Abstract
Rationale
The enhanced formation of intracellular reactive oxygen species (ROS) induced by oxidized low density lipoprotein (OxLDL) promotes macrophage death, a process likely to contribute to the formation of necrotic cores and the progression of atherosclerotic lesions. Yet macrophage deficiency of phagocytic NADPH oxidase (Nox2), the primary source of ROS in macrophages, does not reduce atherosclerotic lesion development in mice. This suggests an as yet unidentified NADPH oxidase may be present in macrophages and responsible for the intracellular ROS formation induced by OxLDL.
Objective
The aim of this study was to identify the source of intracellular ROS involved in macrophage death.
Methods and Results
Nox4 was expressed in human monocytes and mature macrophages, and was localized to the ER and to defined foci within the nucleus. Nox4 colocalized with p22phox, and both proteins were up-regulated in response to OxLDL stimulation, whereas Nox2/gp91phox levels remained unchanged. Induction of Nox4 expression, intracellular ROS formation and macrophage cytotoxicity induced by OxLDL were blocked by MEK1/2 inhibition, but not by inhibitors of p38-MAPK, JNK or JAK2. SiRNA knockdown of Nox4 inhibited both intracellular ROS production and macrophage cytotoxicity induced by OxLDL, while Nox4 over-expression enhanced both OxLDL-stimulated ROS formation and macrophage death.
Conclusions
Nox4 is a novel source of intracellular ROS in human monocytes and macrophages. Induction of Nox4 by OxLDL is mediated by the MEK1/ERK pathway and required for OxLDL cytotoxicity in human macrophages, implicating monocytic Nox4 in atherogenesis.
Keywords: NADPH oxidase, macrophage death, reactive oxygen species, redox signaling, atherosclerosis
INTRODUCTION
Hydrogen peroxide, superoxide anion and other reactive oxygen species (ROS) have long been considered toxic by-products of metabolic processes and mediators of oxidative stress. Only recently has the essential role of ROS in cell signaling and cell function been recognized 1–3. Although ROS are generated by a variety of cellular sources including mitochondria, cytochrome P450 enzymes and the uncoupling of NOS, these ROS are byproducts and not the primary products of these systems. In contrast, NADPH oxidases (Nox) are professional ROS producers. For example, Nox2/gp91phox, the prototype of NADPH oxidases, is responsible for the oxidative burst in neutrophils and macrophages required for bacterial killing 4;5. The discovery of Nox2 homologues including Nox1, 3, 4 and more distantly related Nox5, Duox1 and Duox2 in numerous different tissues suggests a much broader role for Nox enzymes and the ROS they generate 6;7. For example, Nox4–dependent ROS production is activated in response to an array of extracellular stimuli including BMP4 8 and TGF-β 9;10, and mediates a number of different biological functions, such as angiogenesis 11, cell adhesion 8, cell migration 12, and vascular remodeling 13. However, to date only a limited number of targets of these signaling ROS have been identified and validated as bona fide targets of ROS signaling, with reactive cysteine residues of protein tyrosine phosphatases being one of the best established ones.
Oxidized lipoprotein (OxLDL) is an important biomarker of cardiovascular diseases. OxLDL levels are elevated in patients with chronic metabolic disorders 14;15, and in atherosclerosis-prone apoE-null mice, Itabe and coworkers demonstrated a transient increase in circulating OxLDL levels during the progression of atherosclerosis 16. OxLDL is found in atherosclerotic lesions and one of its many proatherogenic properties is its cytotoxicity toward all vascular cells and macrophages 17–19. Enhanced macrophage death in atherosclerotic lesions promotes the formation of necrotic cores, a characteristic feature of advanced atherosclerotic plaques 20;21. Increased intracellular ROS formation plays a critical role in OxLDL-induced macrophage death 18;22 but the source of these intracellular ROS has not been known. Nox2 is the primary source of superoxide in macrophages, but Nox2 deficiency in macrophages does not reduce atherosclerotic lesion development in mice 23. Nox2 activity is regulated by various cytosolic factors 24–26, but the ROS generated by the Nox2 system are either directed to extracellular space or to the phagosomes 27; they are therefore not a likely source of the intracellular ROS observed in macrophages in response to OxLDL stimulation. We thus hypothesized that an as yet unidentified NADPH oxidase may be present in macrophages and is responsible for intracellular ROS formation induced by OxLDL. In the present study, we now provide the first evidence for the existence of a second NADPH oxidase, Nox4, in human monocytes and macrophages. We show that Nox4 is localized in intracellular compartments and co-localizes with its dimerization partner, p22phox. We also provide evidence that OxLDL, but not native LDL, induces the expression of Nox4/p22phox system, and that Nox4, not Nox2, is the source of the OxLDL-induced intracellular ROS responsible for macrophage death.
METHODS
Materials
All chemicals were obtained from Sigma (St. Louis, MO) unless stated otherwise. PDI antibody was purchased from BD Biosciences. p22phox, β-actin and Nox2 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and the Lamp-1 antibody from Abcam (Cambridge, MA). Secondary anti-mouse IgG Cy3 and anti-rabbit IgG Cy5 antibodies were purchased from JacksonImmuno Research (West Grove, PA). Protein-G Sepharose was purchased from GE Healthcare (Piscataway, NJ).
Human Monocyte-derived Macrophage (HMDM) Isolation and Culture
Mononuclear cells were isolated from blood obtained from healthy donors (South Texas Blood and Tissue Center), and mature HMDM were prepared as described previously 28. For inhibitor studies, HMDM were pretreated for 2 h with vehicle or inhibitor in the absence of serum before OxLDL was added for up to 24 h.
LDL Isolation and Oxidation
LDL was isolated by ultracentrifugation from pooled plasma from health blood donors, oxidized with copper sulphate at 37 °C for 24 h, and oxidized LDL was purified by gel-filtration chromatography, filter-sterilized and characterized as described previously 18.
RT-PCR
RNA was extracted by Trizol (Invitrogen) and cDNA was synthesized according to the manufacturer’s protocol (Ambion). Primer sequences and amplification profiles of Nox1 to Nox5 used are described in Table 1 29. The number of PCR cycles and amount of cDNA used in semi-quantitative PCR was first normalized against β-actin and the expression levels were then quantified according to β-actin normalization.
Table 1.
Sequences of Primers Used for RT-PCR
| Forward primers (5′ → 3′) | Reverse primers (5′ → 3′) | |
|---|---|---|
| Nox1 | GTACAAATTCCAGTGTGCAGACCAC | CAGACTGGAATATCGGTGACAGCA |
| Nox2 | GGAGTTTCAAGATGCGTGGAAACTA | GCCAGACTCAGAGTTGGAGATGCT |
| Nox3 | GGATCGGAGTCACTCCCTTCGCTG | ATGAACACCTCTGGGGTCAGCTGA |
| Nox4 | CTCAGCGGAATCAATCAGCTGTG | AGAGGAACACGACAATCAGCCTTAG |
| Nox5 | ATCAAGCGGCCCCCTTTTTTTCAC | CTCATTGTCACACTCCTCGACAGC |
Generation of Rabbit Monoclonal Antibodies Directed Against Nox4
Antibodies were raised against a peptide sequence within the NADPH binding domain of Nox4 that is unique among human and mouse NADPH oxidase sequences but this sequence is conserved between human and mouse Nox4 protein sequences. The antibodies were generated in collaboration with and are available through Epitomics, Burlingame, CA. Hybridoma clones were generated from B cells of one of the immunized rabbits and screened. Protein-G Sepharose-purified monoclonal antibodies were used to perform Western blot and immunohistochemistry experiments.
Immunoprecipitation
HMDM were lysed with co-IP lysis buffer (MES-buffered saline with 1% Triton X-100, 1mM EDTA and 0.5% NP-40) containing protease inhibitor cocktail (Roche). Lysates were cleared at 15,000 x g for 10 minutes, and supernatants were collected and split into two equal samples. Nox4 mAb (10 μg) or the same volume of RPMI with 10% FBS was added to the supernatants, and after constant overnight agitation at 4 °C, 10 μl pre-washed Protein G Sepharose (GE Healthcare) was added to all samples. After overnight mixing at 4 °C, samples were pelleted and washed extensively with co-IP lysis buffer. Samples were resuspend in Laemmli buffer, heated, and subjected to Western blot analysis.
Western Blot Analysis
HMDM protein lysates were subjected to Western blot analysis according to standard protocols. Bands were detected by chemiluminescence on a KODAK Image Station 4000MM and normalized to β-actin (Santa Cruz Biotechnology).
Immunostaining and Confocal Microscopy
Immunostaining and confocal microscopy were carried out as described in detail in the Online Supplement. HMDM were fixed and permeabilized, and the samples were blocked with BSA and donkey serum prior to staining with anti-Nox4 monoclonal antibodies. Fluorescence images were acquired with an Olympus FV-1000 Laser Scanning Confocal Microscopy at Core Optical Imaging Facility at the UTHSCSA.
Subcellular fractionation
Subcellular fractionation and isolation of macrophage nuclei was performed according the protocol described Lorenzen et al. 30. HMDM were washed with PBS and lysed with fractionation buffer buffer (20 mM HEPES pH = 7.4, 250 mM sucrose, 10 mM KCl, 1.5 mM MgCl2, 1.5 mM EDTA with protease inhibitor (Roche)). Cell lysates were passed through 25G needles 10 times with syringe. The lysates were then left on ice for 20 min and centrifuged at 720 g for 5 min. The pellet representing the nuclear fraction was washed twice with fractionation buffer, passed through 25G needles 10 times and pelleted at 720 x g for 5 min, and subjected to Western blot analysis.
Intracellular ROS and Cytotoxicity Assays
HMDM were loaded with DCFH-DA (Molecular Probes) and 3H-labeled adenine (GE Healthcare) for 2 h, washed to remove excess dye and radiolabel, and subsequently. stimulated with LDL or OxLDL. Intracellular ROS formation and cytotoxicity were measured as described previously 18;19.
Adenovirus Generation
Doxycycline-inducible adenoviruses expressing human Nox4 under the control of a tet-on promoter construct were generated using the pAdEasy system as described elsewhere 31. The cDNA for human Nox4 was kindly provided by Dr. Lena Serrander 32. Adenoviruses expressing Nox4-siRNA and control-siRNA were kind gifts from Dr. Kai Chen 33. All infections were performed at 50 MOI.
Statistical Analysis
Results are expressed as mean +/− standard deviation of at least 3 independent experiments. Data were analyzed either by paired two-tailed t test or by ANOVA with the Tukey post-hoc test for multi-group comparisons where appropriate. A p-value of less than 0.05 was considered statistically significant.
RESULTS
Human monocytes and monocyte-derived macrophages (HMDM) express Nox4
To search for new Nox isoforms that may be responsible for OxLDL-induced intracellular ROS formation in macrophages we performed expression profiling by RT-PCR for Nox1 through 5. In addition to Nox2, freshly isolated, adhesion-purified human monocytes and 2-week-old mature and fully differentiated HMDM expressed Nox4, but we did not detect mRNA for Nox1, 3 or 5 in either monocytes or HMDM (Figure 1). Importantly, the treatment of HMDM with OxLDL induced Nox4 mRNA levels up to 4.6-fold (Figure 3A), indicating that Nox4 may be the source of OxLDL-induced ROS formation. Western blot analysis of HMDM lysates with commercially available antibodies directed against Nox4 failed to demonstrate a band at 66 kD, which would correspond to full-length Nox4 protein. We therefore generated new rabbit polyclonal and rabbit monoclonal antibodies directed against a region in the Nox4 NADPH binding domain. The polyclonal and monoclonal antibodies detected endogenous Nox4 proteins in both human and murine cells at the predicted molecular weight (Online Figure I). Using lysates from both Nox4 over-expressing cells and cells from Nox4 knockout mice, we confirmed the specificity of both the rabbit polyclonal (not shown) and monoclonal Nox4 antibodies (Online Figure I).
Figure 1. Human blood monocytes and mature human monocyte-derived macrophages (HMDM) express Nox4 mRNA.

Human mononuclear cells were isolated by Ficoll gradient centrifugation. Human monocytes were allowed to adhere for 1 h and non-adhering cells were removed. Mature HMDM were generated from the same mononuclear cell fraction and allowed to differentiate as described under “Methods”. Expression profiling was performed by semi-quantitative RT-PCR using the gene specific primers listed in Table 1. Representative images of three independent experiments are shown. Mono: human monocytes; HMDM: human monocyte-derived macrophages.
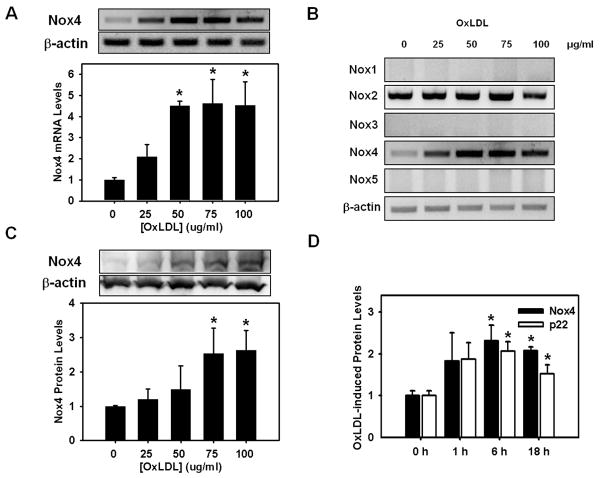
Figure 3. OxLDL induces Nox4 and p22phox protein expression.
HMDM were stimulated with OxLDL for 18 h at the concentrations indicated. Expression levels of Nox1 through 5 mRNA were determined were determined by RT-PCR (Panel A and B). Nox4 protein levels were determined by Western blot analysis (Panel C). Nox2 protein expression was not increased by OxLDL under any of the conditions tested (see Supplemental Figure IIA). Panel D: Time course of Nox4 and p22phox protein expression in response to OxLDL (75μg/ml). Quantitative results from Western blot analyses are shown as mean ± standard deviation from 3 independent experiments. *: P<0.01 versus vehicle or 0 h (n=3)
Endogenous macrophage Nox4 localizes to the endoplasmic reticulum and the nucleus, and co-localizes with p22phox
The minimal requirement for Nox4 activity appears to be the dimerization with p22phox 34;35. Optical sectioning of HMDM stained with fluorescently-labeled anti-Nox4 monoclonal antibodies revealed within the macrophages a high degree of colocalization of Nox4 with its dimerization partner p22phox (Figure 2A). We also found co-immunoprecipation of p22phox with Nox4, suggesting that endogenous Nox4 in macrophages may indeed be active and account for at least some of the intracellular ROS we detected in resting HMDM 18.
Figure 2. Nox4 protein colocalizes with p22phox and is localized in the endoplasmic reticulum and the nucleus of HMDM.

Immunohistochemistry and confocal microscopy was performed on HMDM stained with our new rabbit monoclonal antibody directed against Nox4 (red) and p22phox (Panel A; green) or the ER-specific marker PDI (Panel B; green), or DAPI (Panel C; blue; arrows identify discrete Nox4 loci). The overlays show the extent of co-localization (yellow) of Nox4 with each of these markers. Bar = 10 μm. Panel D: HMDM lysates were prepared and Nox4 protein was immunoprecipitated as described under “Methods”. Immunoprecipitates were subjected to Western blot analysis and probed for Nox4 and of p22phox.
Nox4 protein also co-localized with PDI, an ER marker (Figure 2B), confirming similar findings reported by Chen et al. for HAEC cells 33. However, Nox4 staining was not restricted to the ER, indicating that Nox4 also localizes to other intracellular sites within macrophages. For example, we observed Nox4 staining associated with discrete foci within macrophage nuclei (Figure 2C). Z-plane sectioning confirmed that these Nox4-positive foci are located inside the nucleus (not shown). The presence of Nox4 within macrophage nuclei was confirmed by Western blot analysis of nuclear lysates generated by cell fractionation of HMDM (Online Figure II).
To examine whether Nox4 is expressed by macrophages found in atherosclerotic lesions, we performed immunofluorescence studies in aortic root sections from dyslipidemic LDL-receptor deficient mice. While a significant fraction of Nox4 staining did not overlap with the macrophage-specific marker CD68, we observed significant colocalization of CD68 with Nox4, suggesting that macrophages in atherosclerotic lesion express Nox4 (Online Figure III).
OxLDL induces the concomitant expression of Nox4 and p22phox
Previously, we showed that OxLDL induces intracellular ROS formation and promotes macrophage death 18;19. Since OxLDL stimulation increases Nox4 mRNA levels (Figure 3A) but not Nox2 mRNA in HMDM (Figure 3B), we next examined whether Nox4 is the source of the intracellular ROS responsible for OxLDL cytotoxicity. However, to have Nox4 account for OxLDL-induced ROS formation would require the up-regulation of both Nox4 and p22phox by OxLDL. Stimulation of HMDM with increasing concentrations of OxLDL increased Nox4 mRNA and protein levels (Figure 3A and 3C), suggesting that Nox4 activity is indeed transcriptionally regulated in macrophages as has been reported for other cell types 32, rather than being regulated by cytosolic factors as with Nox2 36. Importantly, OxLDL-induced increases in Nox4 protein levels were paralleled by increased p22phox protein expression, both reaching maximal expression levels after 6 h (Figure 3D). In contrast, Nox2 protein expression levels in HMDM remained unchanged in response to OxLDL (Online Figure IV, PanelA). Because p22phox is the obligate dimerization partner required for Nox4 activity 37, OxLDL appears to induce both components necessary for increased ROS formation, suggesting a role for Nox4/p22phox in OxLDL-induced macrophage death.
Native LDL did not induce Nox4 expression in HMDM (Online Figure IV, Panel B), implicating lipid and/or protein oxidation products of OxLDL in the induction of the Nox4/p22phox complex. This finding is consistent with previous studies, demonstrating that native LDL neither induces ROS formation nor promotes cytotoxicity in HMDM 18;38. Trolox, a peroxyl radical scavenger, protects HMDM from OxLDL-induced cytotoxicity 19, but did not block OxLDL-induced Nox4 up-regulation (Online Figure IV, Panels C and D). This suggests that the transcriptional activation of Nox4 does not appear to be mediated by peroxyl radicals, which are generated both during the oxidation of LDL and the breakdown of lipid peroxides within OxLDL. This finding is also consistent with a role for Nox4 upstream of intracellular ROS production and cytotoxicity induced by OxLDL.
Inhibition of MEK prevents Nox4 expression, intracellular ROS formation and cytotoxicity induced by OxLDL in HMDM
To gain insights into the mechanisms involved in OxLDL-induced Nox4 expression and its potential role in macrophage death, we examined the effect of different kinase inhibitors on OxLDL-stimulated intracellular ROS formation and cytotoxicity. HMDM were pre-incubated with the MEK1/2 inhibitor U0126, the p38-MAPK inhibitor SB203580, or the JNK inhibitor SP600125, and were subsequently stimulated with 75 μg/ml of OxLDL. Inhibition of MEK1/2 by U0126 completely blocked OxLDL-induced Nox4 expression, whereas neither p38-MAPK nor JNK or JAK2 inhibitors showed any effect on Nox4 expression (Figure 4A and 4B, Online Figure V, Panel A). A second MEK1/2 inhibitor, PD98059, showed inhibitory effects very similar to U0126 (not shown), confirming the role of MEK1/2 in the OxLDL-induced up-regulation of Nox4. Inhibition of MEK also blocked p22phox expression induced by OxLDL, whereas Nox2 protein levels were unaffected by MEK inhibition (Online Figure VI). MEK1/2 inhibitors not only blocked OxLDL-stimulated Nox4 expression, they inhibited intracellular ROS formation and macrophage death induced by OxLDL. In contrast, p38-MAPK, JNK and JAK2 inhibitors showed no protective effects (Figure 4C and 4D, Online Figure V, Panel B). These results further support our hypothesis that OxLDL-induced up-regulation of Nox4 is responsible for the increase in intracellular ROS formation and cytotoxicity observed in HMDM exposed to OxLDL.
Figure 4. Inhibition ofMEK1/2 blocks OxLDL-induced Nox4 expression, intracellular ROS formation and cytotoxicity.

HMDM were pre-incubated for 2 h with either vehicle (DMSO), U0126 (U, 5μM), SB203580 (SB, 5μM), or SP600125 (SP, 10μM) and then exposed to OxLDL (75 μg/ml) for 6 h (protein expression, ROS formation) or 18 h (cytotoxicity). For each experiment, we determined Nox4 protein expression by Western blot analysis (Panel A and B), intracellular ROS production (Panel C), and cytotoxicity (Panel D). For each parameter measured, data were normalized to the OxLDL-induced signal determined in vehicle-treated HMDM versus vehicle-treated cells incubated in the absence of OxLDL, and that signal was set at 1.0 to allow for comparisons of all three signals measured. Nox4 protein levels were normalized against β-actin. *: P <0.01 versus DMSO (n=3).
Nox4 knockdown protects HMDM from the cytotoxicity of OxLDL while Nox4 overexpression sensitizes HMDM to OxLDL toxicity
To confirm the role of Nox4 in OxLDL-induced cytotoxicity, we infected HMDM with adenoviruses expressing either Nox4 siRNA or a scrambled control siRNA (scrRNAi) 33. Nox4 siRNA completely suppressed OxLDL-induced Nox4 mRNA expression (not shown) and blocked Nox4 protein expression by 59 % compared to scrRNAi (Figure 5A), but it did not affect Nox2 protein levels (Online Figure VII, Panel A), confirming the specificity of the anti-Nox4 siRNA sequence. Knockdown of Nox4 reduced OxLDL-induced ROS formation by 39 % and cytotoxicity by 42% (Figure 5B and 5C). In contrast, knockdown of Nox2 using anti-sense oligonucleotides resulted in a 33% reduction in total Nox2 protein levels (Online VII, Panels B and C) but had no effect on OxLDL-induced cytotoxicity (Online Figure VII, Panel D). Together, these results provide further evidence that Nox4 is the source of ROS that mediate OxLDL-induced macrophage death. Surprisingly, even though Nox4 siRNA routinely reduced baseline Nox4 mRNA levels by over 50% in unstimulated HMDM (not shown), Nox4 protein expression was not significantly decreased in these cells (Figure 5A), suggesting that the turnover of Nox4 in HMDM is very slow. This finding also implies that the reduction in Nox4 levels by Nox4 siRNA that we observed in OxLDL-stimulated HMDM is primarily due to the inhibition of de novo synthesized Nox4 expressed in response to OxLDL stimulation.
Figure 5. Knockdown of Nox4 attenuates intracellular ROS formation and macrophage death induced by OxLDL whereas overexpression of Nox4 sensitizes macrophages to OxLDL-induced ROS formation and cell injury.
For the knockdown experiments, HMDM were infected overnight with adenoviruses carrying either siRNA directed against either Nox4 (Nox4i) or a random sequence (Scr) (Panel A, B, C). To overexpress Nox4, HMDM were infected with adenoviruses carrying a doxycycline-inducible construct with the sequence for human Nox4 (Panel D, E, F). Nox4 expression was induced by adding 1 μg/ml of doxycycline (Dox) to the culture medium. HMDM were stimulated with OxLDL (75μg/ml) for 6 h to measure Nox4 protein expression and ROS production, and for 18 h to determine cytotoxicity. Nox4 protein levels were normalized against β-actin. Representative Western blots are shown in panels A and D. To allow for a direct comparison of the effect of Nox4 knockdown or overexpression on Nox4 expression levels (Panels A and D), ROS production (Panels B and E) and cytotoxicity (Panels C and F), data were normalized as described in Figure 4. *: P <0.05 versus Scr (n=3).
To increase constitutive Nox4 expression in macrophages, we generated a doxycycline (Dox)-inducible adenoviral vector that contains both the Tet-on transcriptional activator and the Tet-responsive element (TRE) and carries the human Nox4 sequence. Induction of Nox4 expression with Dox (1 μg/ml) in HMDM elevated total Nox4 protein levels by 41 % (Figure 5D), which coincided with an increase of both OxLDL-induced intracellular ROS formation (+30 %, Figure 5E) and cytotoxicity (+65 %; Figure 5F). Nox4 over-expression did not affect Nox2 expression in HMDM (Online Figure VIII, Panel A). In the absence of the adenoviral vector, doxycycline alone had no effect on Nox4 protein levels in HMDM (Online Figure VIII, Panel B) and did not affect ROS production or cell viability in HMDM (not shown). These results confirm that Nox4 is the source of intracellular ROS production stimulated by OxLDL in HMDM and that Nox4 induction mediates OxLDL-induced macrophage death. In the absence of OxLDL, induction of Nox4 expression did not significantly alter baseline ROS production in HMDM or macrophage viability. This finding suggests that p22phox levels may be rate-limiting in resting HMDM, but sufficient p22phox is synthesized in response to OxLDL stimulation to dimerize with the overexpressed transgenic Nox4.
DISCUSSIONS
The goal of this study was to identify the source of the ROS that mediate OxLDL-induced cytotoxicity in macrophages. Here we demonstrated for the first time that human monocytes and macrophages express the NADPH oxidase Nox4 and that this enzyme is inducible by OxLDL. Our highly specific monoclonal antibody detected Nox4 not only in human blood monocytes and HMDM, but also in mouse peritoneal macrophages. Nox4 was previously reported to be present in murine osteoclasts 39, suggesting that Nox4 may be expressed throughout the monocytic cell lineage. Compared to Nox2, the major source of ROS in phagocytic cells 40, Nox4 is expressed at lower levels in monocytes and HMDM, which is consistent with the proposed role for Nox4 in cell signaling and may explain why previous studies failed to detect this enzyme in human monocytes 41. Confocal microscopy revealed that Nox4 was expressed throughout human and murine macrophages, again consistent with a potential role of this enzyme in redox signaling in human and mouse monocytic cells. We confirmed the localization of Nox4 in the ER previously reported by Chen et al. 33 and also detected Nox4 in discrete sites within the nucleus, similar to findings in human airway smooth muscle cells reported by Sturrock and colleagues 10. This latter finding is particularly intriguing as emerging evidence strongly suggests that processes located in the nucleus such as binding of transcription factors like NF-κB and AP-1 are redox regulated 42. We could not detect Nox4 protein in lysosomes (Online Figure VII), indicating that Nox4 expression is not ubiquitous within the cell and is instead limited to select intracellular membranes and organelles.
Most Nox family members, including Nox2, generate superoxide. However, using the redox sensitive dye DCFH we were able to monitor changes in intracellular ROS that resulted from either increased or suppressed Nox4 expression. Thus, it appears that Nox4 produces H2O2 in macrophages. While DCFH is by no means specific, this dye does not react with superoxide and is selective for H2O2 though a catalyst is needed for H2O2 to oxidize DCFH. Nevertheless, we cannot rule out that monocytic Nox4 is able to generate superoxide, which would dismutate to H2O2. In agreement with our findings, results from two recent studies suggest that Nox4 primarily produces H2O2 43;44, the primary ROS acting as the intracellular messenger in redox signaling 45. Together, its intracellular localization, inducibility and ability to generate H2O2 strongly suggest that as in non-phagocytic cells Nox4 may primarily perform signaling functions in macrophages.
We also identified Nox4 as the inducible source of ROS production required for OxLDL-induced macrophage death (Figure 6). This finding nicely illustrates the dichotomy of ROS: on the one hand, ROS are required for physiological redox signaling, yet they are harmful when generated in excess. Induction of Nox4 by OxLDL is mediated by the MEK/ERK pathway, but the upstream components of this signaling mechanism in macrophage have not yet been clearly identified. Clavreul et al. demonstrated that in endothelial cells, OxLDL activates the MEK/ERK pathway via the peroxynitrite-induced S-glutathionylation of p21ras 46. OxLDL promotes S-glutathionylation in HMDM 19, but p21ras S-glutathionylation has not been reported in HMDM. While intriguing, this mechanism would place yet another ROS-generating system upstream of OxLDL-mediated Nox4 expression and Nox4-dependent ROS production.
Figure 6. Hypothetical model for the role of Nox4 in OxLDL-mediated macrophage cytotoxicity.

OxLDL induces Nox4 expression via the MEK/ERK pathway and is blocked by MEK inhibitors UO126 and PD98059. Knock-down of Nox4 (Nox4 RNAi) protected macrophages from increased ROS production and cell injury. The putative site of action for the peroxyl radical scavenger Trolox is indicated. Overexpression of Nox4 (pAdNox4) does not affect basal ROS production or macrophage viability, but sensitizes macrophages to OxLDL-induced ROS formation and cell death.
Nox4 requires a second transmembrane protein, p22phox, to form a functional ROS-generating enzyme complex 37. We showed not only that Nox4 colocalizes with p22phox in HMDM, but that Nox4 and p22phox showed similar induction profiles in response to OxLDL. This suggests that a majority of Nox4 in HMDM is present as Nox4/p22phox dimers and most likely is active. If that is the case, and if the majority of Nox4 is indeed generating H2O2, how then could this system serve its signaling functions? One possibility is that the target(s) is recruited to the membrane where the Nox4/p22phox dimer is localized. Alternatively, the Nox4/p22phox system could be assembled and transported in vesicles or similar structures, and could then be recruited to signaling complexes such as lipid raft, caveolae or focal adhesion sites 47.
OxLDL accumulates in atherosclerotic lesions and promotes macrophage death, thereby contributing to the development of the necrotic core 17–19. While increased intracellular ROS production is essential for OxLDL-induced macrophage death 19, Nox2 deficiency in macrophages does not reduce atherosclerotic lesion development in mice 23. It appeared paradoxical that macrophage-derived ROS would not contribute to this process as the report from Kirk et al would suggest 23, especially in light of the large body of evidence linking oxidative stress to the development of atherosclerosis, as well as our recent finding that the cellular thiol redox state of macrophages is a strong predictor of lesion development 48. With the identification of Nox4 as an alternative source of ROS and a mediator of macrophage death, our data offer a possible explanation for this paradox and suggest that the role of macrophage-derived ROS in atherogenesis should be revisited.
In summary, we identified Nox4 as a novel, inducible source of ROS in monocytes and macrophages, and we demonstrated that Nox4-derived ROS mediate OxLDL-induced macrophage death. Our results suggest that monocytic Nox4 may play an important role in macrophage function and the development and progression of atherosclerosis.
Novelty and Significance.
What is known?
Macrophage death contributes to the formation of necrotic cores within atherosclerotic plaques and promotes the progression of these lesions.
Increased formation of reactive oxygen species (ROS) induced by oxidatively modified low density lipoprotein (OxLDL) promotes macrophage death.
The source of these ROS in macrophages is not known.
What new information does this article contribute?
In addition to the classic phagocytic NADPH oxidase Nox2, we identified the inducible NADPH oxidase Nox4 as a novel source of ROS in monocytes and macrophages.
Expression of the ROS-generating Nox4-p22phox complex is induced by OxLDL
Nox4 but not Nox2 is required for OxLDL to promote cell death in macrophages.
OxLDL accumulates in atherosclerotic lesions and is known to be cytotoxic not only to vascular cells but also to macrophages. Macrophage death induced by OxLDL requires the generation of intracellular ROS; but the source of these ROS in macrophages has not been identified. While the phagocytic NADPH oxidase Nox2 was a likely candidate, studies in Nox2 knockout mice showed that deletion of Nox2 in macrophages has no effect on atherogenesis. We therefore wanted to know whether macrophages express a second as yet unidentified member of these professional ROS-generating enzymes. Using expression profiling approaches and highly-specific monoclonal antibodies, we identified Nox4 as a novel inducible source of ROS in monocytes and macrophages. We found that knockdown of Nox4, but not Nox2, prevented OxLDL-induced ROS formation and macrophage death, and that overexpression of Nox4 enhanced OxLDL cytotoxicity. We also identified the MEK/ERK pathway as a mediator of OxLDL-induced Nox4 expression and ROS production. Furthermore, we observed that Nox4 localizes not only to the ER but also to discrete foci within nuclei, implicating Nox4 in nuclear redox signaling. These data are the first to document Nox4 in human monocytes and macrophages and to identify a role for Nox4 in macrophage redox signaling and cell death.
Supplementary Material
Acknowledgments
ACKNOWLEGMENTS
None
FUNDING SOURCES
This work was supported by grants to R.A. from the NIH (HL-70963) and the AHA (0855011F). Confocal images were generated in the Core Optical Imaging Facility which is supported by UTHSCSA, NIH-NCI P30 CA54174 (San Antonio Cancer Institute), NIH-NIA P30 AG013319 (Nathan Shock Center) and (NIH-NIA P01AG19316).
ABBREVIATIONS
- BMP4
Bone morphogenic protein 4
- DCFH-DA
Dichlorofluorescin diacetate
- Dox
Doxycycline
- EGF
Epidermal growth factor
- ER
Endoplasmic Reticulum
- HAEC
Human aortic endothelial cells
- HMDM
Human monocyte-derived macrophages
- JAK2
Janus Kinase 2
- JNK
Jun N-terminal kinase
- MAPK
Mitogen-activated protein kinase
- MEK
MAPK/ERK kinase 1
- MNC
Human mononuclear cells
- MPM
Mouse peritoneal macrophages
- NADPH
Nicotinamide adenine dinucleotide phosphate
- Nox
NADPH oxidase
- OxLDL
Oxidized low density lipoprotein
- PTP1B
Protein tyrosine phosphatase 1B
- ROS
Reactive oxygen species
- TGF-β
transforming growth factor beta
Footnotes
DISCLOSURES
There are no conflicts to disclose.
References
- 1.Guzik TJ, Griendling K. NADPH oxidases - molecular understanding finally reaching the clinical level? Antioxid Redox Signal. 2009;11:2365–2370. doi: 10.1089/ars.2009.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 3.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 4.Segal AW. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 1987;326:88–91. doi: 10.1038/326088a0. [DOI] [PubMed] [Google Scholar]
- 5.Teahan C, Rowe P, Parker P, Totty N, Segal AW. The X-linked chronic granulomatous disease gene codes for the beta-chain of cytochrome b-245. Nature. 1987;327:720–721. doi: 10.1038/327720a0. [DOI] [PubMed] [Google Scholar]
- 6.Nauseef WM. Biological roles for the NOX family NADPH oxidases. J Biol Chem. 2008;283:16961–16965. doi: 10.1074/jbc.R700045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banfi B, Maturana A, Jaconi S, Arnaudeau S, Laforge T, Sinha B, Ligeti E, Demaurex N, Krause KH. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 2000;287:138–142. doi: 10.1126/science.287.5450.138. [DOI] [PubMed] [Google Scholar]
- 8.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 9.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 10.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 11.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 12.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a Novel Regulator of Nox4 and Cytoskeletal Integrity in Vascular Smooth Muscle Cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 14.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR., Jr Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299:2287–2293. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato R, Mori C, Kitazato K, Arata S, Obama T, Mori M, Takahashi K, Aiuchi T, Takano T, Itabe H. Transient increase in plasma oxidized LDL during the progression of atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:33–39. doi: 10.1161/ATVBAHA.108.164723. [DOI] [PubMed] [Google Scholar]
- 17.Heinloth A, Heermeier K, Raff U, Wanner C, Galle J. Stimulation of NADPH oxidase by oxidized low-density lipoprotein induces proliferation of human vascular endothelial cells. J Am Soc Nephrol. 2000;11:1819–1825. doi: 10.1681/ASN.V11101819. [DOI] [PubMed] [Google Scholar]
- 18.Asmis R, Begley JG. Oxidized LDL promotes peroxide-mediated mitochondrial dysfunction and cell death in human macrophages: a caspase-3-independent pathway. Circ Res. 2003;92:e20–e29. doi: 10.1161/01.res.0000051886.43510.90. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Qiao M, Mieyal JJ, Asmis LM, Asmis R. Molecular mechanism of glutathione-mediated protection from oxidized low-density lipoprotein-induced cell injury in human macrophages: role of glutathione reductase and glutaredoxin. Free Radic Biol Med. 2006;41:775–785. doi: 10.1016/j.freeradbiomed.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 20.Ball RY, Stowers EC, Burton JH, Cary NR, Skepper JN, Mitchinson MJ. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 1995;114:45–54. doi: 10.1016/0021-9150(94)05463-s. [DOI] [PubMed] [Google Scholar]
- 21.Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. 2009;50 (Suppl):S382–S387. doi: 10.1194/jlr.R800032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teissier E, Nohara A, Chinetti G, Paumelle R, Cariou B, Fruchart JC, Brandes RP, Shah A, Staels B. Peroxisome proliferator-activated receptor alpha induces NADPH oxidase activity in macrophages, leading to the generation of LDL with PPAR-alpha activation properties. Circ Res. 2004;95:1174–1182. doi: 10.1161/01.RES.0000150594.95988.45. [DOI] [PubMed] [Google Scholar]
- 23.Kirk EA, Dinauer MC, Rosen H, Chait A, Heinecke JW, LeBoeuf RC. Impaired superoxide production due to a deficiency in phagocyte NADPH oxidase fails to inhibit atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2000;20:1529–1535. doi: 10.1161/01.atv.20.6.1529. [DOI] [PubMed] [Google Scholar]
- 24.Volpp BD, Nauseef WM, Clark RA. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science. 1988;242:1295–1297. doi: 10.1126/science.2848318. [DOI] [PubMed] [Google Scholar]
- 25.Nunoi H, Rotrosen D, Gallin JI, Malech HL. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science. 1988;242:1298–1301. doi: 10.1126/science.2848319. [DOI] [PubMed] [Google Scholar]
- 26.Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254:1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- 27.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- 28.Wintergerst ES, Jelk J, Asmis R. Differential expression of CD14, CD36 and the LDL receptor on human monocyte-derived macrophages. A novel cell culture system to study macrophage differentiation and heterogeneity. Histochem Cell Biol. 1998;110:231–241. doi: 10.1007/s004180050285. [DOI] [PubMed] [Google Scholar]
- 29.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzen JA, Dadabay CY, Fischer EH. COOH-terminal sequence motifs target the T cell protein tyrosine phosphatase to the ER and nucleus. J Cell Biol. 1995;131:631–643. doi: 10.1083/jcb.131.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao M, Kisgati M, Cholewa JM, Zhu W, Smart EJ, Sulistio MS, Asmis R. Increased expression of glutathione reductase in macrophages decreases atherosclerotic lesion formation in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:1375–1382. doi: 10.1161/ATVBAHA.107.142109. [DOI] [PubMed] [Google Scholar]
- 32.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 35.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 36.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 38.Hardwick SJ, Hegyi L, Clare K, Law NS, Carpenter KL, Mitchinson MJ, Skepper JN. Apoptosis in human monocyte-macrophages exposed to oxidized low density lipoprotein. J Pathol. 1996;179:294–302. doi: 10.1002/(SICI)1096-9896(199607)179:3<294::AID-PATH590>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 39.Yang S, Madyastha P, Bingel S, Ries W, Key L. A new superoxide-generating oxidase in murine osteoclasts. J Biol Chem. 2001;276:5452–5458. doi: 10.1074/jbc.M001004200. [DOI] [PubMed] [Google Scholar]
- 40.Royer-Pokora B, Kunkel LM, Monaco AP, Goff SC, Newburger PE, Baehner RL, Cole FS, Curnutte JT, Orkin SH. Cloning the gene for an inherited human disorder--chronic granulomatous disease--on the basis of its chromosomal location. Nature. 1986;322:32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- 41.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 42.Toledano MB, Leonard WJ. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A. 1991;88:4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helmcke I, Heumuller S, Tikkanen R, Schroder K, Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- 45.Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Clavreul N, Adachi T, Pimental DR, Ido Y, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. Faseb J. 2006;20:518–520. doi: 10.1096/fj.05-4875fje. [DOI] [PubMed] [Google Scholar]
- 47.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;349:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 48.Qiao M, Zhao Q, Lee CF, Tannock L, Smart EJ, LeBaron RG, Phelix CF, Rangel Y, Asmis R. Thiol Oxidative Stress Induced by Metabolic Disorders Amplifies Macrophage Chemotactic Responses and Accelerates Atherogenesis and Kidney Injury in LDL Receptor-Deficient Mice. Arterioscler Thromb Vasc Biol. 2009;29:1779–1786. doi: 10.1161/ATVBAHA.109.191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.