Abstract
Three anti-rabies virus (RABV) nucleoprotein (N) monoclonal antibodies (Mab) were characterized by immunofluorescence assays, western blotting, and immunohistochemistry. One of these Mabs recognized the antigen by all of the assays, while the other two recognized N only in the native form in the immunofluorescence assay. These data, together with epitope mapping studies, suggest that two anti-N Mabs recognize conformational epitopes located within the N-terminal region of the RABV N protein. The availability of Mabs specific for both linear and epitope-specific antibodies should prove valuable for rabies diagnosis as well as for RABV N protein structure–function studies.
Rabies virus (RABV) has five structural proteins: the nucleoprotein (N), phosphoprotein (P), the RNA-dependent RNA polymerase (L), the surface glycoprotein (G), and the matrix protein (M) [18]. RABV N protein is 450 amino acids long and serves the critical function of tightly packaging the RNA genome into an RNase-resistant core [24, 26]. Encapsidation of the genomic RNA by newly synthesized N is believed to switch viral RNA from transcription to replication [24]. Furthermore, N is a major antigen for RABV to stimulate Th cells and antibody production, which has been shown to play a protective role against lethal infection in mice and dogs [3, 8]. It has been shown that the N protein has strain-specific and group-specific antigenic determinants [7]. Monoclonal antibodies (Mab) reacting with the group-specific determinants recognize not only all rabies viruses but also rabies-related viruses [4], and these Mabs, when conjugated with FITC or peroxidase, have been used for diagnosis and detection of rabies and rabies-related viruses around the world [14, 20, 22]. Mabs reacting with strain-specific determinants can be used to differentiate not only RABV from rabies-related viruses but also various strains of RABV, for example, RABV strains circulating in different animal reservoirs or isolated from different geographic locations [4]. In this study, we generated a panel of monoclonal antibodies from mice immunized with inactivated RABV and characterized three Mabs to the N protein. Two of them were found to be conformation-specific recognizing the N-terminal of the N protein.
To develop more Mabs specific for RABV, Balb/c mice (6–8 weeks old, NCI-Frederick, MD, USA) were immunized with sucrose-radient-purified and β-propiolactone-inactivated rabies virus strain L16 as described [15, 19, 23]. When the serum antibody titers reached a 1:204,800 endpoint dilution as measured by ELISA assay (coated with inactivated RABV), immunized mice were sacrificed. Splenocytes were obtained and used to fuse with Sp2/0 myeloma cells following the procedure manual of the ClonaCell-HY Hybridoma Cloning Kit (StemCell Technologies, Vancouver, Canada). Serial dilutions of the hybridoma cells were plated into 96-well tissue culture plates (Corning, NY, USA), and the supernatants were screened for the production of anti-rabies antibodies by an ELISA test. Forty-five clones demonstrated strong reactivity to RABV as evidenced by OD values above 0.5 and were further screened in an immunofluorescence assay in BSR cells infected with RABV. Fluorescent foci were observed in the supernatants of 25 hybridoma clones.
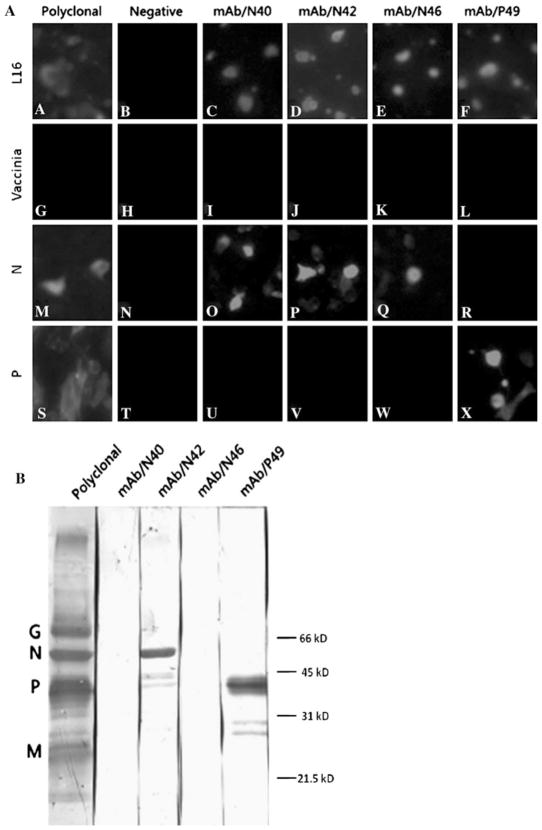
To identify Mabs recognizing RABV N, BSR cells were infected with recombinant vaccinia virus (vTF7-3) [10], followed by transfection with plasmids (pGEM-3Z) expressing RABV N or P (as a control) [26]. Alternatively, BSR cells were infected with RV strain L16 at an moi of one fluorescent focus unit (ffu) per cell. After 48 h infection or transfection, cells were fixed with 80% acetone and reacted with supernatants collected from hybridoma cultures. Goat anti-mouse IgG labeled with fluor 555 (Invitrogen, Carlsbad, CA, USA) was used as the secondary antibody. As shown in Fig. 1a, none of the monoclonal antibodies showed fluorescent staining of cells infected with vaccinia virus alone. In contrast, three of the monoclonal antibodies reacted with cells expressing the RABV N protein (MAbs N40, N42, and N46), and one (MAb P49) reacted with cells expressing the RABV P protein.
Fig. 1.
Characterization of RABV Mabs by immunofluorescence assay and by western blotting. In the immunofluorescent assay, a BSR cells were infected with RABV strain L16 or infected with vTF7-3 (MOI of 1), followed 1 h later by transfection with N- or P-expressing plasmid. At 48 h after infection or infection/transfection, cells were fixed with 80% acetone, reacted with individual Mabs, followed by goat anti-mouse IgG labeled with fluor 555. Panels A–F L16, cells infected with virus strain L16 only; Panels G–L Vaccinia, cells infected with vTF7-3 only; Panels M–R N, cells infected with vTF7-3 and transfected with N-expressing cDNA; Panels S–X P, cells infected with vTF7-3 and transfected with P-expressing cDNA. For Western blotting, b purified RABV L16 virions were subjected to SDS-PAGE, and the separated proteins were transferred to a nitrocellulose membrane. After blocking, the membrane was incubated with each of the Mabs, followed by incubation with peroxidase-conjugated secondary antibody. Reactivity of Mabs was determined by development with DAB. Each of the viral proteins recognized by the polyclonal antibodies is marked, and molecular weight markers were indicated as well
In addition to immunofluorescence assays, western blotting was also performed after separation of purified RABV (L16) proteins by SDS-PAGE [13]. After transferred to a positively charged PVDF membrane (Bio-Rad, CA, USA), RABV proteins on the membrane were reacted with each of the Mabs, followed by goat anti-mouse antibodies conjugated with peroxidase (Promega, Madison, WI, USA). As shown in Fig. 1b, two Mabs (N42 and P49) reacted with the respective RABV antigens present under denaturing and reducing conditions. However, Mabs N40 and N46 failed to recognize N protein by western blotting. These results suggest that two of the three anti-N Mabs recognize conformational eptiopes, while the other anti-N (N42) and the anti-P Mabs are specific for linear epitopes present within their respective proteins.
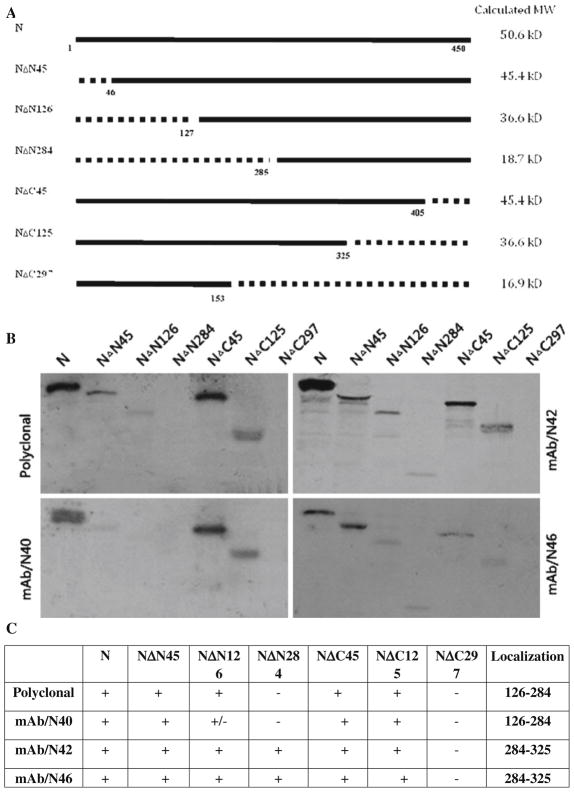
To map the epitopes of the anti-N MAbs N40, N42, and N46, mutants of the RABV N protein lacking the N-terminal 45, 126, and 284 amino acid residues or the C-terminal 45, 125, and 297 amino acid residues were generated (Fig. 2a) by PCR cloning [26], using plasmid pRN as template [8]. These plasmids were designated as NΔ45, NΔ126, NΔ284, CΔ45, CΔ125, and CΔ297, respectively. RABV N (pRN) and P (pRP) as well as the mutant N plasmids (NΔ45, NΔ126, NΔ284, CΔ45, CΔ125, and CΔ297) were each transcribed and translated using a TNT-coupled transcription and translation kit (Promega) as described previously [26]. The in vitro-translated products were subjected to immunoprecipitation with each of the anti-N Mabs followed by SDS-PAGE and autoradiographic analysis in a BAS 2000 Bio-Imaging Analyzer (Media Cybernetics, Bethesda, MD, USA). As shown in Fig. 2b, polyclonal serum precipitated the intact N protein, NΔN45, NΔN126, NΔC45, and NΔC125. Interestingly, the polyclonal antisera failed to react with NΔN284 and NΔC297, suggesting that the majority of the humoral immune response against inactivated RABV in vivo is directed against the central region of the protein (Fig. 2b, c). Likewise, Mab N40 precipitated the intact N, NΔN45, NΔC45, and NΔC125 in a pattern similar to the polyclonal antisera, suggesting reactivity with an epitope within the middle of the N. In contrast, Mabs N42 and N46 precipitated the intact N and all of the N mutants with the single exception of NΔC297. Taken together with the western blotting data, these results suggest that the two MAbs (N40 and N46) recognize different conformational epitopes present within the RABV N. A comparison of the data obtained with polyclonal antisera versus Mab N46 also suggests that the recognition of this conformational epitope by the host immune system is a rare event.
Fig. 2.
Epitope mapping of anti-N Mabs. RABV N with N-terminal or C-terminal deletions were constructed by PCR (a). The amplified fragments were cloned into pGEM-3Z at the PstI and XbaI sites as described previously [8, 26]. After in vitro transcription and translation, RABV N or mutant N proteins were used in immunoprecipitation reactions with individual anti-N Mabs. The precipitated proteins were subjected to SDS-PAGE and autoradiography (b). The results of the immunoprecipitation experiments are summarized in table form (c)
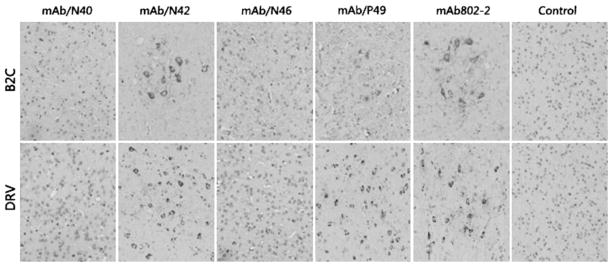
Antibodies to RABV N have been used widely for rabies diagnosis using immunofluorescent assay [20, 22] or immunohistochemistry [6]. Although all the three anti-N MAbs recognized viral antigens in RABV-infected cells by immunofluorescent assay, it is not known if these MAbs can recognize RABV antigen in fixed tissue with immunohistochemistry. To this end, mice were infected with a street virus isolated from a dog (DRV) [5] or a laboratory-adapted virus (B2C) [17] and perfused with neutral formalin as described [25]. The brains were harvested and sectioned for immunohistochemistry with each of the anti-N or anti-P antibodies as described [25]. MAb 802-2 (Kindly provided by Dr. Charles Rupprecht from the Center for Disease Control and Prevention) was used as a positive control [11]. As shown in Fig. 3, only those MAbs (N42, P49) with linear epitopes recognized RABV antigen in the fixed tissue, whereas MAb/N40 and MAb/N46 failed to detect RABV antigens in the fixed tissue. These results further confirm that these two monoclonal antibodies recognize conformational epitopes on RABV N.
Fig. 3.
Characterization of anti-N Mabs in immonuhistochemical analysis. RABV-infected brain tissues were fixed in formalin and embedded in paraffin. Coronal sections were prepared and reacted with each of the Mabs, followed by biotinylated secondary antibody (goat anti-mouse) and avidin-peroxidase. Finally, diaminobenzidine (DAB) was used as a substrate for color development. B2C brain tissues from mice infected with RABV B2C. DRV brain tissues from mice infected with RABV B2C
Mabs to RABV N have been developed and used widely for diagnosis of rabies in samples collected from patients and rabid or rabid-suspicious animals in assays such as the direct immunofluorescent antibody assay (dFA), direct rapid immunohistochemical test (dRIT), or immunohistochemical test [11, 14, 20, 22]. Furthermore, anti-N Mabs have also been used to differentiate RABV strains isolated in different animal species and/or from different geographic locations [4] as well as to probe the structure–function relationship of the N protein [1, 21]. In the present study, we characterized three anti-N Mabs and found that two of them were directed against conformational epitopes. These Mabs may be useful in rabies diagnosis and probing the structure of RABV N.
The dFA test in which anti-N Mabs was conjugated with FITC is extremely useful in identifying rabid animals and implementing post-exposure treatment for human victims who are bitten [14, 20, 22]. In the developed countries, animals that bite humans are sacrificed, and their brains are removed for detection of RV antigens using the dFA. Detection of positive antigens will indicate that the biting dog is infected with RABV, and thus the victims need to have post-exposure treatment. The dFA has also been used for antemortem diagnosis of human rabies to detect RABV antigens in skin biopsy samples [2]. The commercial dFA is sensitive and specific. However, a fluorescent microscope is required to read the slide in the dFA, and this is not readily available in many developing countries. To overcome this problem, a direct RV immunohistochemical test (dRIT) has been developed recently [14]. In this test, the anti-RV antibodies are labeled with horse-radish peroxidase and used to react with antigens in the brain tissue. The positive reaction can be detected under a light microscope. Thus, there is no need for a fluorescent microscope, and the test can be performed almost anywhere. The test has similar sensitivity and specificity as the dFA test. This test has been used in remote areas in developing countries in Africa and Asia [14, 27]. However, the peroxidase-labeled anti-RV antibodies are not commercially available. The anti-N and anti-P Mabs, particularly the two that recognize linear epitopes, could be used in dFA and/or dRIT. Indeed, these antibodies recognized antigens from both lab-adapted and wild-type RABV when used in the immunohistochemical studies reported here.
Linear and conformational Mabs to RABV N have been developed previously [4, 12, 16, 21]. While Mabs to linear epitopes are suitable for diagnosis, Mabs to linear and conformational epitopes have been useful in probing the structure of the N protein, including identifying epitopes [3] and the site of phosphorylation [12]. Apart from antigen epitopes, RABV N has structural domains that interact with genomic RNA for genomic encapsidation [26] and/or with P for specific RNA encapsidation [9]. In this study, the Mab N40 has been crudely mapped to epitopes located between amino acids 45 or 126–284. The conformational epitopes have not been mapped precisely, and further studies are warranted.
Acknowledgments
This work is supported partially by Public Health Service grant AI-051560 from the National Institute of Allergy and Infectious Diseases.
Contributor Information
Yan Jiang, Department of Pathology, College of Veterinary Medicine, University of Georgia, 501 D.W. Brooks Drive, Athens, GA 30602, USA, Department of Pharmacological Sciences, School of Traditional Chinese Medicine, Southwest University, Chongqing 400716, China.
Yonghuang Luo, Department of Pharmacological Sciences, School of Traditional Chinese Medicine, Southwest University, Chongqing 400716, China.
Frank Michel, Department of Anatomy and Radiology, College of Veterinary Medicine, University of Georgia, Athens, GA 30602, USA.
Robert J. Hogan, Department of Anatomy and Radiology, College of Veterinary Medicine, University of Georgia, Athens, GA 30602, USA, Department of Infectious Diseases, College of Veterinary Medicine, University of Georgia, Athens, GA 30602, USA
Ying He, Department of Pathology, College of Veterinary Medicine, University of Georgia, 501 D.W. Brooks Drive, Athens, GA 30602, USA.
Zhen F. Fu, Email: zhenfu@uga.edu, Department of Pathology, College of Veterinary Medicine, University of Georgia, 501 D.W. Brooks Drive, Athens, GA 30602, USA, Department of Infectious Diseases, College of Veterinary Medicine, University of Georgia, Athens, GA 30602, USA
References
- 1.Anzai J, Takamatsu F, Takeuchi K, Kohno T, Morimoto K, Goto H, Minamoto N, Kawai A. Identification of a phosphatase-sensitive epitope of rabies virus nucleoportein which is recognized by a monoclonal antibody 5-2-26. Microbiol Immunol. 1997;41:229–240. doi: 10.1111/j.1348-0421.1997.tb01195.x. [DOI] [PubMed] [Google Scholar]
- 2.Blenden D, Creech W, Torres-Anje M. Use of immunofluorescence examination to detect rabies virus antigen in the skin of humans with clinical encephalitis. J Infect Dis. 1986;154:698–701. doi: 10.1093/infdis/154.4.698. [DOI] [PubMed] [Google Scholar]
- 3.Dietzschold B, Wang HH, Rupprecht CE, Celis E, Tollis M, Ertl H, Heber-Katz E, Koprowski H. Induction of protective immunity against rabies by immunization with rabies virus ribonucleoprotein. Proc Natl Acad Sci USA. 1987;84:9165–9169. doi: 10.1073/pnas.84.24.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietzschold B, Rupprecht CE, Tollis M, Lafon M, Mattei J, Wiktor TJ, Koprowski H. Antigenic diversity of the glycoprotein and nucleocapsid proteins of rabies and rabies-related viruses: implications for epidemiology and control of rabies. Rev Infect Dis. 1988;10(suppl 4):S785–S798. doi: 10.1093/clinids/10.supplement_4.s785. [DOI] [PubMed] [Google Scholar]
- 5.Dietzschold B, Morimoto K, Hooper DC, Smith JS, Rupprecht CE, Koprowski H. Genotypic and phenotypic diversity of rabies virus variants involved in human rabies: implications for postexposure prophylaxis. J Human Virol. 2000;3:50–57. [PubMed] [Google Scholar]
- 6.Fekadu M, Greer PW, Chandler FW, Sanderlin DW. Use of the avidin-biotin peroxidase system to detect rabies antigen in formalin-fixed paraffin-embedded tissues. J Virol Methods. 1988;19:91–96. doi: 10.1016/0166-0934(88)90152-8. [DOI] [PubMed] [Google Scholar]
- 7.Flamand A, Wiktor TJ, Koprowski H. Use of hybridoma monoclonal antibodies in the detection of antigenic differences between rabies and rabies-related virus proteins. I. The nucleo-capsid protein. J Gen Virol. 1980;48:97–104. doi: 10.1099/0022-1317-48-1-97. [DOI] [PubMed] [Google Scholar]
- 8.Fu ZF, Dietzschold B, Schumacher CL, Wunner WH, Ertl HC, Koprowski H. Rabies virus nucleoprotein expressed in and purified from insect cells is efficacious as a vaccine. Proc Natl Acad Sci USA. 1991;88:2001–2005. doi: 10.1073/pnas.88.5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu ZF, Wunner WH, Dietzschold B. Immunoprotection by rabies virus nucleoprotein. Curr Top Microbiol Immunol. 1994;187:161–172. doi: 10.1007/978-3-642-78490-3_9. [DOI] [PubMed] [Google Scholar]
- 10.Fuerst TR, Niles EG, Studier FW, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamir AN, Moser G, Fu ZF, Dietzschold B, Rupprecht CE. Immunohistochemical test for rabies: identification of a diagnostically superior monoclonal antibody. Vet Rec. 1995;136:295–296. doi: 10.1136/vr.136.12.295. [DOI] [PubMed] [Google Scholar]
- 12.Kawai A, Anzai J, Honda Y, Morimoto K, Takeuchi K, Kohno T, Wakisaka K, Goto H, Minamoto N. Monoclonal antibody #5-2-26 recognizes the phosphatase-sensitive epitope of rabies virus nucleoprotein. Microbiol Immunol. 1997;41:33–42. doi: 10.1111/j.1348-0421.1997.tb01170.x. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Lembo T, Niezgoda M, Velasco-Villa A, Cleaveland S, Ernest E, Rupprecht CE. Evaluation of a direct, rapid immunohistochemical test for rabies diagnosis. Emerg Infect Dis. 2006;12:310–313. doi: 10.3201/eid1202.050812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhusudana SN, Shamsundar R, Seetharaman S. In vitro inactivation of the rabies virus by ascorbic acid. Int J Infect Dis. 2004;8:21–25. doi: 10.1016/j.ijid.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Minamoto N, Tanaka H, Hishida M, Goto H, Ito H, Naruse S, Yamamoto K, Sugiyama M, Kinjo T, Mannen K, et al. Linear and conformation-dependent antigenic sites on the nucleoprotein of rabies virus. Microbiol Immunol. 1994;38:449–455. doi: 10.1111/j.1348-0421.1994.tb01806.x. [DOI] [PubMed] [Google Scholar]
- 17.Morimoto K, Hooper DC, Carbaugh H, Fu ZF, Koprowski H, Dietzschold B. Rabies virus quasispecies: implications for pathogenesis. Proc Natl Acad Sci USA. 1998;95:3152–3156. doi: 10.1073/pnas.95.6.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose J, Whitt M. Rhabdoviridae: the viruses and their replication. 4. Lippincott-Reven; Philadelphia: 2000. [Google Scholar]
- 19.Schnell MJ, Mebatsion T, Conzelmann KK. Infectious rabies viruses from cloned cDNA. EMBO J. 1994;13:4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith A, Yager P, Baer G. A rapid reproducible test for determining rabies neutralizing antibody. In: Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. World Health Organization; Geneva: 1996. pp. 181–192. [Google Scholar]
- 21.Toriumi H, Kawai A. Structural difference recognized by a monoclonal antibody #404-11 between the rabies virus nucleocapsid (NC) produced in virus infected cells and the NC-like structures produced in the nucleoprotein (N) cDNA-transfected cells. Microbiol Immunol. 2005;49:757–770. doi: 10.1111/j.1348-0421.2005.tb03666.x. [DOI] [PubMed] [Google Scholar]
- 22.Trimarchi C, Debbie JG. The fluorescent antibody in rabies. In: Baer G, editor. The natural history of rabies viruses. CRC Press; Boca Raton: 1991. pp. 220–229. [Google Scholar]
- 23.Wu X, Gong X, Foley HD, Schnell MJ, Fu ZF. Both viral transcription and replication are reduced when the rabies virus nucleoprotein is not phosphorylated. J Virol. 2002;76:4153–4161. doi: 10.1128/JVI.76.9.4153-4161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wunner WH, Larson JK, Dietzschold B, Smith CL. The molecular biology of rabies viruses. Rev Infect Dis. 1988;10(suppl 4):S771–S784. doi: 10.1093/clinids/10.supplement_4.s771. [DOI] [PubMed] [Google Scholar]
- 25.Yan X, Prosniak M, Curtis MT, Weiss ML, Faber M, Dietzschold B, Fu ZF. Silver-haired bat rabies virus variant does not induce apoptosis in the brain of experimentally infected mice. J NeuroVirol. 2001;7:518–527. doi: 10.1080/135502801753248105. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Hooper DC, Wunner WH, Koprowski H, Dietzschold B, Fu ZF. The specificity of rabies virus RNA encapsidation by nucleoprotein. Virology. 1998;242:107–117. doi: 10.1006/viro.1997.9022. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Xiong C, Kuzmin I, Niezgoda M, Fu Z, Rupprecht C. Investigation of the role of healthy dogs as carriers of rabies virus. Vector-Borne Zoon Dis. 2008;8:313–320. doi: 10.1089/vbz.2007.0209. [DOI] [PubMed] [Google Scholar]