Abstract
Magnetic MFe2O4 (M = Fe, Co, or Mn) nanoparticles with uniform diameters in the 4–20 nm range and with excellent material properties, reported previously, can be rendered soluble in water or aqueous buffers using a combination of alkylphosphonate surfactants and other surfactants such as ethoxylated fatty alcohols or phospholipids. Surfactant-modified oligonucleotides can be incorporated into the particles’ organic shell. The particles can withstand salt concentrations up to 0.3 M, temperatures up to 90 °C, and various operations such as concentration to dryness, column or membrane separations, and electrophoresis. The particles can be selectively hybridized to DNA-functionalized gold surfaces with high coverages using a two-story monolayer structure. These particles may find valuable applications involving the magnetic detection of small numbers of biomolecules using spin valves, magnetic tunnel junctions, or other sensors.
I. Introduction
Magnetic sensors have undergone great advances in the past decade, leading to suggestions that biological detection methods involving surface-bound fluorescent tags may be rivaled by magnetic analogues.1–4 In a prototype of such a scheme, a probe DNA oligomer is attached to the sensor surface, and a magnetic tag is attached to the target oligomer. Under the right conditions, a complementary target oligomer will hybridize to the probe, bringing the magnetic tag close to the sensor, whereas noncomplementary targets are washed away. Induced dipoles of the magnetic tags in proximity to the sensor cause a measurable signal variation.
To achieve detection limits approaching a single biomolecular binding event, it is important that the magnetic tags have uniform, high moments, which requires their sizes and shapes to be similar. Their sizes should be comparable to those of the biomolecules to allow for efficient mass transport and control of the number of biomolecules per tag. Ideally, they will be slightly below the size at which they become superparamagnetic, so they have the maximum possible saturation moment while still having zero coercivity and high permeability.
Previous work has used magnetic tags composed of micron-sized polymer spheres or 100-nm-scale dextran globules embedded with multiple iron oxide particles with various sizes and shapes.1,2,5
Our group recently reported a high-yield synthetic procedure for ferrite particles that meet these requirements.6 We have since improved the procedure, allowing particles in the 10–20 nm range to be synthesized reliably in a one-pot reaction on the 100 mg scale.7 The particles have uniform size distributions and are single crystals with moments comparable to bulk values, so we infer that individual particles have nearly identical magnetic properties. Using such particles with planar spin valve sensors, we have been able to demonstrate a detection limit of about 20 nanoparticles without the help of lock-in detection.8
These particles cannot immediately be used in biological applications, because they are only soluble in hexane and other organic solvents. For such particles to be useful to biologists, they must be soluble in water in a pH range of about 5–9, at salt concentrations ranging from zero to a few hundred mM and temperatures ranging from 5 to 95 °C; these reflect typical conditions of DNA hybridization and denaturation reactions, as well as some enzymatic reactions and of storage conditions. Our previous work reported the use of a surfactant that could stabilize particles at salt concentrations less than 10 mM.6 Others have found ways to stabilize particles in water by cycling to high pH, which presumably hydrolyzes extant ligands from the surface, in the presence of capping proteins,9 or with block copolymers,10 cyclodextrins,11 or other surfactants.12 Perhaps due to the specific size and surface structure of our hydrophobic particles, these methods were not effective for us.
We have obtained particles that are stable throughout this range by replacing the hydrophobic ligands surrounding the hexane-soluble particles with a combination of surfactants. One of the surfactants, an alkylphosphonate, binds strongly to the metal oxide particle surface, and others, such as ethoxylated fatty alcohols, compose the outer part of a bilayer or intercalated structure and help confer water solubility. The affinity of alkylphosphonates for metal oxide surfaces and their ability to bind to particles has been reported by several groups, but the solubility properties of such particles have not been previously addressed or optimized.13–15
Because the outer surfactant layer is relatively labile, it is easy to substitute functionalized surfactants onto the particle surface. An aminohexyl or mercaptohexyl DNA oligomer can be chemically modified with a surfactant end group that plugs into the particle and resists being washed off.
To functionalize sensor surfaces with DNA, we assume that it is possible to apply a gold coating to the top of the sensor and use alkanethiol chemistry to attach molecules to the gold. It was found that the straightforward approach of Herne and Tarlov, in which mercaptohexyl DNA oligomers are applied directly to the gold surface and then diluted with mercaptohexanol, did not result in adequate particle coverage in our application.16 Instead, we modified a procedure by Persson et al.17 to build a multistory structure composed of a well-packed mixed monolayer composed of decanethiol and mercaptoundecanol18 which is then reacted with 4-maleimidophenyl isocyanate.19 The isocyanate group reacts with the alcohol groups of the monolayer. The thiol group of the mercaptohexyl DNA oligomer adds across the electrophilic double bond of the maleimide group, covalently tethering the DNA to the underlying monolayer. These surfaces selectively capture magnetic nanoparticles decorated with complementary DNA oligomers.
II. Experimental Section
All materials were purchased from Sigma-Aldrich unless otherwise indicated and used as received.
A. Preparation of Water-Soluble 13 nm Ferrite Particles
Hydrophobic 13 nm ferrite particles were prepared according to the procedures in refs 6 and 7, dried, weighed, and resuspended at 10–40 mg/mL concentrations in hexane. Tetramethylammonium hydrogen tetradecanephosphonate was prepared from the alkylphosphonic acid (Alfa) by adding one equivalent of methanolic tetramethylammonium hydroxide to a methanol solution, drying, and recrystallizing in tetrahydrofuran. A suspension of 10 mg of the alkylphosphonate and 30 mg of an ethoxylated fatty alcohol (steareth 10 and/or oleth 10)20 was formed in 2.5 mL of dichloromethane, and 10 mg of the particles in hexane solution was added. The mixture was shaken for 1h, and then the particles were separated with a bar magnet. The solvent was decanted, and the particles were rinsed with additional dichloromethane. The particles were then resuspended in 1 mL of a pH 7 aqueous buffer composed of 50 mM hemisodium morpholinopropanesulfonate (MOPS) and 1 mM disodium EDTA and heated in a water bath at 60–70 °C for 4 h. Overnight, a gray surfactant residue settled at the bottom; the particle suspension was decanted or filtered to remove this. Stable colloids can also be formed using other surfactants along with the alkylphosphonate, including steareth 2, ceteth 2, tetramethylammonium octadecanoate, tetramethylammonium oleate, oleth 10 carboxylate (Emulsogen COL-100, Clariant), and dipalmitoyl (or stearoyl) phosphatidylethanolamine grafted with polyoxyethylene 8, 12, or 45 (Avanti). With some of these, the magnetic separation was ineffective, so the solvent was removed by evaporation. Particles dried from deionized water, as well as free surfactants, were characterized by thermal gravimetric analysis (TA Instruments 2950, from room temperature to 600 °C at 20 °C/min), transmission electron microscopy (Phillips CM-12, 120 kV, amorphous carbon-coated copper grids), and infrared spectrometry (Nicolet Nexus 670) in potassium bromide pellets. Magnetic properties were measured by SQUID magnetometry (Quantum Designs MPMS2) of particles dried on tared silicon substrates; masses were corrected by the percent losses observed in the thermal gravimetric analyses to reflect the properties of the ferrite material only. Colloidal stability was tested by heating to 70–90 °C in various 100 mM buffers including cyclohexylaminoethanesulfonate (CHES, pH 9) and acetate (pH 5).
B. Attachment of DNA Oligomers to Particles
The complementary DNA oligomers aminohexyl-TTTTTGCTGGAATTCGTCAGACTG and aminohexyl-TTTTTCAGTCTGACGAATTCCAGC were obtained from Integrated DNA Technologies and stored frozen as 1 mM solutions in otherwise deionized water. Other oligomers with a longer poly-T segment (25 total) were also used. The succinimidyl ester of Emulsogen COL-100 was prepared by dissolving equimolar amounts of it and N-hydroxysuccinimide in dichloromethane (1 mmol/5 mL) and then adding an equimolar amount of a solution of dicyclohexylcarbodiimide in dichloromethane (1 mmol/mL) and stirring in a room-temperature water bath. The precipitated dicyclohexylurea byproduct was removed by filtration and the solvent removed by rotary evaporation. The product was stored at −20 °C. A fresh 3 mg/mL solution in the MOPS buffer mentioned above was prepared, and 5 µL (12 nmol) was added to 1 µL (1 nmol) of DNA and incubated at room temperature for 2 h. A 5 µL portion of the aqueous particle solution (14 pmol) was added, and the mixture was incubated for 1 h at 50 °C. Excess DNA and surfactant were removed by centrifugal filtration (YM-100, Millipore) to dryness three times using 0.5 mL volumes of buffer. The particles were then resuspended in 15–50 µL of 50 mM trisodium citrate (pH 7). The presence of DNA on the particles was confirmed by differences in electrophoretic migration rates of particles (0.6% agarose in the MOPS buffer with 1 µL of 1% ethidium bromide/100 mL of gel) and by specific hybridization of a Cy5 dye-terminated oligomer to only one of the two sequences above. A 1 µL portion of 0.1 mM Cy5-DNA was added to each of the batches of these particles and incubated at 37 °C for 12 h. The particles were filtered as above in warm citrate buffer, resuspended, spotted onto a glass slide, and imaged on a microarray scanner (Axon GenePix 4000A). Removal of excess DNA by filtration was confirmed by UV imaging of the above gel.
C. Preparation of Surfaces
A 100 nm gold film was evaporated onto a silicon wafer with a 5 nm Ti seed layer. After being coated with a protective photoresist layer, the wafer was diced into 5 mm squares on an adhesive backing. A similar wafer was prepared by sputtering 5 nm of tantalum and 5 nm of gold. Individual samples were prepared by sonicating in acetone for 10 min to remove the protective polymer, rinsing with ethanol and blowing dry, and then cleaning for 3 min in a UV–ozone cleaner (UVO-42, Jelight). The samples were then rinsed in ethanol, added to a 1 mL ethanol solution of 1 mg of mercaptoundecanol and 1 µL of decanethiol, and incubated for 3–12 h at room temperature in a dark cabinet. The samples were rinsed with ethanol, thoroughly blown dry, and added to a freshly prepared, pale yellow 5 mg/mL solution of 4-maleimidophenyl isocyanate (PMPI, Pierce) in anhydrous o-xylene (Aldrich sure-seal). This solution was incubated at 37 °C for 1 h, rinsed with ethanol, and blown dry. These surfaces were characterized by ellipsometry and X-ray photoelectron spectroscopy at each reaction stage and after quenching with a small, heteroatom-containing thiol, cystamine hydrochloride (1 mg/mL in MOPS, 2 h at room temperature, rinsed with deionized water).
Mercaptohexyl DNA oligomers with the above sequences were obtained from Integrated DNA Technologies as disulfides and deprotected by adding 1 µL of 1 M dithiothreitol in water to 140 nmol of DNA dissolved in 100 µL of deionized water and incubating for 15 min at room temperature. To remove byproducts from this reaction, we used a variation of a method described elsewhere.21 A 3 mL portion of a Sephadex G-50 slurry was added to a 3 mL syringe tube and spun in a 15 mL centrifuge tube to remove excess water, leaving a 1.5 mL column that is compressed to conform to the tube. The DNA was added and spun through the column and stored at −20 °C. The presence of deprotected DNA was confirmed by electrophoresis in a stained gel as above and by the change in migration through the gel of gold nanoparticles exposed to the DNA. A 2 µL portion of this solution was added to 30 µL of MOPS buffer, and the mixture was placed on a maleimide-terminated gold sample, kept in a box containing a large reservoir of water for 2 h, and then rinsed with buffer and blown dry.
D. Surface Hybridization
The particle suspensions from section B were added to the substrates from section C and incubated overnight at room temperature, from 1 to 4 h at 37 °C, or both in a box containing a large reservoir of water at the given temperature (similar results were obtained under all conditions). The substrates were rinsed with citrate buffer at the given temperature and blown dry. As a control experiment, xylyldithiol monolayers were prepared on gold substrates22 (cleaned as above, soaked 16 h in a 1 mg/mL dichloromethane solution of xylyldithiol, and rinsed with ethanol) which were then exposed to citrate-capped 10 nm gold particles (BBI) for 30 min at room temperature and rinsed with deionized water. Samples were mounted with graphite paste and imaged at 5 kV in an FEI Sirion scanning electron microscope.
III. Results and Discussion
A. Characterization of the Surfactant Layer
A wide range of surfactants was tested with the hydrophobic nanoparticles, and clear patterns emerged regarding the stability of the particles at salt concentrations on the order of 100 mM and at elevated temperatures. A key component is tetradecanephosphonate. Other chain lengths such as octadecanephosphonate or hexanephosphonate do not result in stable dispersions. However, tetradecanephosphonate alone does not either.
Sahoo et al. have put forth a strong argument for the formation of a quasi-bilayer structure by phosphonates on metal oxide surfaces, based on their own results and those of others.15 There is also reason to believe that the chains between layers are at least partially intercalated.10 The large headgroup of an alkylphosphonate surfactant is likely to result in large interchain spacing and a loosely packed hydrophobic region if it were only a monolayer. Particles treated with only tetradecanephosphonate, and presumably bearing alkylphosphonates in an outer surfactant layer, can form loose suspensions in deionized water that settle out within minutes, while untreated particles do not break apart from a dried assembly upon exposure to water. From this observation, it appears that phosphonate groups on the outer surface are conferring some hydrophilicity, but such groups are known to stick together23 when mediated by transition metal ions, and this hydrophilicity is likely to be affected by salt concentration and pH, so it is not surprising that alkylphosphonates alone do not result in a stable aqueous colloid. This suggests the strategy of replacing the outer phosphonates with a different surfactant. Because the inner phosphonate layer is strongly bound, it can be expected to remain unaffected by such a process.15
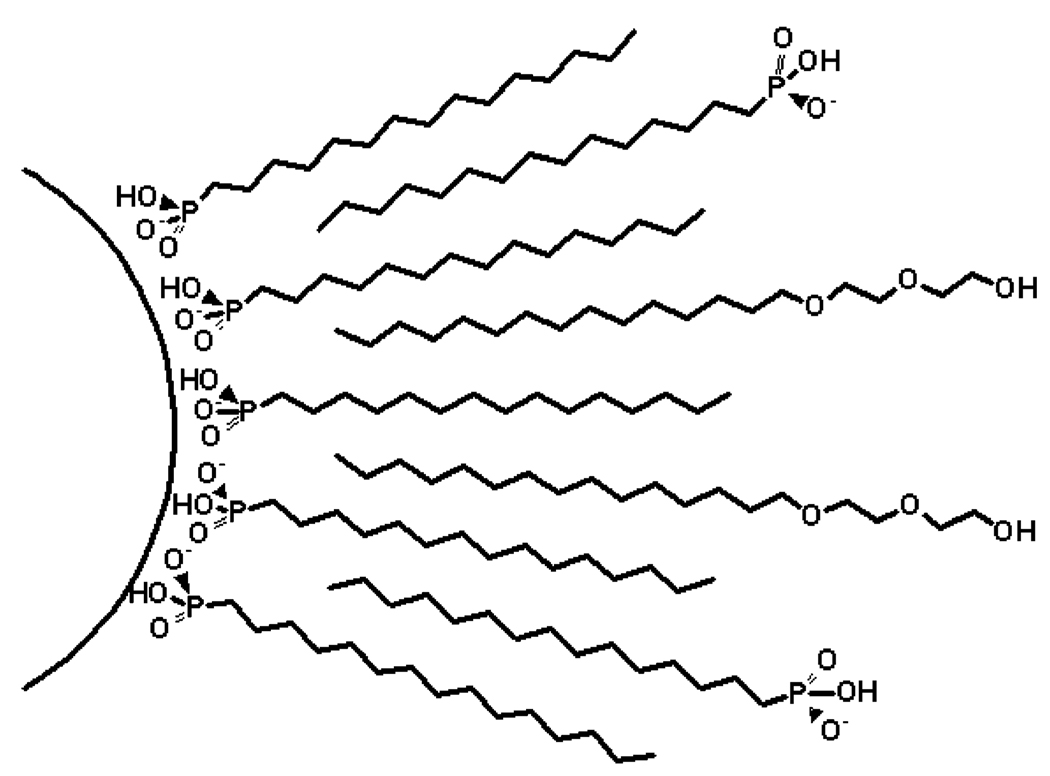
Ethoxylated fatty alcohols are good candidates for this task. Both the hydrophobic and hydrophilic ends are commercially available at a low cost over a wide range of lengths, and ethylene oxide chains confer a water solubility that is relatively independent of other solution components. We have found an ideal surfactant treatment that combines tetradecanephosphonate with steareth 10 and oleth 10. The resulting colloids have shown stability for more than 9 months when stored at room temperature. As discussed in the Experimental Section, a variety of related surfactants can be used with satisfactory results, forming colloids that are stable for as many months. A hypothetical structure for the surfactant layers on these particles is shown in Figure 1.
Figure 1.
Schematic illustration of the nanoparticle surface after the treatment with an alkyl phosphonic acid and poly-oxyethylene 2 alkyl ether.
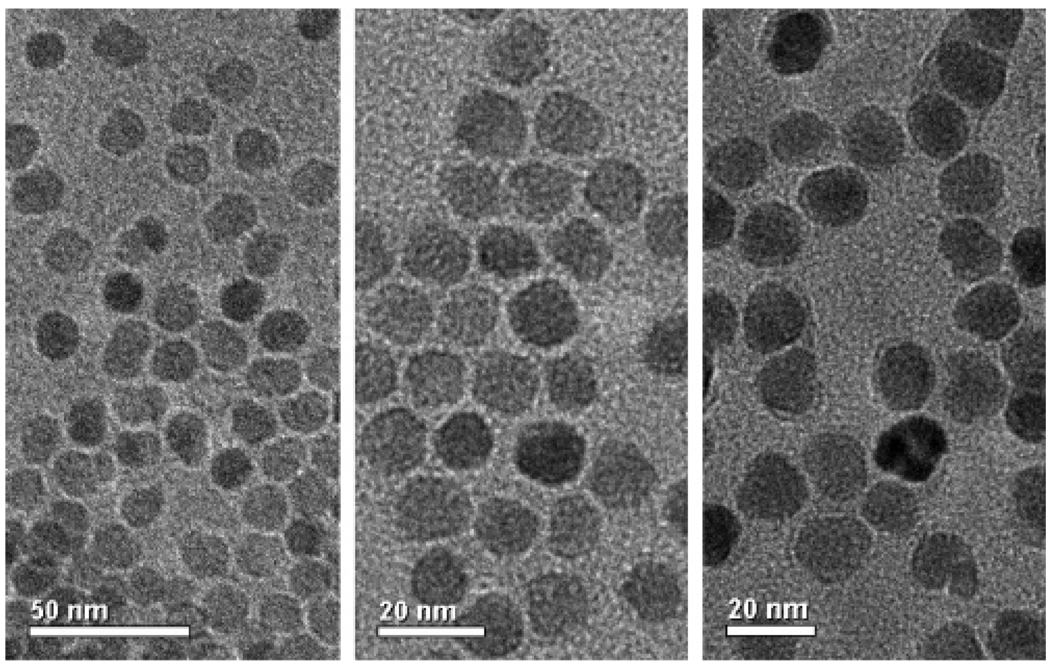
Transmission electron microscopy shows that, after the surfactant treatment, the particles have not significantly changed in size or shape24 and that, based on interparticle spacing, the organic layers around the particles are ~1–2 nm thick, consistent with the proposed structure.
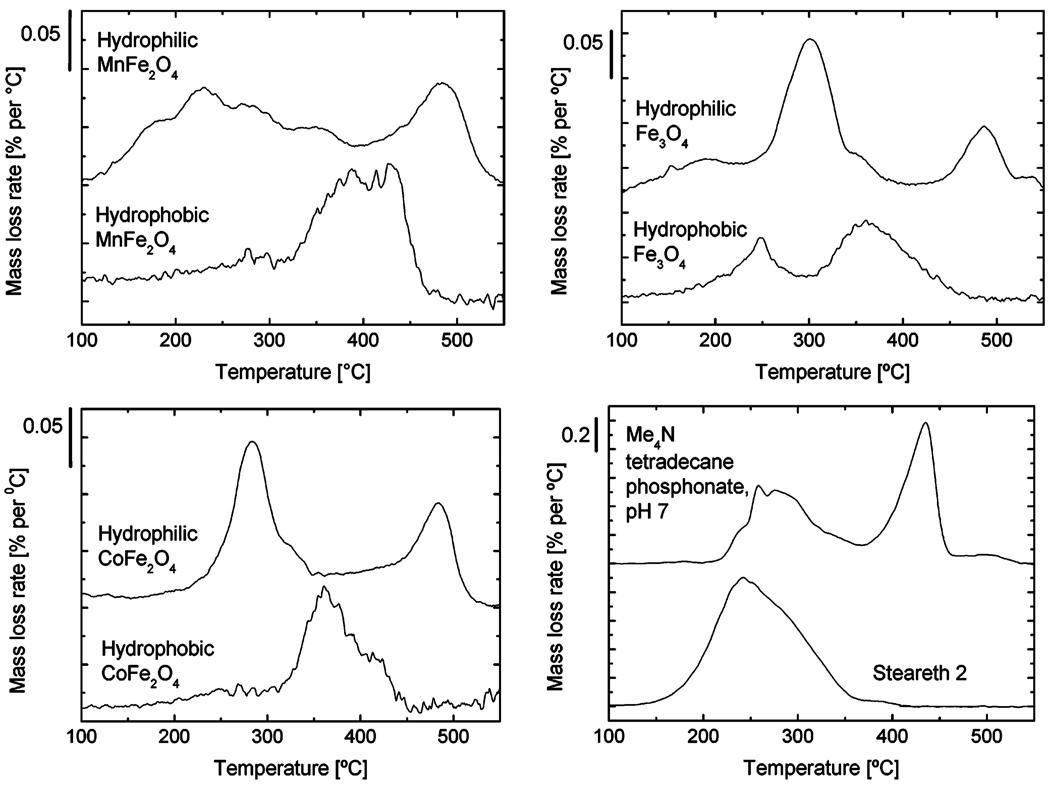
Thermal gravimetric analysis provides additional evidence for this structure. In this experiment, a small sample is heated slowly under flowing nitrogen and changes in mass are recorded. Substances that are bound more strongly volatilize at higher temperatures. Figure 3 shows a consistent, very strongly bound peak at 480 °C attributable to a surface-bound alkylphosphonate layer, in agreement with the observations of Sahoo et al.15 and different from the peak shown by the free surfactant. At much lower temperatures are more loosely bound substances. The hydrophobic particles show evidence of mostly a monolayer of surfactants with an intermediate binding strength, along with some more loosely bound material in the case of M = Fe.
Figure 3.
Thermal gravimetric analyses of the particles in Figure 2, along with the hydrophobic particles from which they were formed, and of the two free surfactants used to treat these particles.
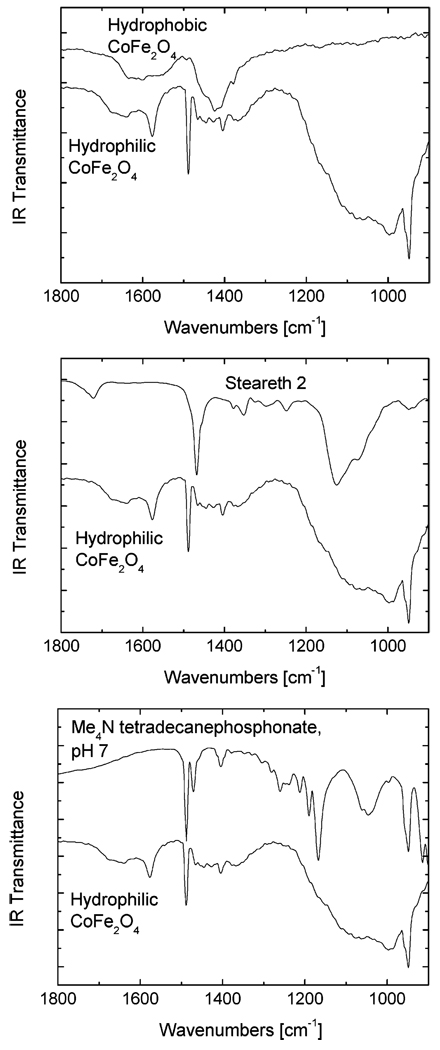
The infrared spectra show some evidence of the presence of the new surfactants. Figure 4 compares the water-soluble particles to the hydrophobic particles and each of the two surfactants. Steareth 2 shows broad ether absorbances in the 1200 cm−1 range, and there is absorbance in this region for the water-soluble particles but not the hydrophobic particles. The phosphonate P=O vibrationis also expected in this region; other phosphonate absorbances appear in the 1000–1100 range, where the water-soluble particles also absorb. Some broadening of these features can be expected on the particles due to the inhomogeneous environment at the particle surface.25
Figure 4.
Infrared transmittance spectra of water-soluble cobalt ferrite particles compared to hydrophobic particles and the two surfactants with which they were treated.
B. Material Properties
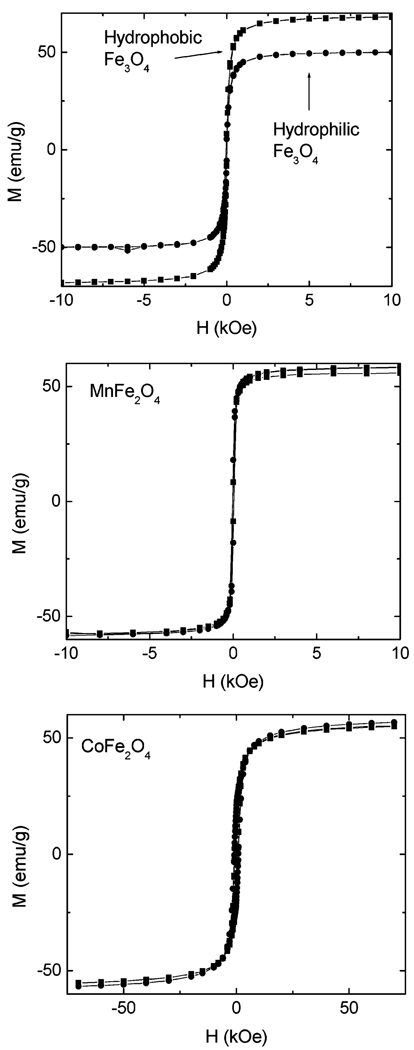
Though their solubility and surface properties are similar, important differences between the three types of particles (manganese ferrite, magnetite, and cobalt ferrite) can be seen in their magnetic properties. Figure 5 shows magnetic measurements of each type. The cobalt particles have a large room-temperature coercivity (700 Oe). The coercivity of the water-soluble particles at 10 K was measured to be 10 kOe, with a saturation magnetization of ~65 emu/g. The magnetite particles are superparamagnetic: they have no room-temperature coercivity and a steeper slope at low fields. There is a significant decrease in saturation magnetization when these particles are transferred to water, and this decrease is comparable to the difference in this property between Fe3O4 and Fe2O3. Such an oxidation reaction is plausible, especially during the step in which the particles are heated in an aqueous buffer. The manganese ferrite is also superparamagnetic, and its saturation magnetization does not change upon transfer to water. While this value is about 10% lower than that of the hydrophobic magnetite, it is greater than that of the hydrophilic iron oxide particles. For both water-soluble manganese ferrite and iron oxide, the 10 K coercivity is 200 Oe and the saturation magnetization increases by about 10% above the room-temperature value.
Figure 5.
Magnetometry of MFe2O4 particles (M = Mn, Fe, and Co) at 300 K. The data marked with circles are for water-soluble particles, and the data marked with squares are for hydrophobic particles. Note that the scale of the abscissa differs for the case of cobalt.
C. Attachment of DNA Oligomers to Particles
The bilayer on the particle surface provides a convenient platform for biofunctionalization. The particles remain stable in aqueous buffers when a range of outer-layer surfactants are used, as long as the alkylphosphonate is present to anchor the bilayer to the particle surface. One of those outer-layer surfactants could be a DNA oligomer with a hydrophobic tail. It is most convenient to use aqueous chemistry when using either DNA or water-soluble nanoparticles, so it is easier to attach a surfactant to DNA when it has finite water solubility. Oleth 10 is soluble in water, as is its terminal active ester form, succinimidyl oleth 10 carboxylate. Oleth 10 carboxylic acid is commercially available; similar ethoxylated fatty alcohols can be oxidized at the glycol end group with permanganate.26 The active ester reacts readily with the primary amine group on an aminohexyl DNA oligomer under mild conditions in water. A competing hydrolysis reaction also occurs. By incubating this modified DNA oligomer with nanoparticles, a surfactant substitution is effected.
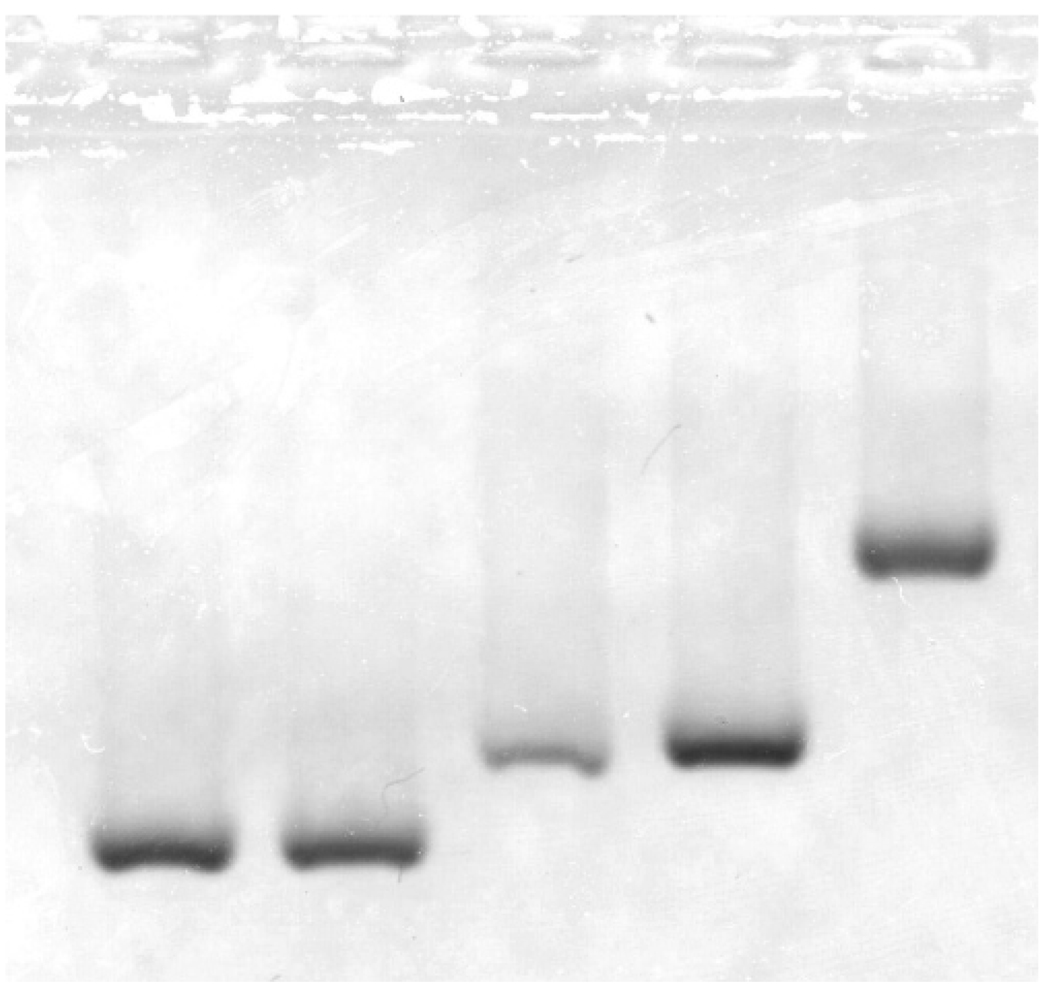
Evidence for this can be seen by electrophoresis, as shown in Figure 6. Migration through the spongy pores of the gel is expected to be affected by the overall particle size (including attached surfactants) as well as by charges associated with the particle, which may come from surface charges on the metal oxide, charges on the alkylphosphonate and DNA, and charges of ions that may coordinate to the surfactants. The two left lanes show that DNA has no measured effect on the particles in the absence of an active ester group at the end of the surfactant that can couple to the DNA. The active ester causes a shift even in the absence of DNA, as the middle lane shows, but with DNA, there is a much larger shift (rightmost lane), indicating that the DNA is attaching to the particles and slowing their migration.
Figure 6.
Electrophoresis of plain and DNA-substituted MnFe2O4 nanoparticles, reacted with (from left to right) (1) oleth 10 carboxylate, (2) oleth 10 carboxylate and DNA, (3) succinimidyl oleth 10 carboxylate, (4) a mixture of 1 and 5, prepared at room temperature less than 5 min before starting the migration; (5) succinimidyl oleth 10 carboxylate and DNA. The DNA is functionalized with an aminohexyl group at the 5′ end and is 24 bases long. Migration is toward the anode.
The second lane from the right shows that a mixture of particles with and without DNA appears in a single band in an approximately average position rather than separating into two bands coincident with the original particles. This can occur if surfactant molecules are able to transfer between particles. Because these particles have been thoroughly filtered, we do not expect that this proceeds by a mechanism in which a surfactant completely dissociates from a particle but instead exchange when the particles themselves interact closely.
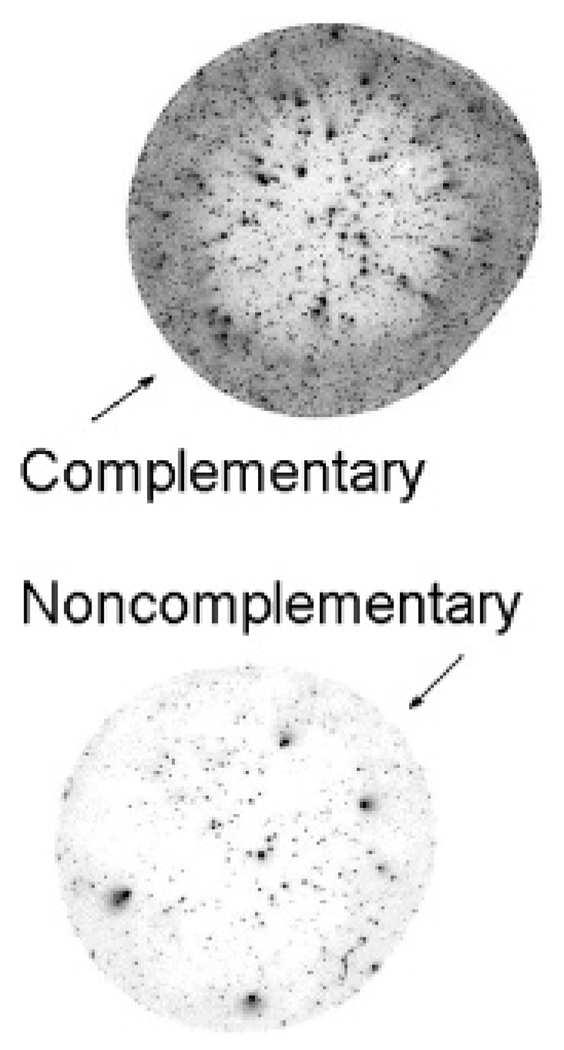
Additional evidence for the substitution of DNA onto particles is seen when an attempt is made to hybridize particles with two types of DNA to a fluorescent dyelabeled DNA oligomer. Figure 7 shows a significant contrast between particles with DNA complementary versus noncomplementary to the dye-labeled oligomer. Because the particles were thoroughly filtered before and after the hybridization reaction, such specificity would not be expected without a stable degree of substitution of DNA onto the surfaces of the particles. This also confirms that, although Figure 6 showed that surfactants can be rapidly exchanged, they strongly prefer to be on the particles rather than free in solution; otherwise, one would expect them to be removed during the filtration.
Figure 7.
Inverted image of DNA-functionalized MnFe2O4 particles spotted onto a glass slide, as seen in a microarray scanner, after attempted hybridization with a fluorescent dye-labeled oligomer. For the top spot, the dye-labeled oligomer is complementary to the oligomers on the particles. For the bottom spot, the two oligomers are noncomplementary.
D. Attachment of DNA Oligomers to Surfaces
The use of DNA-functionalized magnetic nanoparticles with magnetic sensors requires an appropriate chemical interface at the sensor surface. We have chosen to work with gold surfaces because of the relatively well understood chemistry of alkanethiol monolayers on this material. However, we were not able to obtain sufficient particle attachment using published methods to attach DNA oligomers to gold surfaces, including direct attachment of mercaptohexyl DNA to gold and the use of active esters on an existing monolayer.16,27 With direct attachment, according to these reports, there are some conditions under which thiol adsorption is eclipsed by nonspecific physisorption of DNA, a situation where the DNA is less accessible for hybridization and coverage is suboptimal. Coverage was also shown to be a strong function of buffer concentration under conditions similar to those reported here.
Such concerns led Boncheva et al. to develop approaches for the covalent attachment of DNA to the top of a thicker, preformed monolayer using active ester chemistry.27 The preformed monolayer minimizes direct DNA–gold interaction and provides a firmer, more hydrophobic anchor to the gold surface that can be expected to withstand a wider range of conditions. Unfortunately, active esters are prone to hydrolysis, and the combined steric constraints of the monolayer geometry and the bulky leaving group can hinder reactivity toward the desired species. To mitigate these problems, their reported procedures use DNA with unusual modifications or solvent conditions.
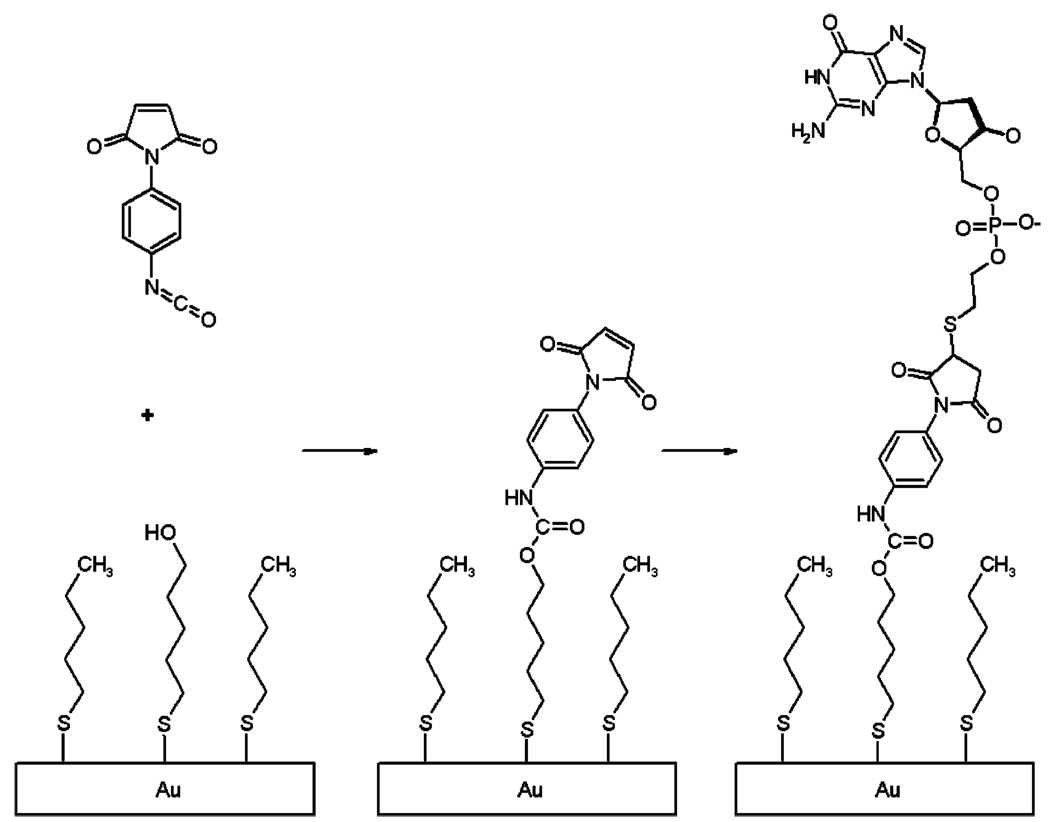
We have adopted another method for gold surface functionalization17 that builds on a preformed, well-packed monolayer, uses coupling chemistry that is more hydrolytically stable and less sterically hindered, and works with common DNA modifications and buffers. First, a mercaptoundecanol monolayer is formed, and then a heterobifunctional coupling agent28 is attached, in which an aryl isocyanate reacts with the monolayer’s terminal hydroxyl groups to form a urethane bond in an aprotic solvent. Mercaptohexyl DNA oligomers are then coupled to the electrophilic double bond of a maleimide group on the coupling agent, a process that involves no leaving group. Figure 8 illustrates this scheme.
Figure 8.
Two-story monolayer structure for DNA attachment. A heterobifunctional coupling agent is added to a preformed mercaptoundecanol monolayer, which is then reacted with mercaptohexyl DNA.
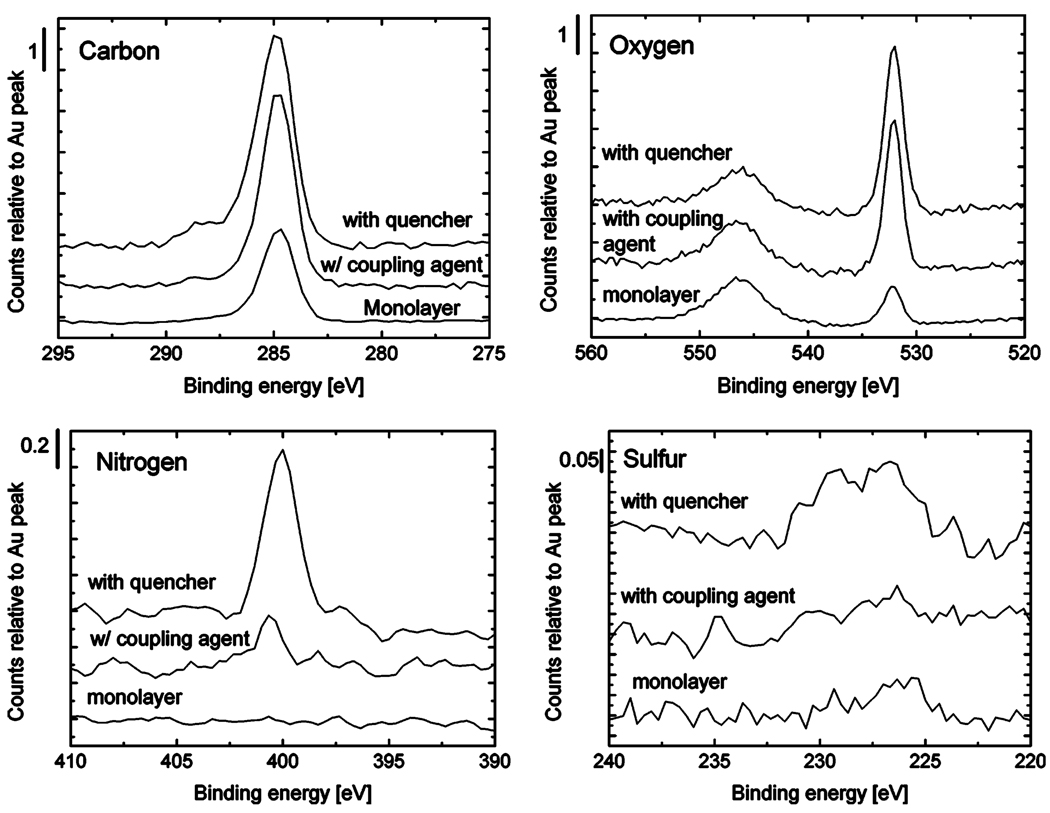
Each step of this process can be monitored by X-ray photoelectron spectroscopy and by ellipsometry. Figure 9 shows the change in surface composition as components are added. The original monolayer shows a significant amount of carbon, some oxygen, no nitrogen, and very little sulfur, which exists in only monolayer amounts and is expected to be buried under a long path length of carbon. The ellipsometric thickness is 1.1 nm, about what one would expect for a well-packed monolayer. The coupling agent adds carbon, oxygen, and nitrogen to the top of the layer, all of which appear in the spectra. These peaks may appear larger than one would expect for the number of atoms added because the thicker monolayer (measured as 1.7 nm)29 is expected to reduce the relative size of the gold peak, to which all other peaks for a given sample are normalized. Cystamine was added to test the reactivity of the maleimide, and additional nitrogen, sulfur, and carbon are seen. Additional oxygen also appears, perhaps due to hydration of the amine group.
Figure 9.
Narrow-scan X-ray photoelectron spectra of a two-story monolayer structure, starting with a mercaptoundecanol monolayer, then adding 4-maleimidophenyl isocyanate as a coupling agent, and then adding cystamine as a quencher. Background was subtracted for all peaks, and peaks for each sample were normalized with respect to the gold peak at 546 eV.
E. Hybridization of Particles to Surfaces
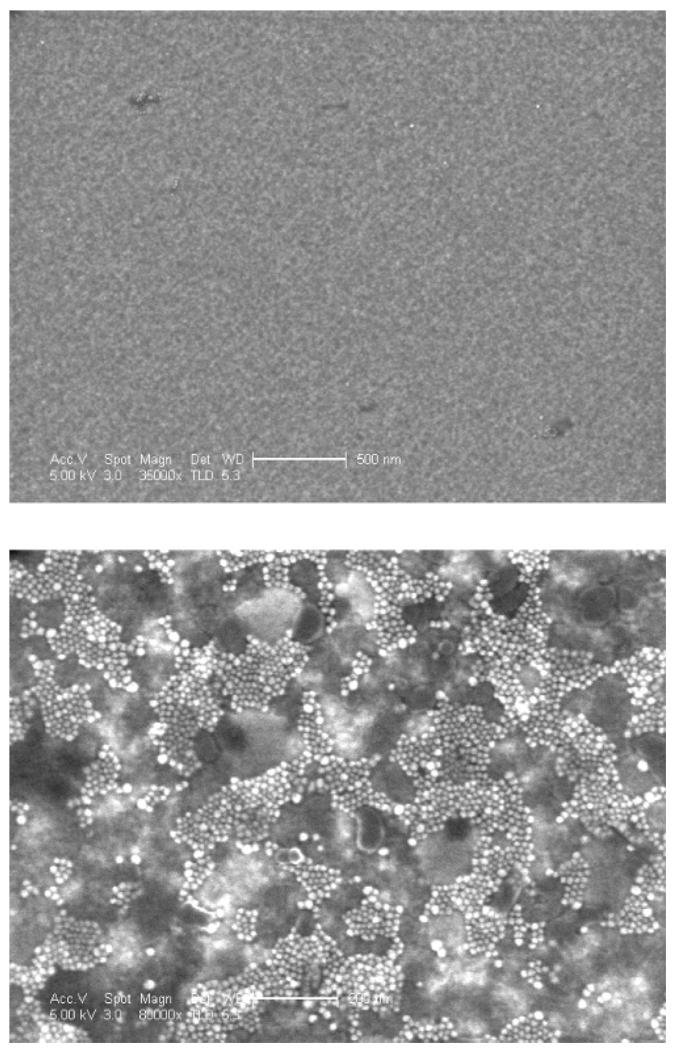
A direct test of the feasibility of the hybridization of magnetic particles to surfaces is by observation of them by electron microscopy. Figure 10 shows the results of attempts to hybridize particles with two types of DNA to a surface functionalized as above. When the particle and surface oligomers are noncomplementary, very few particles are seen (the low-contrast texture is due to the substrate topography).When the two oligomers are complementary, we see many two-dimensional domains of high-contrast, 15 nm particles on the surface. Similar results have been observed after varying the substrate thickness (as discussed in the Experimental Section), varying the mole fraction of mercaptoundecanol in the monolayer (including 100%), and using a few of the different types of particle surfactants discussed above. This provides clear evidence that the surface coupling and hybridization reactions behave as desired. It should benoted that, while this result was reliably obtained on unpatterned gold surfaces, this was not the case when gold was patterned with micron-scale features on other substrates for which the surface chemistry was less carefully controlled.
Figure 10.
Scanning electron micrographs of DNA-functionalized MnFe2O4 particles exposed to DNA-functionalized gold substrates. For the top image, the particle DNA is noncomplementary to the surface DNA (500 nm scale bar). For the bottom image, the particle DNA is complementary to the surface DNA (200 nm scale bar). The particles appear as high-contrast dots that are ~15 nm wide.
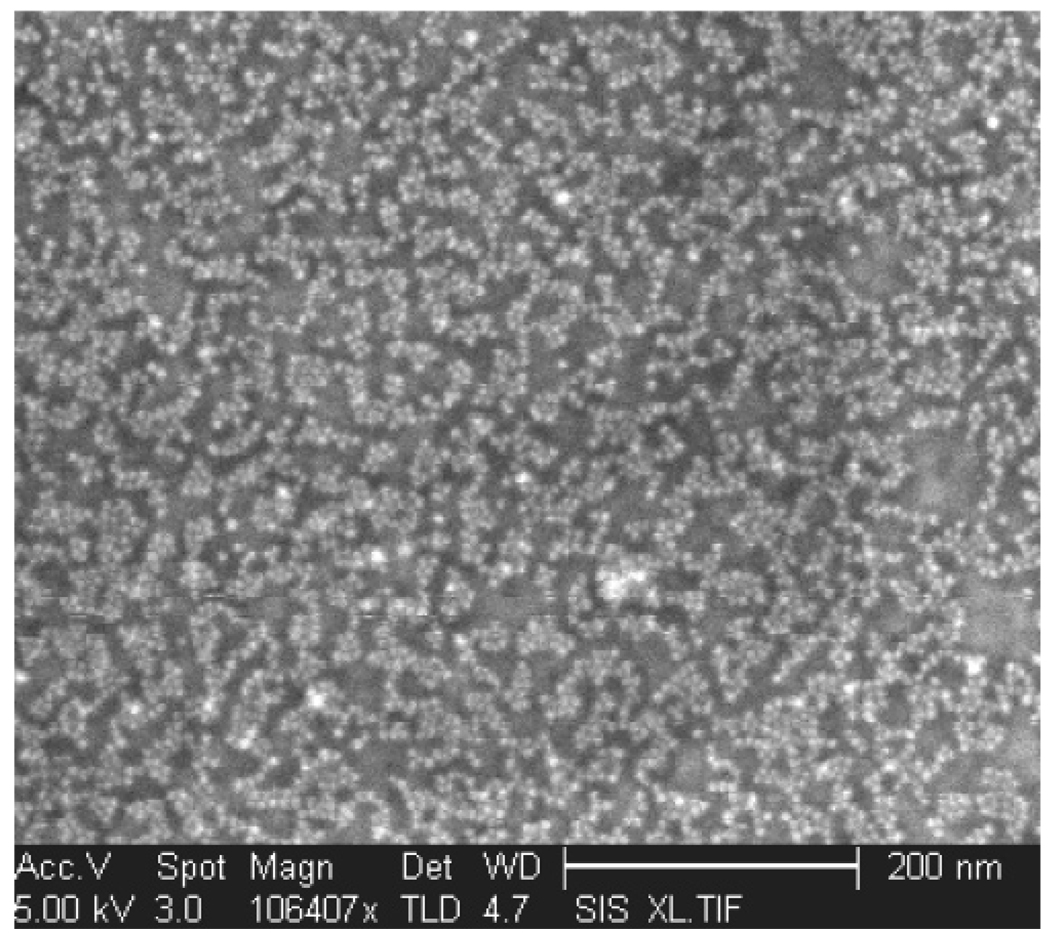
Figure 10 invites the question of why more uniform coverage is not obtained on the 100 nm length scale. It may be that it is, but many particles come off during the rinsing step. The dark patches in this image may be places where hydrophobic material has bound to free surfactant-modified DNA from a particle that fell off or a micelle that was not removed in the filtration step. Alternatively, particles may bind randomly and uniformly at lower coverage and then rearrange into islands, perhaps leaving some organic material behind. In a step toward understanding this, we compare the results of Figure 10 with an analogous arrangement that uses much simpler chemistry. It has been shown previously that gold nanoparticles can bind to gold surfaces mediated by a xylyldithiol monolayer; one end of this molecule attaches to the surface, and exposed thiol groups can capture aqueous gold particles. An electron micrograph of such a layer is shown in Figure 11. Coverage is not complete, and similar two-dimensional clustering is observed, although the length scale of the nonuniformity is slightly smaller. This result leads us to believe that the clustering observed in Figure 10 is not a fluke associated with our surface chemistry but instead reflects a more universal property of nanoparticle assembly on functional monolayer surfaces.
Figure 11.
10 nm gold particles adsorbed on a xylyldithiol monolayer on a gold substrate.
IV. Conclusions
Through simple treatments with low-cost surfactants, monodisperse ferrite nanoparticles can be made to form stable colloids in aqueous buffers over a useful range of temperatures and salt concentrations and further functionalized with DNA oligomers. The combination of a strongly bound alkylphosphonate inner surfactant layer and an outer surfactant layer that is more readily exchanged provides a flexible platform for particle functionalization. Because of its stable, high saturation magnetization and room-temperature superparamagnetism, manganese ferrite is an especially promising material for potential applications of these particles.
Monolayer-coated gold surfaces can be prepared that allow for high coverages of water-soluble thiols with short alkyl chains (such as a mercaptohexyl DNA oligomer) to be firmly attached. DNA-functionalized ferrite particles can be selectively hybridized to these surfaces: few particles adsorb when the DNA oligomers are noncomplementary, whereas many do when they are complementary. For the complementary case, an interesting clustering phenomenon occurs that seems to be a generalizable result worthy of further study.
These results constitute a prototypical chemical platform for the development of a biological detection system in which the proximity of a magnetic particle to a magnetic sensor acts as a proxy for a biochemical binding event. Such a sensor may be a spin valve, in which an external field modulates the magnetoresistance of a thin film structure, or a magnetic tunnel junction, in which the field modulates the tunneling of electrons through an insulating barrier from one electrically conducting magnetic material to another.3,30 Both of these are suited to the application of gold–thiol surface chemistry with appropriate modifications to their fabrication. However, the detection of a small number of binding events–a key reason for using such uniformly sized particles–would require the use of small detector areas, approaching the length scale of the nonuniformity seen in Figure 10; for this specific application, it would be desirable to have greater control over this, as well as over the surface chemistry of areas adjacent to the sensor. These are opportunities for additional work that may also be of more general utility.
The gold surface attachment chemistry developed to solve problems encountered here may find valuable applications to other types of biosensors that rely on other phenomena, such as electrochemical effects. Water-soluble magnetic nanoparticles have also been considered for applications such as drug delivery, contrast agents for magnetic resonance imaging, and hyperthermia treatments for cancer patients.31 The well-defined material properties, stability, and chemical flexibility of the particles presented here may facilitate these efforts.
Figure 2.
Bright-field transmission electron micrographs of MFe2O4 (M = Mn, Fe, and Co from left to right) treated with tetradecanephosphonate and steareth 2 and cast from deionized water.
Acknowledgment
This work is supported in part by DARPA through ONR under Grant Nos. N00014-01-1-0885.
References
- 1.Baselt DR, Lee GU, Natesan M, Metzger SW, Sheehan PE, Colton RJ. Biosens. Bioelectron. 1998;13:731. doi: 10.1016/s0956-5663(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira HA, Graham DL, Freitas PP, Cabral JMS. J. Appl. Phys. 2003;93:7281. [Google Scholar]
- 3.Li GX, Wang SX, Sun SH. IEEE Trans. Magn. 2004;40:3000. [Google Scholar]
- 4.Brzeska M, Panhorst M, Kamp PB, Schotter J, Reiss G, Puhler A, Becker A, Bruckl H. J. Biotechnol. 2004;112:25. doi: 10.1016/j.jbiotec.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Graham DL, Ferreira H, Bernardo J, Freitas PP, Cabral JMS. J. Appl. Phys. 2002;91:7786. [Google Scholar]
- 6.Sun SH, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li GX. J. Am. Chem. Soc. 2004;126:273. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- 7.Zeng H, Rice PM, Wang SX, Sun S. J. Am. Chem. Soc. 2004;126:11458. doi: 10.1021/ja045911d. [DOI] [PubMed] [Google Scholar]
- 8.Wang SX, Bae S-Y, Li GX, Sun S, White RL, Kemp JT, Webb CD. J. Magn. Magn. Mater. in press. [Google Scholar]
- 9.Grancharov S, Held G. Personal communication [Google Scholar]
- 10.Shen LF, Laibinis PE, Hatton TA. Langmuir. 1999;15:447. [Google Scholar]
- 11.Wang Y, Wong JF, Teng XW, Lin XZ, Yang H. Nano Lett. 2003;3:1555. [Google Scholar]
- 12.Xu XQ, Shen H, Xu JR, Li XJ. Appl. Surf. Sci. 2004;221:430. [Google Scholar]
- 13.Gao W, Dickinson L, Grozinger C, Morin FG, Reven L. Langmuir. 1996;12:6429. [Google Scholar]
- 14.Textor M, Ruiz L, Hofer R, Rossi A, Feldman K, Hahner G, Spencer ND. Langmuir. 2000;16:3257. [Google Scholar]
- 15.Sahoo Y, Pizem H, Fried T, Golodnitsky D, Burstein L, Sukenik CN, Markovich G. Langmuir. 2001;17:7907. [Google Scholar]
- 16.Herne TM, Tarlov MJ. J. Am. Chem. Soc. 1997;119:8916. [Google Scholar]
- 17.Persson HHJ, Caseri WR, Suter UW. Langmuir. 2001;17:3643. [Google Scholar]
- 18.Chidsey CED, Loiacono DN. Langmuir. 1990;6:682. [Google Scholar]
- 19.Jin L, Horgan A, Levicky R. Langmuir. 2003;19:6968. [Google Scholar]
- 20.These are more systematically known as polyoxyethylene 10 octadecyl (or oleyl) ether.
- 21.Gullberg M, Gustafsdottir SM, Schallmeiner E, Jarvius J, Bjarnegard M, Betsholtz C, Landegren U, Fredriksson S. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8420. doi: 10.1073/pnas.0400552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson JI, Feng S, Ferrence GM, Bein T, Kubiak CP. Inorg. Chim. Acta. 1996;242:115. [Google Scholar]
- 23.Schilling ML, Katz HE, Stein SM, Shane SF, Wilson WL, Buratto S, Ungashe SB, Taylor GN, Putvinski TM, Chidsey CED. Langmuir. 1993;9:2156. [Google Scholar]
- 24.Compare to Figure 1b and Figure 7 in ref 6.
- 25.Hostetler MJ, Stokes JJ, Murray RW. Langmuir. 1996;12:3604. [Google Scholar]
- 26. Carbonell RG, Kilpatrick PK, Torres JL, Guzman R. 5,045,190. U.S. Patent. 1991. See example 10. The procedure can be modified to allow the oxidation of other surfactants by using dioxane–water mixtures and changing the amount of solvent.
- 27.Boncheva M, Scheibler L, Lincoln P, Vogel H, Akerman B. Langmuir. 1999;15:4317. [Google Scholar]
- 28.Annunziato ME, Patel US, Ranade M, Palumbo PS. Bioconjugate Chem. 1993;4:212. doi: 10.1021/bc00021a005. [DOI] [PubMed] [Google Scholar]
- 29.0.6 nm was added to the thickness; this is comparable to the 0.9 nm length of the coupling agent as claimed by the vendor.
- 30.Reiss G, Bruckl H, Hutten A, Koop H, Meyners D, Thomas A, Kammerer S, Schmalhorst J, Brzeska M. Phys. Status Solidi A. 2004;201:1628. [Google Scholar]
- 31.Murray CB, Sun SH, Doyle H, Betley T. MRS Bull. 2001;26:985. [Google Scholar]