Abstract
Bone marrow colony formation in soft gel culture may be stimulated by substances elaborated by human peripheral blood leukocytes. In order to determine the cell type responsible for colony stimulation, peripheral leukocytes were separated by Ficoll-Hypaque gradients and differential glass adhesion. Morphologic, histochemical, and functional criteria were applied to determine the purity of the monocyte, lymphocyte, and neutrophil fractions.
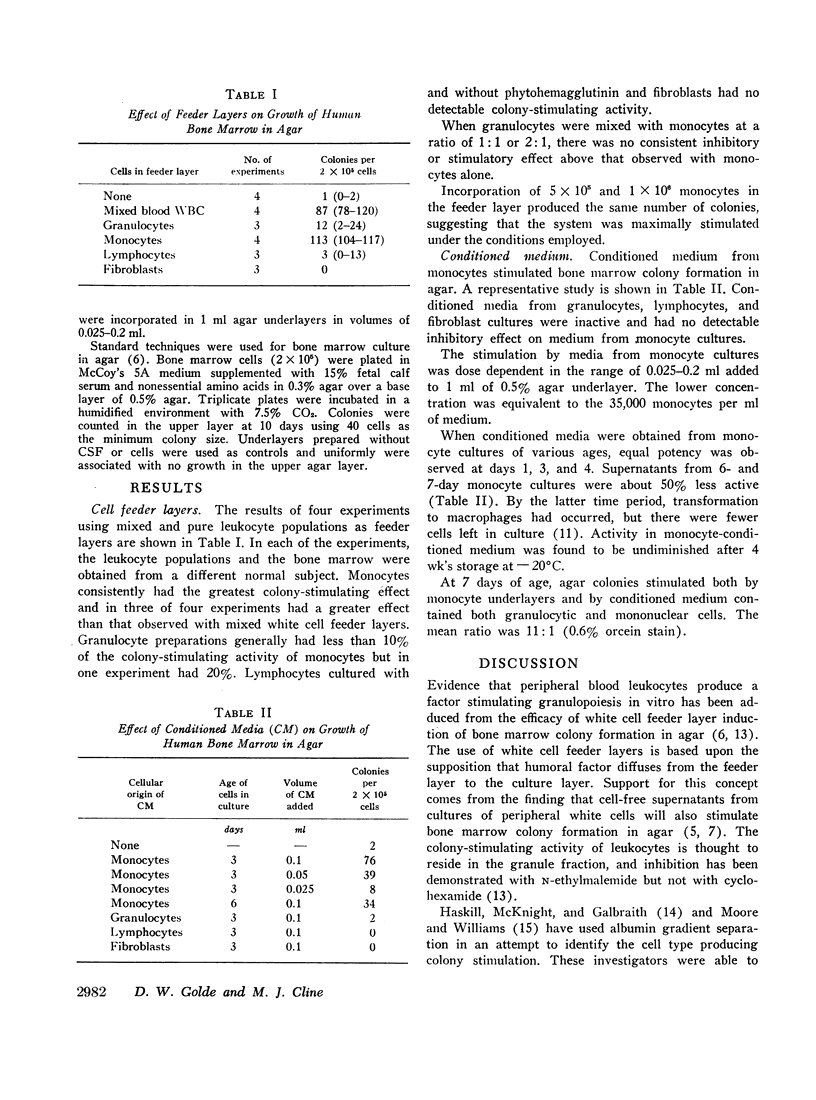
Using these cells as feeder layers and as a source of conditioned medium, evidence was obtained that the monocyte is the colony-stimulating cell of human peripheral blood. Activity greater than that of mixed white cells was obtained with monocyte underlayers, and only monocyte- and macrophage-conditioned media were shown to have significant colony-stimulating activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan S. H., Metcalf D. Inhibition of bone marrow colony formation by normal and leukaemic human serum. Nature. 1970 Aug 22;227(5260):845–846. doi: 10.1038/227845a0. [DOI] [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R. Bone marrow colonies: stimulation in vitro by supernatant from incubated human blood cells. Science. 1970 Aug 14;169(3946):691–692. doi: 10.1126/science.169.3946.691. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Sumner M. A. Bone marrow macrophage precursors. I. Some functional characteristics of the early cells of the mouse macrophage series. Blood. 1972 Jul;40(1):62–69. [PubMed] [Google Scholar]
- Epstein L. B., Cline M. J., Merigan T. C. The interaction of human macrophages and lymphocytes in the phytohemagglutinin-stimulated production of interferon. J Clin Invest. 1971 Apr;50(4):744–753. doi: 10.1172/JCI106545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALLON H. J., FREI E., 3rd, DAVIDSON J. D., TRIER J. S., BURK D. Leukocyte preparations from human blood: evaluation of their morphologic and metabolic state. J Lab Clin Med. 1962 May;59:779–791. [PubMed] [Google Scholar]
- Hanifin J. M., Cline M. J. Human monocytes and macrophages. Interaction with antigen and lymphocytes. J Cell Biol. 1970 Jul;46(1):97–105. doi: 10.1083/jcb.46.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Senn J. S., Till J. E., McCulloch E. A. Colony formation by normal and leukemic human marrow cells in culture: effect of conditioned medium from human leukocytes. Blood. 1971 Jan;37(1):1–5. [PubMed] [Google Scholar]
- Metcalf D. The nature of leukaemia: neoplasm or disorder of haemopoietic regulation? Med J Aust. 1971 Oct 9;2(15):739–746. [PubMed] [Google Scholar]
- Pike B. L., Robinson W. A. Human bone marrow colony growth in agar-gel. J Cell Physiol. 1970 Aug;76(1):77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Metcalf D. Enzyme treatment of colony stimulating factor: evidence for a peptide component. Aust J Exp Biol Med Sci. 1971 Jun;49(3):281–290. doi: 10.1038/icb.1971.28. [DOI] [PubMed] [Google Scholar]


