Abstract
Good adherence is critical for antiretroviral therapy (ART) in sub-Saharan Africa. We report on the characteristics of medicine companions (MCs) chosen by Ugandan patients enrolling on ART, and on how MCs were chosen, and what roles they played. Baseline data on MCs of 1453 participants in a randomized controlled trial comparing facility and home-based delivery of ART in Jinja, Uganda were analyzed. Textual data on experience with MCs were collected through in-depth interviews among a subsample of 40 trial participants equally divided by sex and trial arm. Significantly more women (71%) than men (29%) were recruited. The majority (75%) of women participants were either widowed (51%) or separated or divorced (24%), whereas most of the men (66%) were married. Women were most likely to choose a child as their MC while men were most likely to choose their spouse; 41% of women chose an MC under 21 compared with only 14% of men. Only 31% of married women chose their husband, compared with 66% of married men who chose their wife. Qualitative interviews suggested MCs proved useful for reminding and other supportive tasks in the first three months but were generally less essential by six months and beyond. Convenience, reliability, and trust were key considerations in choosing an MC. Children provided the only alternative for many unmarried women, but even some married women felt children made more reliable MCs than husbands. Participants who had disclosed their serostatus usually received drug-taking reminders from multiple household members. One participant in the qualitative sample with poor family relations delayed starting treatment due to unwillingness to identify an MC. MCs were generally welcome and useful in supporting early adherence. However, disclosure to an MC should not be a condition of obtaining treatment.
Keywords: ART delivery, medicine companion, adherence, stigma, disclosure
Introduction
Antiretroviral therapy (ART) is rapidly being scaled up to meet the needs of patients with HIV, particularly in Africa. A main feature of some programs is the requirement or strong suggestion for patients to choose a “medicine companion” (MC) to provide support and reminders for ART patients to take their medications consistently and on schedule (Médecins sans frontières (MSF), Department of Public Health of the University of Cape Town, and The Provincial Administration of the Western Cape. Published by WHO, Geneva, 2003). Directly observed treatment for tuberculosis infection (DOTS), the WHO standard recommended therapy since 1993, usually involves observation of the dose by a health professional, despite doubts expressed about the effectiveness and impact of the direct observation of therapy, (Cox, Morrow, & Deutschmann, 2008; Davies & Squire, 2008). DOTS usually requires that the patient travel to a place where the treatment can be observed, whereas MCs for ART often involve non-health professionals, typically family members, who support the therapy in the home setting. More importantly, the MC model requires an act of disclosure for an illness which is still highly stigmatized in some settings. There is little scientific evidence evaluating the demographic and social characteristics of MCs, how they are chosen, and whether and for how long they are perceived to be useful in supporting adherence. This paper reports on the baseline characteristics of MCs of 1453 Ugandan trial participants receiving ART from an nongovernmental organization (NGO) that had a routine policy of requiring MCs. It also documents the experience with MCs of a small subset of 40 trial participants who were interviewed several times over the first 36 months on ART.
Methods
Methods of the clinical trial have been described in detail (Amuron et al., 2007). The goal of the trial was to evaluate the effectiveness as well as the costs of home-based (HB) ART care as compared with facility-based (FB) ART care. All ART provision and services are being carried out by The AIDS Support Organization (TASO) Clinic in Jinja, southeast Uganda, in a real-life health care setting. TASO is a large non-governmental organization with 11 centers in Uganda offering counseling, out-patient medical care, and a range of social support services to HIV-infected patients. TASO also conducts counselor training, AIDS advocacy and community outreach, and has been a global pioneer in AIDS care (Ssebbanja, 2007).
Registration and care are provided for free in this setting. TASO started providing ART in early 2004 and recruitment of participants into the trial began in February 2005. Follow-up ended in 2008. Patients were assessed for eligibility by TASO staff, their WHO HIV stage was determined, and a CD4 count and viral load obtained. If CD4 count was below the eligibility threshold of 200 × 106/1, or their WHO HIV stage was determined to be late Stage III or IV, they were offered ART in accordance with Ministry of Health guidelines. Patients were then randomized to either HB or FB care, using cluster randomization. Clusters were defined as geographical areas—a sub-county, town, village, or part of Jinja town. Fortyfour such areas were defined and randomized in strata according to the estimated number of HIV-infected patients and the distance from TASO Jinja.
Patients receiving HB care are visited monthly by a field officer on a motorcycle who delivers drugs and monitors the patients using a checklist. Field officers have mobile phones and can discuss a case with a physician at TASO if necessary, and determine whether referral to the clinic is necessary. HB care patients visit the TASO clinic every six months for a routine clinical and counseling review. FB patients visit the clinic every month to collect drugs and be seen by a nurse; they are assessed routinely every three months by a counselor and physician. Patients in both arms can visit the TASO clinic any time they feel unwell, and they have access to a telephone hotline. In the FB arm, patients eligible for ART were given Voluntary Testing and Counseling (VCT) vouchers for each family member and for the MC, and were encouraged to bring them to the facility for free VCT. In the HB arm, ART patients were visited by TASO field officers and VCT was provided at their homes to household members and to the MC if requested.
All patients are requested to appoint a MC to assist them in remembering to take their medicines and to encourage them when they are not feeling well (Amuron et al., 2007). MCs were also supposed to assist by picking up the patient's drugs if the TASO team could not reach them in time. Of note, however, is that direct observation of therapy is not expected of the MC. In the FB arm, eligible patients were asked to come along with their MCs on their enrollment visit. This was a condition for ART initiation. The MCs were provided with counseling and ART adherence information on how to support their patients. A flat fee of UGX 2500 (approximately $1.50) was provided to compensate their cost of transport. In the HB arm, patients identified the MCs when the field officers visited their homes before the enrollment visit. Adherence support information was then given at this visit. This home visit was a condition for ART initiation. The MCs of FB patients were interviewed directly. Information on the MCs of HB patients was obtained from the patients themselves.
A complementary qualitative study was designed to chronicle life changes and evolving challenges of adherence over the first three years on ART using qualitative methods among a subsample of trial participants. A subset of 41 patients (21 men and 20 women) were chosen and 40 were interviewed in-depth at baseline (at the final enrollment visit before patients had started medication), at three and six months, and at 36 months after initiation of treatment. One man was recruited but died soon after recruitment and was replaced so the final number was 40; not all patients were available for interview at all rounds. The qualitative study sample was stratified to ensure equal numbers of participants by sex, trial arm, and clinical/immunological stage contrasting early (CD4 counts above 150 × 106/1 or Stages I and II defining conditions) and advanced (CD4 counts below 100 × 106/1 or Stage III and IV defining conditions) categories. The patients were asked about the role of their MC, how they chose their MC, and how helpful the MC was in their therapy.
Quantitative data were analyzed using Stata v. 9 (Stata Corp., College Station, TX, USA) and Microsoft Excel (Microsoft Corp., Redmond, WA, USA). Textual data on rationales and experiences with MCs were transcribed, translated, and coded using NVivo v.7 (QSR International, Melbourne, Australia) and analyzed by two independent reviewers. This paper reports on the data collected at baseline on the characteristics of MCs in both arms of the study, and on the qualitative data about MCs collected through in-depth interviews at baseline, months 3, 6, and 36 (no questions on the MC were asked at the 18th month interview). Ethical approval was given by the Uganda National Council of Science and Technology, and the Institutional Review Boards of the Uganda Virus Research Institute, the US Centers for Disease Control, and the London School of Hygiene & Tropical Medicine.
Results: baseline characteristics of the patients and of their chosen “medicine companion” (MC)
Significantly more women (71%) than men (29%) were recruited into the trial, and both men and women presented at an advanced stage with very low median CD4 count and high plasma viral load (Amuron et al., 2007). Women patients had a mean age of 36 and men, 40. The mean age of the MCs chosen is 30.8 years and the median is 30 (33.8 for men, 29.5 for women, p = 0.000), but the mode is 14 (Table 1 and Figure 1). The median age of children under 18 who act as MC was 14 for male patients and 13 for female patients. It is notable that 41.4% of women chose an MC under 21 compared with only 14% of men. Two of the MCs are reported to be five years old, and 11% or 160 of MCs are 12 years of age or under. The majority (75%) of women were either widowed (51%) or separated or divorced (24%), whereas most of the men (66%) were married (Amuron et al., 2007). Only 23% of the women are currently married, and of these, only 31% chose their husband as MC, compared with 66% of men who chose their wife (p = 0.000) (Table 1). Women, including the widowed women, were most likely to choose a child or other relative or household member. Of those who were accompanied to TASO, the MC was the accompanying person in 90% of cases. The median cost of transport for the accompanying person was 2000 USh (approximately US$1.15) (mean of 2888 USh, US$1.65).1 The mean transport cost reported by patients was 3000 USh, and these lower transport costs for the MC reflects the fact that many of the patients have chosen their children as their MC, and children under about age 12 (or able to sit on the lap of the adult) benefit from a lower fare on public or shared private transport.
Table 1.
Characteristics of medicine companions of patients on antiretroviral therapy.
| Men | Women | All | ||
| Choice of medicine companion: all patients, n = 1453 | ||||
| Spouse/partner | 186 (44) | 75 (7) | 261 (18) | P <0.001 |
| Biological child | 64 (15) | 409 (40) | 473 (33) | |
| Other relative/HH member | 154 (37) | 497 (48) | 651 (45) | |
| Neighbor/friend | 14 (3) | 47 (5) | 61 (4) | |
| Other | 4(1) | 3 (0) | 7 (0) | |
| Total | 422 | 1031 | 1453 | |
| Age of medicine companion: all patients, n = 1453 | ||||
| Under 21 | 60 (14) | 427 (41) | 487 (33) | P <0.001 |
| 21-30 | 127 (30) | 175 (17) | 302 (21) | |
| 31-40 | 137 (33) | 168 (16) | 305 (21) | |
| 41-50 | 43 (10) | 134 (13) | 177 (12) | |
| 51-65 | 31 (7) | 67 (7) | 98 (7) | |
| Over 65 | 10 (2) | 36 (3) | 46 (3) | |
| Missing | 14 (3) | 24 (2) | 38 (3) | |
| Occupation of medicine companion: n = 750a | ||||
| Business/self employed | 50 (23) | 84 (16) | 134 (18) | P <0.001 |
| Professional | 26 (12) | 39 (7) | 65 (9) | |
| Student | 35 (16) | 200 (38) | 235 (31) | |
| Farmer | 60 (27) | 106 (20) | 166 (22) | |
| Unemployed | 17 (8) | 63 (12) | 80 (11) | |
| Housewife | 30 (14) | 22 (4) | 52 (7) | |
| Other | 1 (0) | 17(3) | 18 (2) | |
| HIV serostatus of medicine companionb: all patients, n = 1453 | ||||
| Positive | 112 (27) | 103 (10) | 215 (15) | P <0.001 |
| Negative | 144 (34) | 457 (44) | 601 (41) | |
| Don't know | 165 (39) | 472 (46) | 637 (44) | |
| Choice of medicine companion among married patients: n = 516 | ||||
| Spouse/partner | 185 (66) | 73 (31) | 258 (50) | P <0.001 |
| Biological child | 32 (11) | 85 (36) | 117 (23) | |
| Other relative/HH member | 55 (20) | 64 (27) | 119 (23) | |
| Neighbor/friend | 5 (2) | 15(6) | 20 (4) | |
| Other | 2 (1) | 0 (0) | 2 (0) |
aIt includes only medicine companions of facility-based patients who accompanied patients to TASO.
bIt is as reported by patient.
Figure 1.
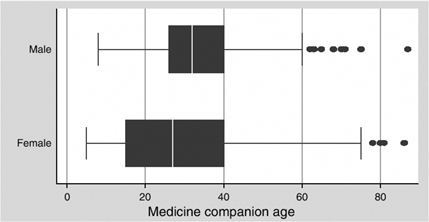
Age of medicine companions chosen, by gender of patient.
The occupation of the MCs who accompanied the (FB) patients (note that about half of the patients—those in the HB arm - came unaccompanied) are shown in Table 1. “Student” was the most frequently reported occupation (31%), again reflecting the importance of children as MCs, followed by “farmer” (22%), and “business or self-employed” (18%, p = 0.000).
Patients were asked about the HIV status of their MC. In most cases (44%) patients reported that they did not know (or perhaps were not willing to disclose) the HIV status of their MC. Of those who did report the status, 15% reported that their MC was HIV positive, but there were significant differences between men and women in this regard, with 27% of men but only 10% of women reporting that their MC was HIV positive (p <0.05). Among the married patients, 55% of wives, and 73% of husbands chosen as MC were HIV positive. 16% of women and 20% of men who chose their spouse as MC reported not knowing that person's HIV status (Table 1). Thirty percent of HIV-positive MCs were reported to be on ART by the patient. Nine of the children under 18 who are MC were reported to be HIV positive by the patient, and two of them were reported to be on ART.
Role of the medicine companion (MC) in assisting patients with adherence: data from qualitative interviews
At baseline patients gave a variety of reasons for their choice of MC, including that he/she would give financial support; that person would help with house chores, e.g., cooking, washing, etc.; the person would always be around when needed or was someone they lived with; the person was also on antiretroviral drugs and/or was a TASO patient and so could easily remember; and finally, that the person cared about their well-being.
As noted above, most of the men chose their wife, and one male patient's response about how he chose his wife as his MC is typical of the reactions of the men in particular: “She is the one I stay with and besides, she is also on drugs and she too chose me as her medicine companion, so, each reminds the other.” On the other hand, women tended to choose their children or other immediate family like their mother, father, sister, nephew, or aunt. Since many of the women are widowed, separated, or divorced, the children were the people they lived with. They claimed that children were home most of the time; they could be trusted and also they cared about the health of their mother. Respondents did not mention any concerns or discomfort about having children as their MCs, or any misgivings about the responsibility they were entrusting to their children. One woman responded as follows: “My mother is now old; I can't trust my brother more than the kids. Even his wife … You never know, he could also be sick and [she could] decide to steal the what, the drugs.” Another woman noted that “As she is a child, she feels obliged and concern for her mother and so has to remind me.” One male respondent also chose his son: “I live with my children in the same house and the elder son is in senior four, I wanted him to know the problems he would face in case I died at this moment now that he is the first-born child.”
Only one woman in the sub-sample had a problem with disclosing her status, claiming that she didn't trust anyone, even her family. She refused to present an MC and neither did she allow TASO officers to visit her home as was required for all HB patients before they were started on drugs. This delayed her getting on ART and she started a month later, but this was after she had been convinced by the counselor who was following her up to choose an MC, and to be visited at home. She described the situation as follows: “I don't have any one, I am alone. And I even told [her counselor] that I keep my things to myself and if I hadn't thought that I could reach a time whereby I couldn't manage on my own [if I thought I could continue to manage by myself], I wouldn't have told that niece. They would have known about it from the documents that I had HIV/AIDS and was getting treatment at TASO. Now that I never had children, my relatives turned against me and isolated me and it was after seeing that I had acquired property [riches] that they wanted to come back to me but I had already had enough.”
At three months, 29 patients were again interviewed (at this round, only 29 were interviewed because four had died, and eight weren't interviewed for other reasons), and most still felt they needed the support of their MCs. One man whose wife (his MC) had left him was asked “How do you manage now that you don't have a medicine companion?” He responded that “I am now depending on the watch alone to remind me, it's what I look at and know that it's time for taking drugs.”
At six months, 36 patients were interviewed, and by this time most said they had become used to taking the drugs and thus could manage on their own even without this MC, and that the MC was only necessary in the early stages of ART when they were becoming used to the drugs and were still weak and had more illnesses. Some MCs continued to do their work though some patients felt that it was not very necessary as the drug companion could also forget. Drug taking had become routine for them: “Eeh, how would I forget my life, ah, and wait to be reminded!”
In contrast, one female patient was lonely and missed having an MC (her mother), and said “I have faced many problems and it is the reason why I sometimes miss my drugs because I have no one to tell my needs yet she was there before and she could plan what to eat. I now sit back and think of what to do in vain, and by the time I realize, the time for drug taking has already gone.”
At 36 months, 33 patients were interviewed at the final round. By this time, dependency on MCs had greatly reduced and all but one said they were very used to taking their drugs and so could manage on their own. Most of the men, even those who weren't married, said a “wife” would make the best drug companion “because you are together all the time, she is the one that takes care of you and she knows your health.” Women on the other hand said children were best, because they cared and were home most of the time. Only 3 of the 16 women who were interviewed at the final round said a spouse would make the ideal MC. By this time many of the household members or family had become involved in the role of reminding the patient to take their medicines. One patient commented as follows:
Even my sister in-law reminds me and even her husband, and besides, there is a radio at home [to indicate the time]. All the children here know about it and they also tell me when they see that it's time [for taking drugs], “take your drugs Aunt.” There is one who is six years but she tells me “don't forget the drugs, Aunt.”
Sometimes the situation involves multiple family members. One woman was initially staying with her father who is her MC. At the six month follow-up visit, she had rented a room about 300 meters from the father. She had a new partner and one day she found him examining the drugs, and she snatched them from him and ran out with them. Her maternal aunt who lived next door advised her not to store the drugs in the house again but keep them at the aunt's place, and that is where she goes to take the drugs. Her father still stores the drugs; he gives her drugs for few days and refills her stocks whenever they get finished. So her drugs are kept by two people—her father who replenishes them regularly, and her aunt who lives next door.
Discussion
The main rationale for requiring an MC—which thus entails disclosure of one's HIV status—was that the support provided by the MC would lead to improved adherence. This study did not measure the impact of the MC on outcomes (all patients had MCs), but it nonetheless documents the main characteristics and changing role of the MCs as the patients got accustomed to being on lifelong ART. Unlike some other programs (Behforouz, Farmer, & Mukherjee, 2004; Farmer et al., 2001), direct observation of therapy was not stressed in this model of support. There are many factors which can affect adherence; a study on 304 patients of three programs in Uganda (Byakika-Tusiime et al., 2005) found that “Age, gender, education, religions, treatment duration, dosing interval, pill burden, drug and alcohol consumption, confidence in ART, distance from treatment centre, cost of medications, depression, social support and number of concurrent conditions did not predict adherence.” The main predictor of adherence in their study was shortage of drugs due to lack of money. Fear of disclosure has been identified as a major barrier to adherence (Mills et al., 2006). External factors can also play an important role in adherence; in one Ugandan study, 1.2% of patients became convinced by religious authorities that they had been “spiritually healed” and discontinued their therapy (Wanyama et al., 2007).
Somewhat surprisingly, only one other similar program was found in the literature in which the patients themselves select an MC – the Khayelitsha program in South Africa, which requires patients to choose, and disclose their HIV status, to a “treatment assistant.” They describe the process as follows: “After the patient has been counselled about ARV treatment, a clinic worker assesses the social and support structures available by conducting a home visit. The home visit also verifies the person's family environment and disclosure to at least one person who will act as a treatment assistant” (Mèdecins sans frontiéres (MSF) et al., 2003). That person “is usually someone living in the household, aware of the person's status and willing to assist with medication as necessary.” No mention is made of specifically observing therapy.
In two other cases, programs focus on directly observed therapy and assign a person to assist the patient in taking their medicines. In Haiti, a program of ART provision relied heavily on “accompagnateurs,” community health promoters, but these were appointed by the health staff rather than chosen by the patients themselves. Their role was to “accompany” the patients by directly observing therapy and providing other types of assistance (Farmer et al., 2001). Another study examined the feasibility of integrating ART into the tuberculosis DOT programme (Friedland et al., 2004), and refers to the need for “treatment supporters” who are appointed by the health services rather than chosen by the patient.
Adherence and outcomes in this group of TRIAL patients were very good in comparison with other studies. Although the study was carried out within a normal resource-constrained health service setting (Amuron et al., 2007), the mortality rates of about six deaths per 100-person-years and virologic failure rates of about eight per 100-person-years were better than those reported from most other settings (Jaffar et al., 2009). Others have reported mortality rates ranging between 6 and 15 deaths per 100-person years (Coetzee et al., 2004; Etard et al., 2006; Ferradini et al., 2006; Marazzi et al., 2008; Mermin et al., 2008; Stringer et al., 2006; Toure et al., 2008) and virologie failure from 15 to 40% (Braitstein et al., 2006; Coetzee et al., 2004; Fielding et al., 2008; Laurent et al., 2002; Vicente et al., 2006). However, some caution as to the possible role played by the MC in achieving these results is warranted; since virtually all patients had MCs it is not possible to distinguish between those who had and those who did not have MCs in achieving these outcomes. Other factors such as the intensive counseling and support provided by TASO staff might have also been important in these outcomes.
There were differences between men and women in terms of who they chose as their MC, and why. In many ART programs in sub-Saharan Africa, there are many more women than men among patients on ART therapy; in this trial, 71% of the patients were women. Only 25% of these women were married, as compared with 66% of the men. Married men were more than twice as likely to choose their wife as MC as married women (66% vs 31%), and in both cases the spouse who was chosen as MC was likely to be HIV positive (55% of wives, and 73% of husbands chosen as MC were HIV positive). It seems likely that some of the married women did not choose their husband as MC because they had not yet disclosed their status to their husband and were comfortable with choosing husbands as MC only if the husband already knew the woman's HIV status. Disclosure of HIV status to the husband or partner in Uganda and elsewhere in Africa often has particularly severe consequences for women, including domestic violence, abandonment, and divorce (Karamagi, Tumwine, Tylleskar, & Heggenhougen, 2006; Koenig et al., 2003). For the men, if they did not have a spouse to whom they were willing to disclose, the idea of being required to disclose their status to another relative or friend may have been a deterrent (as evidenced by the small percentage of men (29%) in the trial) to obtaining care at TASO where disclosure is strongly promoted, and may be interpreted by some as essentially mandatory. There are other sources of ART in Jinja which do not require or strongly encourage disclosure. Men may also have chosen their wives knowing that culturally the expectation is that a woman has a duty to look after her husband, no matter what. Women on the other hand seem to have more confidence in their children than in their husband in terms of who would look after them in their illness, rather than punish them for disclosing their HIV status. Hardon et al. (2007) found in a study of ART adherence in three African countries (including Uganda) that in all three, children were involved in reminding patients to take their medicines and they recommend that “adherence support could recognize the potential role of children … and provide them with adequate information on ART … to empower them in their role as treatment supporters” (Hardon et al., 2007).
The relationship between an MC and patient is one of mutual obligation; Ware et al. (2009) explain that the “social expectations of adherence create obligations on the part of patients, who must meet these expectations to preserve relationships with helpers.” Adherence “allows patients to meet social responsibilities by preventing health decline and reducing the need for support”(Ware et al., 2009). They explain that the “social expectations of adherence create obligations on the part of patients, who must meet these expectations to preserve relationships with helpers” (Ware et al., 2009). Adherence “allows patients to meet social responsibilities by preventing health decline and reducing the need for support” (Ware et al., 2009).
In this study patients found the MC to be very useful at the beginning of the treatment, but as time passed they became more autonomous and relied less on their MC. In a South African study, one female patient complained “my treatment supporter is overwhelming me … I needed him when I was very sick … but now I am feeling well and I need him to back off a little bit … to give me some space so I can be in charge with my life again” (Nachega et al., 2006).
The requirement to choose an MC raises a number of important questions, especially regarding what might be termed the “the culture of disclosure.” In this group, only 41 (3%) of participants refused to join, or left the trial early, due to stigma (Jaffar et al., 2009); however, the group might be comprised primarily of those who had opted to seek care at TASO despite knowing that disclosure of their HIV status would be expected of them. If the support provided by an MC is valuable to some patients, it comes with an important trade-off—the loss of confidentiality and autonomy for the patient. Doubts about the advisability of requiring disclosure to an MC or other individual have been expressed by a number of authors. Liechty and Bangsberg (2003) observe that “the program [in Haiti] has demonstrated remarkable success. However, it is impossible to know how much of the effect is due to the merits of community driven and supportive healthcare, stable medication supply and distribution, the medication alone, or witnessed dosing” (Liechty & Bangsberg, 2003). They stress that “witnessed dosing of HIV antiretroviral therapy is complicated by the need for lifelong treatment, daily medication dosing, and the consequent impact on individual rights in the setting of a highly stigmatizing disease.” Finally, they note that “we still must face the question of why calls for directly observed HIV therapy are limited to sub-Saharan Africa, and not North America or Western Europe” (Liechty & Bangsberg, 2003). A study involving focus groups with rural American HIV patients noted that “Some participants … appeared to be more willing to risk their health than risk people learning of their medical condition” (Whetten-Goldstein, Nguyen, & Sugarman, 2001). Could this be the case with certain categories of HIV-positive African patients as well? Disclosure can lead to stigma, and “stigma is feared because it leads to social isolation, undermining relationships that are essential to survival” (Ware et al., 2009).
Conclusions
This paper has tried to answer the basic question of who is chosen as an MC in this Ugandan setting, and how useful they are perceived to be by the patients, while raising a number of additional questions. What is the role of the MC, and how well do they understand this role? How well prepared are they to support the lifelong therapy of the patient? If the patient fails therapy, will the MC feel that they have failed the patient? Will they be blamed by the patient or someone else in the family? What of the responsibility given to a child who is designated as an MC by his or her parent? What is the role and importance of disclosure of HIV status in successful adherence? Perhaps most importantly, is the requirement of disclosure discouraging many who need and are eligible for ART, men in particular, from coming forward?
Our study supports the notion that treatment companions are useful at the start of treatment, but also that after a while, most people no longer need them. Living in a supportive home environment where everyone knows the person's HIV status, and reminds them to take their medicines, may be the optimal form of support for adherence. But disclosure and selection of an MC should be encouraged—not required—because it does mandate an act of disclosure and may delay some people from getting on treatment, both of which may raise ethical concerns, especially in a model where the level of support offered to patients might be less than that provided to TASO patients. These considerations should form the basis for additional research before the model of requiring the selection of an medicine companion is adopted as a standard for ART programs around Africa.
Note
These amounts can be compared with the monthly incomes reported by patients of 15,000 USh for women and 20,000 USh for men, or approximately US$8.60 and US$11.50, respectively.
References
- Amuron B., Coutinho A., Grosskurth H., Nabiryo C., Birungi J., Namara G., et al. A cluster-randomised trial to compare home-based with health facility-based antiretroviral treatment in Uganda: Study design and baseline findings. Open AIDS Journal. 2007;1:21–27. doi: 10.2174/1874613600701010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behforouz H.L., Farmer P.E., Mukherjee J.S. From directly observed therapy to accompagnateurs: Enhancing AIDS treatment outcomes in Haiti and in Boston. Clinical Infectious Diseases. 2004;38(s5):S429–S436. doi: 10.1086/421408. [DOI] [PubMed] [Google Scholar]
- Braitstein P., Brinkhof M.W., Dabis F., Schechter M., Boulle A., Miotti P., et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: Comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- Byakika-Tusiime J., Oyugi J.H., Tumwikirize W.A., Katabira E.T., Mugyenyi P.N., Bangsberg D.R. Adherence to HIV antiretroviral therapy in HIV + Ugandan patients purchasing therapy. International Journal of STD & AIDS. 2005;16(1):38–41. doi: 10.1258/0956462052932548. [DOI] [PubMed] [Google Scholar]
- Coetzee D., Hildebrand K., Boulle A., Maartens G., Louis F., Labatala V., et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18(6):887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- Cox H.S., Morrow M., Deutschmann P.W. Long term efficacy of DOTS regimens for tuberculosis: Systematic review. British Medical Journal. 2008;336:457–458. doi: 10.1136/bmj.39463.640787.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G.R., Squire S.B. Doubts about DOTS. BMJ. 2008;336(7642):457–458. doi: 10.1136/bmj.39464.485208.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etard J.F., Ndiaye I., Thierry-Mieg M., Gueye N.F., Gueye P.M., Laniece I., et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: A 7-year cohort study. AIDS. 2006;20(8):1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- Farmer P., Leandre F., Mukherjee J., Gupta R., Tarter L., Kim J.Y. Community-based treatment of advanced HIV disease: Introducing DOT-HAART (directly observed therapy with highly active antiretroviral therapy) Bulletin of the World Health Organization. 2001;79(12):1145–1151. [PMC free article] [PubMed] [Google Scholar]
- Ferradini L., Jeannin A., Pinoges L., Izopet J., Odhiambo D., Mankhambo L., et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: An effectiveness assessment. Lancet. 2006;367(9519):1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- Fielding K.L., Charalambous S., Stenson A.L., Pemba L.F., Martin D.J., Wood R., et al. Risk factors for poor virological outcome at 12 months in a workplace-based antiretroviral therapy programme in South Africa: A cohort study. BMC Infectious Diseases. 2008;8:93–100. doi: 10.1186/1471-2334-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland G., Abdool Karim S., Abdool Karim Q., Lalloo U., Jack C., Gandhi N., et al. Utility of tuberculosis directly observed therapy programs as sites for access to and provision of antiretroviral therapy in resource-limited countries. Clinical Infectious Diseases. 2004;38(Suppl. 5):S421–428. doi: 10.1086/421407. [DOI] [PubMed] [Google Scholar]
- Hardon A.P., Akurut D., Comoro C., Ekezie C., Irunde H.F., Gerrits T., et al. Hunger, waiting time and transport costs: Time to confront challenges to ART adherence in Africa. AIDS Care. 2007;19(5):658–665. doi: 10.1080/09540120701244943. [DOI] [PubMed] [Google Scholar]
- Jaffar S., Amuron B., Foster S.D., Birungi J., Levin J., Namara G., et al. Rates of virological failure in patients treated in a home-based versus a facility-based HIV-care model in Jinja, southeast Uganda: A cluster-randomised equivalence trial. The Lancet. 2009;374(9707):2080–2089. doi: 10.1016/S0140-6736(09)61674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamagi C.A.S., Tumwine J.K., Tylleskar T., Heggenhougen K. Intimate partner violence against women in eastern Uganda: Implications for HIV prevention. BMC Public Health. 2006;6(1):284. doi: 10.1186/1471-2458-6-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig M.A., Lutalo T., Zhao F., Nalugoda F., Wabwire-Mangen F., Kiwanuka N., et al. Domestic violence in rural Uganda: Evidence from a community-based study. Bulletin of the World Health Organization. 2003;81:53–60. [PMC free article] [PubMed] [Google Scholar]
- Laurent C., Diakhate N., Gueye N.F., Toure M.A., Sow P.S., Faye M.A., et al. The Senegalese government's highly active antiretroviral therapy initiative: An 18-month follow-up study. AIDS. 2002;16(10):1363–1370. doi: 10.1097/00002030-200207050-00008. [DOI] [PubMed] [Google Scholar]
- Liechty C., Bangsberg D. Doubts about DOT: Antiretroviral therapy for resource-poor countries. AIDS. 2003;17(9):1383–1387. doi: 10.1097/00002030-200306130-00013. [DOI] [PubMed] [Google Scholar]
- Marazzi M.C., Liotta G., Germano P., Guidotti G., Altan A.D., Ceffa S., et al. Excessive early mortality in the first year of treatment in HIV type 1-infected patients initiating antiretroviral therapy in resource-limited settings. AIDS Research and Human Retroviruses. 2008;24(4):555–560. doi: 10.1089/aid.2007.0217. [DOI] [PubMed] [Google Scholar]
- Médecins sans frontière (MSF), Department of Public Health of the University of Cape Town, & The Provincial Administration of the Western Cape. Antiretroviral therapy in primary health care: Experience of the Khayelitsha Programme in South Africa. Geneva: WHO; 2003. [Google Scholar]
- Mermin J., Were W., Ekwaru J.P., Moore D., Downing R., Behumbiize P., et al. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: A prospective cohort study. Lancet. 2008;371(9614):752–759. doi: 10.1016/S0140-6736(08)60345-1. [DOI] [PubMed] [Google Scholar]
- Mills E., Nachega J., Bangsberg D., Singh S., Rachlis B., Wu P., et al. Adherence to HAART: A systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Medicine. 2006;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachega J.B., Knowlton A.R., Deluca A., Schoeman J.H., Watkinson L., Efron A., et al. Treatment supporter to improve adherence to antiretroviral therapy in HIV-infected South African adults. Journal of Acquired Immune Deficiency Syndromes. 2006;43(1):S127–133. doi: 10.1097/01.qai.0000248349.25630.3d. [DOI] [PubMed] [Google Scholar]
- Ssebbanja P.K. United against AIDS: The story of TASO. Oxford and Kampala: Strategies for Hope Trust and The AIDS Support Organization (TASO) 2007.
- Stringer J.S., Zulu I., Levy J., Stringer E.M., Mwango A., Chi B.H., et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: Feasibility and early outcomes. JAMA. 2006;296(7):782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- Toure S., Kouadio B., Seyler C., Traore M., Dakoury-Dogbo N., Duvignac J., et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d'Ivoire: 2-year outcomes and determinants. AIDS. 2008;22(7):873–882. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente M., Hodgson J., Massidda O., Tonjum T., Henriques-Normark B., Ron E.Z. The fallacies of hope: Will we discover new antibiotics to combat pathogenic bacteria in time? FEMS Microbiology Reviews. 2006;30(6):841–852. doi: 10.1111/j.1574-6976.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Wanyama J., Castelnuovo B., Wandera B., Mwebaze P., Kambugu A., Bangsberg D.R., et al. Belief in divine healing can be a barrier to antiretroviral therapy adherence in Uganda. AIDS. 2007;21(11):1486–1487. doi: 10.1097/QAD.0b013e32823ecf7f. [DOI] [PubMed] [Google Scholar]
- Ware N.C., Idoko J., Kaaya S., Biraro I.A., Wyatt M.A., Agbaji O., et al. Explaining adherence success in Sub-Saharan Africa: An ethnographic study. PLoS Medicine. 2009;6:e1000011. doi: 10.1371/journal.pmed.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetten-Goldstein K., Nguyen T.Q., Sugarman J. So much for keeping secrets: The importance of considering patients’ perspectives on maintaining confidentiality. AIDS Care. 2001;13(4):457–466. doi: 10.1080/09540120120057987. [DOI] [PubMed] [Google Scholar]


