. doi: 10.1016/0003-4975(92)90314-t. [DOI] [PubMed] [Google Scholar]
- 28.Rena O, Papalia E, Oliaro A, Casadio C, Ruffini E, Filosso PL, Sacerdote C, Maggi G. Supraventricular arrhythmias after resection surgery of the lung. Eur J Cardiothorac Surg. 2001;20:688–693. doi: 10.1016/s1010-7940(01)00890-9. [DOI] [PubMed] [Google Scholar]
- 29.Rubin LJ. Approach to the diagnosis and treatment of pulmonary hypertension. Chest. 1989;96:659–664. doi: 10.1378/chest.96.3.659. [DOI] [PubMed] [Google Scholar]
- 30.Salci H, Kennerman E, Celimli N, Torun S. Use of a Heimlich flutter valve in a dog with spontaneous pneumothorax. Aust Vet Pract. 2005;35:47–51. [Google Scholar]
- 31.Sasaki Y, Kitagawa H, Hirano Y. Relationship between pulmonary arterial pressure and lesions in the pulmonary arteries and parenchyma, and cardiac valves in canine dirofilariasis. J Vet Med Sci. 1992;54:739–744. doi: 10.1292/jvms.54.739. [DOI] [PubMed] [Google Scholar]
- 32.Schober KE, Baade H. Doppler echocardiographic prediction of pulmonary hypertension in West Highland white terriers with chronic pulmonary disease. J Vet Intern Med. 2006;20:912–920. doi: 10.1892/0891-6640(2006)20[912:depoph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Serres FJ, Chetboul V, Tissier R, Carlos Sampedrano C, Gouni V, Nicolle AP, Pouchelon JL. Doppler echocardiography-derived evidence of pulmonary arterial hypertension in dogs with degenerative mitral valve disease: 86 cases (2001-2005) J Am Vet Med Assoc. 2006;229:1772–1778. doi: 10.2460/javma.229.11.1772. [DOI] [PubMed] [Google Scholar]
- 34.Shields TW, Locicero J, Ponn RB, Rusch VW. General Thoracic Surgery. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 564–592. [Google Scholar]
- 35.Tramarin R, Torbicki A, Marchandise B, Laaban JP, Morpurgo M. Doppler echocardiographic evaluation of pulmonary artery pressure in chronic obstructive pulmonary disease. A European multicentre study. Eur Heart J. 1991;12:103–111. doi: 10.1093/oxfordjournals.eurheartj.a059855. [DOI] [PubMed] [Google Scholar]
- 36.Uehara Y. An attempt to estimate the pulmonary artery pressure in dogs by means of pulsed Doppler echocardiography. J Vet Med Sci. 1993;55:307–312. doi: 10.1292/jvms.55.307. [DOI] [PubMed] [Google Scholar]
- 37.Yano T, Yokoyama H, Fukuyama Y, Takai E, Mizutani K, Ichinose Y. The current status of postoperative complications and risk factors after a pulmonary resection for primary lung cancer. A multivariate analysis. Eur J Cardiothorac Surg. 1997;11:445–449. doi: 10.1016/s1010-7940(96)01097-4. [DOI] [PubMed] [Google Scholar]
- 38.Yilmaz Z, Senturk S. Characterisation of lipid profiles in dogs with parvoviral enteritis. J Small Anim Pract. 2007;48:643–650. doi: 10.1111/j.1748-5827.2007.00391.x. [DOI] [PubMed] [Google Scholar]
- 39.Yu CM, Sanderson JE, Chan S, Yeung L, Hung YT, Woo KS. Right ventricular diastolic dysfunction in heart failure. Circulation. 1996;93:1509–1514. doi: 10.1161/01.cir.93.8.1509. [DOI] [PubMed] [Google Scholar]