Abstract
In premenopausal women, elevated C-reactive protein (CRP) concentrations have been associated with an increased risk of negative reproductive outcomes. Whole grain consumption has been associated with lower CRP concentrations in older women; however, less is known about this relationship in younger women. We investigated whether whole grain intake was associated with serum high sensitivity CRP (hs-CRP) concentrations in young women. BioCycle was a prospective cohort study conducted at the University of Buffalo from 2005 to 2007, which followed 259 healthy women aged 18–44 y for le 2 menstrual cycles. hs-CRP concentrations were measured longitudinally le 8 times/cycle with visits standardized to menstrual cycle phase. Whole grain intake was estimated by 24-h recalls le 4 times/cycle. Servings were defined as 16 g or 125 mL of a 100% whole grain food. Whole grain intake was inversely associated with hs-CRP concentrations after adjusting for age, race, BMI, illness, and antiinflammatory drug use. Consumers of between 0 and 1 serving/d of whole grains had, on average, 11.5% lower hs-CRP concentrations (P = 0.02) and consumers of ge 1 serving/d had 12.3% lower hs-CRP concentrations (P = 0.02) compared with nonconsumers. Women who consumed ge 1 serving/d of whole grain had a lower probability of having moderate (P = 0.008) or elevated (P = 0.001) hs-CRP according to the AHA criteria compared with nonconsumers. Given that elevated concentrations of hs-CRP have been linked to adverse reproductive outcomes and pregnancy complications, interventions targeting whole grain consumption may have the potential to improve health status among young women.
Introduction
C-reactive protein (CRP)7 is an acute-phase protein secreted by the liver that is a sensitive marker for subclinical inflammation (1). Elevated concentrations of CRP have recently emerged as a prominent biomarker of chronic disease risk in both young and older women. For instance, in healthy women, elevated CRP is strongly and independently associated with future heightened risk for cardiovascular disease (CVD) and cardiovascular events (2), type 2 diabetes (3), and all cancers (4). Among reproductive-aged women, elevated CRP concentrations have been associated with increased risk of pregnancy complications and adverse reproductive outcomes such as preeclampsia (5), gestational diabetes (6), preterm birth (7), fertility problems (specifically assisted reproductive technology failure) (8), and incidence of polycystic ovary syndrome (9).
Whole grain intake has been associated with lower circulating concentrations of CRP in some (10, 11), but not all (12), cohorts of older women. It is thought that whole grains exert an antiinflammatory effect through synergistic activity of their health-promoting components, which include dietary fiber, minerals, phytoestrogens, and antioxidants (13). Based on this as well as evidence that whole grains have beneficial health effects, the AHA (14), USDA (15), and American Diabetes Association (16) all recommend that at least one-half of an individual's grain intake come from whole grains. However, despite evidence of the protective effects of whole grain foods on CRP concentrations in older women and those with certain health conditions, little research has been conducted to determine whether a similar association exists in younger women. Thus, the aim of this study was to assess the association between whole grain intake and serum high sensitivity CRP (hs-CRP) concentrations in young women and evaluate whether this association was mediated by other metabolic variables.
Methods
Study design.
The BioCycle study was a prospective cohort study of menstrual cycle function among 259 regularly menstruating, healthy, premenopausal volunteers aged 18–44 y recruited from western New York and followed for up to 2 menstrual cycles. Exclusion criteria included current use of oral contraceptives, vitamin and mineral supplements, or prescription medications; pregnancy or breast-feeding in the past 6 mo; and recent history of infections or diagnosis of chronic conditions. Women with a self-reported BMI (kg/m2) <18 or >35 at screening and those planning to restrict their diet for weight loss or medical reasons were excluded. Details of this study have been published elsewhere (17). The University at Buffalo Health Sciences’ Institutional Review Board approved the study and all participants provided written informed consent.
Participants were followed for 1 (n = 9) or 2 (n = 250) menstrual cycles with fasting blood samples collected on approximately d 2 of menstruation, mid and late follicular phase, 2 d around expected ovulation, and early, mid, and late luteal phase, with collection dates scheduled using fertility monitors (Clearblue Easy Fertility Monitor; Inverness Medical) (18). Standardizing serum collection was done, because hs-CRP concentrations, among others, have been observed to vary according to reproductive hormone concentrations across the menstrual cycle in premenopausal women (19). Overall, compliance to the study protocol was high, with 94% of women completing at least 7 clinic visits/cycle and 100% completing 5 visits/cycle.
Dietary assessment.
Dietary intake was assessed up to 4 times/cycle (corresponding to menses, mid follicular phase, ovulation, and mid luteal phase) using a 24-h dietary recall. Dietary intake data were collected and analyzed using the Nutrition Data System for Research software version 2005 developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN. Whole grain servings were derived from total grain servings (defined per the Dietary Guidelines for Americans 2005 as 1 slice of bread, 1 ounce of ready-to-eat cereal, or 1/2 cup of cooked cereal, rice, or pasta) (15). Whole grains were those containing the entire grain kernel (i.e. the bran, germ, and endosperm). If a whole grain ingredient was the first ingredient on the food label, the grain product was classified as a whole grain. Products that contained only some whole grains were not included in the whole grain category. A whole grain serving was defined as 16 g or 1/2 cup (125 mL) of a 100% whole grain food. The majority of women completed 4 dietary recalls/cycle (87%) and all participants completed at least 2 recalls/cycle.
Biological specimens.
Fasting blood specimens were collected were collected between 0700 and 0830 h at each cycle visit. hs-CRP was measured in serum using the IMMULITE 2000 High Sensitivity CRP Chemiluminescent Immunoassay (20). Serum homocysteine was measured at mid follicular, ovulation, and mid luteal visits using an IMMULITE 2000 Homocysteine Competitive Immunoassay (CV < 10.4% at all concentrations). Serum total cholesterol, HDL cholesterol, and triglycerides were determined by an auto chemistry analyzer (<5% CV for all assays). LDL cholesterol was determined using the Friedewald formula (21). Serum insulin was measured using a solid-phase competitive Chemiluminescent Enzymatic Immunoassay by Specialty Laboratories on the DPC Immulite 2000 analyzer (Siemens Medical Solutions Diagnostics) (<10% CV). Fasting plasma glucose was assayed using a hexokinase-based methodology on a Beckman LX20 autoanalyzer (CV < 3%). Insulin resistance (homeostatic model assessment-insulin resistance) was calculated based on the homeostasis model using the equation: fasting insulin (mU/L) × fasting glucose (mmol/L)/22.5 (22). All biomarkers were analyzed at the Kaleida Center for Laboratory Medicine, Buffalo, NY.
Covariate assessment.
Participants completed questionnaires regarding lifestyle, family, and reproductive health history, and physical activity (International Physical Activity Questionnaire long form 2002) (23). High, moderate, and low physical activity categories were created based on standard International Physical Activity Questionnaire cutpoints. At each clinic visit, women reported whether they had been ill (cold, flu, or other) in the past 7 d. In daily diaries, women recorded the type, frequency, and dosage of nonsteroidal antiinflammatory drug (NSAID) use. The mean of 3 blood pressure measurements was calculated at each clinic visit. Height and weight were measured by standardized protocols and used to calculate BMI. All covariates had <5% total missing data.
Statistical analysis.
ANOVA was used to compare dietary intake by visit for each woman to determine whether intake changed significantly over the cycle. No significant differences in whole grain intake were observed across each cycle; therefore, a mean daily intake per cycle was calculated for whole grain and other dietary variables. Whole grain intake per cycle was categorized according to consumption groups: 0 servings/d, >0 but <1 whole grain servings/d, and ≥1 whole grain servings/d (24). Because only 3% of the women consumed the USDA recommended intake (≥3 servings/d) and <10% consumed ≥2 servings/d, there was not adequate power to evaluate the association of whole grain intake with hs-CRP according to these cutpoints.
In all analyses, hs-CRP concentrations were log transformed for normality. Six percent of the hs-CRP measurements were below the limit of detection (0.1) and were accounted for using multiple imputation (25). Less than 1% of the hs-CRP measurements were >10 mg/L, which might be indicative of viral or bacterial infection. Excluding these observations produced little change in the results, so all values were included in the analyses. hs-CRP was categorized by the mean hs-CRP across the cycle according to the AHA CVD risk cutpoints: hs-CRP < 1 mg/L, low; hs-CRP ≥ 1 mg/L and ≤ 3 mg/L, moderate; or hs-CRP > 3 mg/L, elevated (26).
Descriptive statistics were calculated for demographic characteristics, serum biomarkers, and dietary intake. Generalized linear mixed models were used to test for associations between demographic and dietary variables and levels of whole grain intake and hs-CRP. Generalized linear mixed models on the log scale of hs-CRP were used to evaluate the association between mean whole grain consumption per cycle and hs-CRP concentrations. In these models, up to 8 log hs-CRP concentrations/cycle were included and considered as separate observations in the model. Because 250 women were followed for 2 menstrual cycles and 16.4% of these women changed AHA categorizations, we evaluated the risk of moving to a more severe AHA hs-CRP categorization from cycle 1 to cycle 2 based on mean whole grain intake per cycle (27). Generalized linear mixed models with multinormal distributions were used for these comparisons. Generalized linear mixed models were utilized in all analyses, because they appropriately account for the correlation between cycles as well as allow for addition of a random effect to account for differences in baseline levels of hs-CRP between women. Confounding was assessed using a hybrid approach combining prior knowledge and change in point estimates (28). A set of variables was determined by a review of the prior literature and a detailed directed acyclic graph was created that identified the variables that should be included in the models. Important a priori confounders we considered were age, BMI, race, physical activity, smoking status, and several dietary components, particularly unsaturated fatty acid, fruit, vegetable, fiber, and vitamin E intake. Confounders were included in the final adjusted model only if they changed the exposure coefficient by >15% and were significant at P = 0.10. In addition, interactions between whole grain intake and lifestyle factors such as BMI were tested using cross-product terms in the generalized linear mixed model.
The National Cancer Institute (NCI) method for estimating the usual intake of episodically consumed foods was used as a sensitivity analysis to assess potential misclassification of whole grain intake (specifically for nonconsumers) (29). This method takes into account the high number of days where whole grains were not consumed, because episodic consumption may not have been accurately captured by the 24-h recall. Generalized linear mixed models with random intercepts were used to evaluate the association between usual whole grain consumption per cycle and log(hs-CRP) concentrations. SAS version 9.1 (SAS Institute) was used for all statistical analyses.
Results
Demographics and dietary intake.
Overall, this cohort of women was young (mean age 27.3 y), of healthy weight (mean BMI 24.1), moderately to highly physically active (90.5%), nonsmokers (81.9% never smoked), low consumers of whole grains (67.0% consumed <1 serving/d), and had low mean concentrations of hs-CRP across the menstrual cycle (66.0% <1 mg/L) (Table 1). Consumers of ≥1 serving/d of whole grains had lower serum concentrations of hs-CRP and triglycerides and lower insulin resistance and systolic blood pressure. Higher whole grain intake was associated with an overall healthier diet (Table 2). More specifically, whole grain intakes ≥1 serving/d were associated with lower total fat, SFA, and monounsaturated fatty acid (MUFA) intake and higher fiber, magnesium, folate, B vitamins, vitamin E, and whole fruit intakes.
TABLE 1.
Demographic characteristics of healthy, premenopausal women according to whole grain serving and serum hs-CRP concentration per cycle (259 women)1
| Daily servings of whole grain2 |
hs-CRP categories2 |
||||||||
| Total cohort | 0 servings/d | 0.01–0.99 servings/d | ≥1 serving/d | P-value3 | Low, <1 mg/L | Moderate, 1–3 mg/L | Elevated, >3 mg/L | P-value3 | |
| Cycles, n | 509 | 123 | 218 | 168 | 337 | 137 | 35 | ||
| Demographics | |||||||||
| Age, y | 27.3 ± 8.2 | 26.9 ± 8.0 | 27.4 ± 8.1 | 27.8 ± 8.5 | 0.42 | 25.9 ± 7.8 | 30.2 ± 8.4 | 30.6 ± 8.1 | <0.001 |
| BMI, 2 | 24.1 ± 3.9 | 24.6 ± 4.0 | 24.0 ± 3.6 | 23.8 ± 4.0 | 0.15 | 22.8 ± 3.0 | 26.3 ± 4.2 | 27.7 ± 3.9 | <0.001 |
| Physical activity, n (%) | 0.74 | 0.78 | |||||||
| Low | 48 (9.5) | 15 (12.2) | 17 (7.8) | 16 (9.6) | 31 (9.2) | 12 (8.8) | 5 (14.3) | ||
| Moderate | 181 (36.0) | 50 (40.7) | 66 (30.3) | 66 (39.8) | 125 (37.2) | 48 (35.3) | 9 (25.7) | ||
| High | 279 (54.5) | 58 (47.2) | 135 (61.9) | 86 (50.6) | 181 (53.6) | 77 (55.9) | 21 (60.0) | ||
| Race, n (%) | 0.003 | 0.03 | |||||||
| Caucasian | 302 (59.4) | 55 (44.7) | 128 (58.7) | 119 (71.1) | 186 (55.1) | 94 (69.1) | 22 (62.9) | ||
| African-American | 101 (19.8) | 36 (29.3) | 43 (19.7) | 22 (12.7) | 63 (18.8) | 26 (19.1) | 12 (31.4) | ||
| Other | 106 (20.8) | 32 (26.0) | 47 (21.6) | 27 (16.3) | 88 (26.2) | 16 (11.8) | 2 (5.7) | ||
| Past or current smoker, n (%) | 92 (18.1) | 22 (17.9) | 41 (18.8) | 29 (17.5) | 0.77 | 50 (14.9) | 34 (25.0) | 8 (22.9) | 0.02 |
| Past OC use, n (%) | 273 (54.5) | 62 (51.2) | 123 (56.7) | 88 (54.0) | 0.77 | 155 (46.8) | 94 (69.6) | 24 (68.6) | <0.001 |
| Family history of CVD, n (%) | 237 (46.9) | 46 (38.3) | 106 (48.9) | 85 (50.9) | 0.09 | 148 (44.2) | 70 (51.9) | 19 (54.3) | 0.18 |
| Illness in past mo, n (%) | 111 (21.8) | 29 (23.6) | 50 (22.9) | 32 (19.1) | 0.57 | 59 (17.5) | 41 (29.9) | 13 (31.4) | 0.004 |
| NSAID use in past mo, n (%) | 149 (29.3) | 25 (20.3) | 59 (27.1) | 65 (38.7) | 0.001 | 92 (27.2) | 48 (35.0) | 9 (25.7) | 0.27 |
| Serum biomarkers4 | |||||||||
| hs-CRP, mg/L | 0.6 ± 1.2 | 0.7 ± 1.3 | 0.6 ± 1.2 | 0.5 ± 1.0 | 0.01 | 0.3 ± 0.8 | 1.5 ± 0.7 | 4.2 ± 0.8 | <0.001 |
| Homocysteine, μmol/L | 6.0 ± 1.4 | 6.0 ± 1.5 | 6.0 ± 1.3 | 5.9 ± 1.5 | 0.32 | 6.0 ± 1.4 | 5.8 ± 1.5 | 6.4 ± 1.5 | 0.10 |
| Cholesterol,5 mg/dL | 164.3 ± 27.4 | 165.6 ± 25.9 | 162.7 ± 26.5 | 165.7 ± 29.5 | 0.87 | 162.4 ± 26.0 | 166.4 ± 28.5 | 174.8 ± 33.4 | 0.99 |
| LDL-cholesterol, mg/dL | 100.3 ± 26.1 | 103.4 ± 25.4 | 98.7 ± 24.3 | 101.8 ± 27.2 | 0.14 | 99.1 ± 24.5 | 102.7 ± 26.5 | 110.4 ± 29.9 | 0.46 |
| HDL-cholesterol, mg/dL | 52.2 ± 12.0 | 52.8 ± 16.6 | 52.1 ± 11.4 | 53.4 ± 14.7 | 0.64 | 53.6 ± 15.0 | 51.6 ± 10.6 | 48.1 ± 13.3 | 0.12 |
| Triglycerides,6mg/dL | 59.4 ± 30.5 | 63.0 ± 29.2 | 58.3 ± 24.8 | 60.2 ± 26.6 | 0.02 | 55.6 ± 20.8 | 65.6 ± 30.3 | 81.2 ± 42.4 | 0.001 |
| Insulin,7mU/L | 8.3 ± 5.5 | 9.4 ± 5.4 | 8.5 ± 6.2 | 7.4 ± 4.2 | 0.08 | 7.5 ± 4.1 | 9.8 ± 7.7 | 10.5 ± 5.0 | 0.16 |
| Insulin resistance (HOMA-IR) | 1.8 ± 1.9 | 2.1 ± 1.3 | 1.9 ± 1.7 | 1.6 ± 1.0 | 0.01 | 1.6 ± 0.9 | 2.2 ± 2.1 | 2.4 ± 1.2 | 0.02 |
| Glucose,8mg/dL | 86.8 ± 5.1 | 86.8 ± 5.1 | 87.4 ± 5.3 | 86.1 ± 4.8 | 0.28 | 86.4 ± 4.7 | 87.2 ± 5.6 | 89.5 ± 5.8 | 0.30 |
| Blood pressure, mm Hg | |||||||||
| Systolic | 99.3 ± 8.8 | 100.2 ± 9.3 | 99.8 ± 9.0 | 97.9 ± 8.0 | 0.04 | 98.4 ± 8.5 | 99.7 ± 8.4 | 106.3 ± 10.3 | 0.01 |
| Diastolic | 61.3 ± 8.2 | 61.8 ± 8.5 | 61.6 ± 8.1 | 60.5 ± 8.1 | 0.24 | 60.4 ± 7.7 | 62.9 ± 8.3 | 63.5 ± 10.7 | 0.02 |
Values are mean ± SD or (%). OC, oral contraceptives; HOMA-IR, homeostatic model assessment-insulin resistance.
Based on mean whole grain servings per day per cycle and mean serum hs-CRP concentrations per cycle. A whole grain serving was defined as 16 g or 1/2 cup (125 mL) of a 100% whole grain food.
p–values were calculated using generalized linear mixed models with random effects.
All makers were measured in serum except for glucose, which was measured in plasma.
To convert cholesterol (total, HDL, and LDL) from mg/dL to mmol/L, multiply by 0.026.
To convert triglycerides from mg/dL to mmol/L, multiply by 0.011.
To convert insulin from mU/L to pmol/L, multiply by 6.945.
To convert glucose from mg/dL to mmol/L, multiply by 0.0555.
TABLE 2.
Dietary intake of healthy premenopausal women according to whole grain serving and serum hs-CRP concentrations per cycle (259 women)1
| Daily servings of whole grain2 |
hs-CRP category2 |
||||||||
| Total cohort | 0 servings/d | 0.01–0.99 servings/d | ≥1 servings/d | P-value3 | Low, <1 mg/L | Moderate, 1–3 mg/L | Elevated, >3 mg/L | P-value3 | |
| Cycles, n | 509 | 123 | 218 | 168 | 337 | 137 | 35 | ||
| Nutrients | |||||||||
| Energy, kJ/d | 6728 ± 1695 | 6396 ± 1673 | 6757 ± 1760 | 6934 ± 1594 | 0.16 | 6629 ± 1649 | 6924 ± 1684 | 6923 ± 2102 | 0.44 |
| Carbohydrate, % energy | 50.9 ± 8.2 | 48.6 ± 8.3 | 50.7 ± 7.9 | 52.8 ± 8.2 | <0.001 | 51.8 ± 7.4 | 49.4 ± 8.5 | 48.1 ± 12.3 | 0.002 |
| Protein, % energy | 15.7 ± 3.4 | 16.3 ± 3.9 | 15.5 ± 3.1 | 15.7 ± 3.5 | 0.28 | 15.5 ± 3.4 | 16.0 ± 3.2 | 16.6 ± 4.7 | 0.007 |
| Total Fat, % energy | 33.9 ± 6.3 | 35.2 ± 6.2 | 34.0 ± 6.6 | 32.7 ± 5.8 | 0.001 | 33.4 ± 5.8 | 34.6 ± 6.7 | 35.3 ± 8.7 | 0.10 |
| PUFA, % energy | 7.0 ± 2.0 | 7.1 ± 1.8 | 7.0 ± 2.0 | 7.1 ± 2.0 | 0.94 | 7.0 ± 1.9 | 7.1 ± 2.2 | 7.0 ± 1.8 | 0.83 |
| MUFA, % energy | 12.6 ± 2.8 | 13.2 ± 2.7 | 12.6 ± 2.9 | 12.1 ± 2.7 | 0.001 | 12.4 ± 2.6 | 12.7 ± 3.1 | 13.2 ± 3.6 | 0.25 |
| SFA, % energy | 11.5 ± 2.9 | 12.0 ± 3.1 | 11.7 ± 2.9 | 11.0 ± 2.8 | 0.003 | 11.3 ± 2.8 | 12.0 ± 2.9 | 12.2 ± 3.7 | 0.03 |
| Total fiber, g/d | 13.6 ± 6.0 | 10.3 ± 3.7 | 12.6 ± 4.4 | 17.2 ± 7.1 | <0.001 | 13.8 ± 6.4 | 13.7 ± 5.2 | 11.4 ± 4.4 | 0.007 |
| Magnesium, mg/d | 222.1 ± 74.3 | 181.6 ± 56.8 | 210.5 ± 60.2 | 266.7 ± 79.6 | <0.001 | 224.9 ± 77.1 | 222.9 ± 70.1 | 191.6 ± 54.1 | 0.006 |
| Folate, mg/d | 368.9 ± 148.2 | 296.9 ± 94.8 | 371.4 ± 137.0 | 418.3 ± 171.9 | <0.001 | 374.8 ± 155.7 | 364.0 ± 132.4 | 330.7 ± 127.9 | 0.02 |
| Selenium, μg/d | 88.4 ± 27.9 | 82.7 ± 27.3 | 88.5 ± 26.8 | 92.3 ± 29.4 | 0.16 | 87.5 ± 28.5 | 89.9 ± 26.2 | 90.4 ± 29.4 | 0.74 |
| Vitamin E, mg/d | 9.8 ± 7.2 | 7.0 ± 2.7 | 9.4 ± 6.0 | 12.4 ± 9.5 | <0.001 | 10.4 ± 8.1 | 8.6 ± 4.4 | 9.2 ± 5.5 | 0.02 |
| Vitamin C, mg/d | 70.0 ± 43.1 | 56.8 ± 35.3 | 72.4 ± 42.9 | 76.6 ± 46.5 | 0.03 | 71.7 ± 44.3 | 67.7 ± 42.3 | 62.6 ± 31.6 | 0.18 |
| Thiamine, mg/d | 1.4 ± 0.5 | 1.2 ± 0.35 | 1.4 ± 0.5 | 1.5 ± 0.5 | <0.001 | 1.4 ± 0.5 | 1.4 ± 0.4 | 1.3 ± 0.4 | 0.06 |
| Riboflavin, mg/d | 1.7 ± 0.6 | 1.4 ± 0.52 | 1.7 ± 0.6 | 1.8 ± 0.6 | <0.001 | 1.7 ± 0.6 | 1.7 ± 0.6 | 1.5 ± 0.5 | 0.48 |
| Niacin, mg/d | 19.5 ± 6.7 | 17.8 ± 5.4 | 19.5 ± 6.5 | 20.8 ± 7.5 | 0.01 | 19.4 ± 7.0 | 19.8 ± 5.8 | 19.4 ± 6.4 | 0.37 |
| Vitamin B-6, mg/d | 1.5 ± 0.6 | 1.3 ± 0.4 | 1.4 ± 0.5 | 1.7 ± 0.7 | <0.001 | 1.5 ± 0.6 | 1.5 ± 0.5 | 1.4 ± 0.4 | 0.10 |
| Iron, mg/d | 12.3 ± 5.1 | 10.2 ± 4.2 | 12.0 ± 4.3 | 14.2 ± 5.9 | <0.001 | 12.5 ± 5.3 | 12.0 ± 4.6 | 11.7 ± 4.4 | 0.08 |
| β-Carotene, μg/d | 2339.3 ± 2335.9 | 1942.0 ± 2157.5 | 2212.9 ± 2102.0 | 2794.3 ± 2666.7 | 0.10 | 2403.6 ± 2385.8 | 2459.5 ± 2396.6 | 1249.7 ± 990.4 | 0.06 |
| Whole foods, servings/d | |||||||||
| Whole fruit4 | 0.4 (0.0, 1.0) | 0.3 (0.0, 0.6) | 0.4 (0.0, 0.9) | 0.6 (0.2, 1.3) | 0.03 | 0.4 (0.0, 1.0) | 0.5 (0.5, 1.0) | 0.3 (0.0, 0.8) | 0.48 |
| Total grains5 | 5.3 (4.2, 6.6) | 4.5 (3.3, 5.7) | 5.3 (4.3, 6.6) | 6.0 (4.8, 7.1) | <0.001 | 5.2 (4.2, 6.4) | 5.4 (4.3, 6.8) | 5.3 (3.9, 6.4) | 0.54 |
| Whole grains | 0.5 (0.1, 1.3) | 0.0 (0.0, 0.0) | 0.5 (0.3, 0.7) | 1.6 (1.3, 2.1) | <0.001 | 0.6 (0.2, 1.3) | 0.5 (0.0, 1.2) | 0.3 (0.0, 0.9) | 0.01 |
| Total vegetables6 | 2.2 (1.5, 3.1) | 2.0 (1.4, 2.9) | 2.3 (1.6, 3.0) | 2.4 (1.5, 3.8) | 0.13 | 2.2 (1.5, 3.1) | 2.5 (1.7, 3.4) | 1.8 (1.4, 2.4) | 0.06 |
Values are mean ± SD or median (25th percentile, 75th percentile).
Based on mean whole grain serving per day per cycle and mean serum hs-CRP concentrations per cycle. A whole grain serving was defined as 16 g or 1/2 cup (125 mL) of a 100% whole grain food.
p–values were calculated using generalized linear mixed models with random effects adjusting for total energy intake.
A whole fruit serving was defined as 1 medium fruit, 1/2 cup (125 mL) of chopped, cooked, or canned fruit, or 1/4 cup (62.5 mL) of dried fruit.
A grain serving was defined as 16 g or 1/2 cup (125 mL) of bread, ready-to-eat cereal, cooked cereal, rice, or pasta.
A vegetable serving was defined as 1 cup (250 mL) of raw leafy vegetables, 1/2 cup (125 mL) of other cooked or raw vegetables, or 1/2 cup (125 mL) of vegetable juice.
Women with elevated hs-CRP on average had higher BMI, were older, smokers, more likely to be African American, past oral contraceptive (OC) users, and more likely to have had an illness in the past month. They also had higher levels of triglycerides, insulin resistance, and blood pressure. Elevated hs-CRP was also associated with higher protein and SFA intakes and lower carbohydrate, fiber, magnesium, folate, vitamin E, and whole grain intakes.
Serum hs-CRP concentrations.
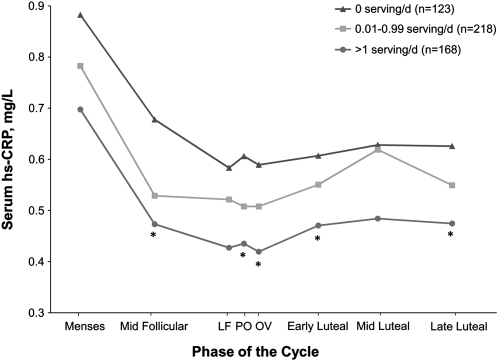
Whole grain intake was inversely associated with serum hs-CRP concentrations (Fig. 1). Specifically, consumption of whole grains between 0 and 1 serving/d (minimal consumers) and ≥1 serving/d (moderate consumers) was associated with decreased concentrations of hs-CRP across the cycle compared with nonconsumers in models adjusted for total energy intake, age, race, BMI, illness, and NSAID use (Table 3) (P = 0.02). For minimal consumers, hs-CRP concentrations across the cycle were 10.5% lower than the concentrations of nonconsumers. Similarly, for moderate consumers, hs-CRP concentrations were 12.1% lower than nonconsumers. To assess the robustness of our findings, we extended our nonconsumer group to women consuming <1 serving/d. When we compared this reference group to women consuming >1 serving/d, the results, while still inverse, were slightly attenuated (data not shown).
FIGURE 1.
Unadjusted mean serum hs-CRP concentrations across the menstrual cycle by category of mean daily whole grain intake in healthy premenopausal women. Mean whole grain intake per cycle was based on up to four 24-h dietary recalls. A whole grain serving was defined as 16 g or 1/2 cup (125 mL) of a 100% whole grain food. P-values were calculated using generalized linear mixed models with random effects where nonconsumers were the reference category. *Different from 0 servings/d, P < 0.05 and ≥1 servings/d. LF, late follicular; PO, peri-ovulatory (LH/FSH surge); OV, ovulation.
TABLE 3.
Association between whole grain intake and the log of serum hs-CRP concentrations in healthy premenopausal women1
| Whole grain intake per cycle2 | Model | Predicted mean hs-CRP3 (% change from REF) | β (95% CI)4 | P-value |
| Nonconsumers, n = 123 | Energy-adjusted | 0.62 | REF | REF |
| 15 | 0.69 | REF | REF | |
| 26 | 0.65 | REF | REF | |
| 37 | 0.65 | REF | REF | |
| Consumers, n = 386 | Energy-adjusted | 0.52 (14.92) | −0.162 (−0.251, −0.073) | <0.001 |
| 1 | 0.58 (18.05) | −0.160 (−0.248, −0.072) | <0.001 | |
| 2 | 0.58 (12.25) | −0.116 (−0.216, −0.015) | 0.02 | |
| 3 | 0.59 (9.47) | −0.100 (−0.201, 0.002) | 0.06 | |
| Nonconsumers, n = 123 | Energy-adjusted | 0.62 | REF | REF |
| 1 | 0.69 | REF | REF | |
| 2 | 0.65 | REF | REF | |
| 3 | 0.65 | REF | REF | |
| Consumers < 1 serving/d, n = 218 | Energy-adjusted | 0.53 (14.65) | −0.158 (−0.250, −0.066) | <0.001 |
| 1 | 0.61 (14.31) | −0.154 (−0.245, −0.064) | <0.001 | |
| 2 | 0.58 (10.46) | −0.111 (−0.215, −0.006) | 0.04 | |
| 3 | 0.59 (9.45) | −0.099 (−0.204, 0.006) | 0.06 | |
| Consumers ≥1 serving/d, n = 168 | Energy-adjusted | 0.52 (15.65) | −0.170 (−0.279, −0.061) | 0.002 |
| 1 | 0.59 (16.12) | −0.184 (−0.283, −0.069) | 0.001 | |
| 2 | 0.57 (12.09) | −0.129 (−0.251, −0.007) | 0.04 | |
| 3 | 0.58 (9.55) | −0.100 (−0.226, 0.026) | 0.11 |
REF, Reference category.
A whole grain serving was defined as 16 g or 1/2 cup (125 mL) of a 100% whole grain food.
Predicted mean values are estimated for the average woman in the cohort (27.5 y, 24.1 BMI, 6728.3 kJ, Caucasian, not ill, no NSAID use, and 13.6 g/d fiber).
β and 95% CI were obtained used generalized linear mixed models with random effects.
Model 1 adjusted for energy intake (continuous), age (continuous), race (Caucasian, African-American, other), and BMI (continuous).
Model 2 adjusted for factors in model 1 and additionally adjusted for illness during 7 d prior to visit (yes, no) and NSAID use the day before blood draw (yes, no).
Model 3 adjusted for factors in model 2 as well as dietary fiber intake (continuous).
Because whole grain foods are rich sources of magnesium, dietary fiber, iron, B vitamins, vitamin E, and folate, we examined whether the observed significant inverse association between whole grain and CRP could be explained by these nutrients. Inclusion of dietary fiber attenuated some of the effect of whole grain intake on hs-CRP, as seen in model 3 (Table 3). However, dietary fiber was not significantly associated with hs-CRP in these models. Inclusion of magnesium and vitamin E in the model also resulted in a slight attenuation, but the associations remained significant. Further adjustment for other dietary components, specifically SFA, PUFA, MUFA, fruit, and vegetable intake, did not appreciably change the results.
We further investigated whether certain metabolic factors (i.e. total, LDL, and HDL cholesterol; triglycerides; insulin; insulin resistance; glucose; systolic and diastolic blood pressure; homocysteine; and estradiol) were potentially mediating the effect of whole grains on CRP. The inverse association between whole grain and CRP remained significant and did not change materially after adjustment for these traits.
Risk of elevated serum hs-CRP.
To extend the association between whole grain intake and serum hs-CRP to clinically relevant hs-CRP cutpoints, we used the AHA's cardiovascular risk cutpoints. Results from the generalized linear mixed model adjusting for age, race, BMI, illness, and NSAID use demonstrated that consumers at or above 1 serving/d of whole grains had lower odds of moving to a more severe AHA CVD risk categorization from cycle 1 to cycle 2 (Table 4). Specifically, women at low risk for CVD (hs-CRP < 1 mg/L) in cycle 1 who consumed ≥1 serving/d of whole grains had 59% lower odds of moving to a classification of moderate risk for CVD (hs-CRP ≥ 1 mg/L and ≤ 3 mg/L) and 89% lower odds of moving to an elevated classification of hs-CRP (hs-CRP > 3 mg/L) in cycle 2 compared with nonconsumers. Additionally, women at moderate risk for CVD in cycle 1 who consumed ≥ 1 serving/d of whole grains had 73% lower odds of moving to an elevated risk of CVD in cycle 2 compared with nonconsumers. Similar to the continuous analysis, further adjustment for other dietary components and metabolic variables had little effect on these estimates.
TABLE 4.
Association between whole grain intake and risk of elevated serum hs-CRP concentration in healthy premenopausal women1
| Adjusted odds ratio (95% CI)2 |
||||||
| Low to moderate hs-CRP4 |
Low to elevated hs-CRP |
Moderate to elevated hs-CRP |
||||
| Categories of whole grain intake3 | Energy-adjusted | Model 15 | Energy-adjusted | Model 1 | Energy-adjusted | Model 1 |
| Whole grain consumer | ||||||
| No | REF | REF | REF | REF | REF | REF |
| Yes | 0.58 (0.36, 0.92) | 0.51 (0.30, 0.88) | 0.35 (0.15, 0.65) | 0.32 (0.13, 0.74) | 0.54 (0.25, 1.18) | 0.62 (0.27, 1.42) |
| Whole grain categories | ||||||
| 0 servings/d | REF | REF | REF | REF | REF | REF |
| 0.01–1 servings/d | 0.62 (0.38, 1.03) | 0.57 (0.32, 1.03) | 0.47 (0.22, 1.01) | 0.49 (0.20, 1.21) | 0.75 (0.33, 1.71) | 0.86 (0.36, 2.06) |
| >1 servings/d | 0.53 (0.31, 0.90) | 0.41 (0.21, 0.78) | 0.14 (0.04, 0.43) | 0.11 (0.03, 0.41) | 0.26 (0.08, 0.85) | 0.27 (0.08, 0.96) |
| NCI usual intake categories | ||||||
| ≤0.5 servings/d | REF | REF | REF | REF | REF | REF |
| 0.51–0.99 servings/d | 0.53 (0.33, 0.86) | 0.50 (0.28, 1.13) | 0.67 (0.32, 1.41) | 0.67 (0.28, 1.00) | 1.26 (0.56, 2.81) | 1.35 (0.58, 3.13) |
| ≥1.0 servings/d | 0.51 (0.30, 0.86) | 0.40 (0.21, 1.00) | 0.09 (0.02, 0.41) | 0.07 (0.01, 0.87) | 0.18 (0.04, 0.83) | 0.18 (0.04, 0.85) |
REF, reference category.
Analyses used generalized linear mixed models with random effects and multinormal distribution.
A whole grain serving was defined as 16 g or 1/2 cup (125 mL) of a 100% whole grain food.
Based on the AHA's CVD risk cutpoints (26).
Adjusted for age (continuous), race (Caucasian, African-American, other), BMI (continuous), illness in past month (yes, no), and NSAID use before blood draw (yes, no).
NCI sensitivity analysis.
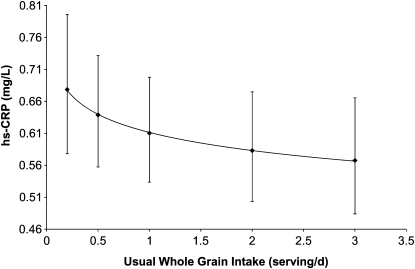
The distribution of whole grain intake observed in this study (based on the mean of up to four 24-h recalls) had a spike at zero (24.1% of cycles). The estimated distribution of whole grain intake based on the NCI method resulted in a smoothed distribution curve with no observations at zero. When usual whole grain intake based on the NCI method was assessed as a continuous, log-transformed variable in a linear mixed effects model with log hs-CRP as the outcome, a significant inverse association was observed adjusting for age, race, and BMI (P = 0.047) (Fig. 2). Compared with usual consumers of a minimal amount of whole grains (0.2 serving/d), women who consumed 1 serving/d had 10.1% lower hs-CRP concentrations and consumers of 3 servings/d had 16.3% lower hs-CRP concentrations.
FIGURE 2.
Serum hs-CRP concentrations in healthy premenopausal women by usual intake of whole grains [NCI method (29)]. Values are predicted means ± 95% CI, n = 509 cycles, 259 women. The analysis used generalized linear mixed models with random effects to evaluate the effect of usual intake of whole grains per cycle on log hs-CRP concentrations (up to 8 measurements/cycle).
Discussion
Consumption of whole grains was significantly and inversely associated with hs-CRP concentrations across the menstrual cycle in this cohort of young, healthy women. Furthermore, women who consumed ≥1 serving/d of whole grain had a significantly lower probability of having a moderate or elevated hs-CRP categorization (based on AHA criteria). The associations remained significant after adjustment for various other demographic characteristics, metabolic variables, and dietary factors. These results highlight the benefits of even moderate whole grain intake as part of a healthy diet. Furthermore, this association raises the possibility of using whole grains as a potential, effective intervention to protect against the adverse reproductive outcomes associated with elevated hs-CRP in reproductive-aged women.
Data on the association between whole grain intakes and hs-CRP concentrations among young, apparently health women are sparse. Findings from the present study are generally consistent with those among older women (mainly postmenopausal) (10–12). For instance, in women from the Nurses’ Health Study II (mean age, 42 y; mean CRP, 0.89 mg/L), an inverse association was observed between increased whole grain intake and CRP concentrations; however, this relationship no longer remained significant after accounting for lifestyle factors (alcohol intake, smoking, BMI, physical activity, and hypercholesterolemia) (12). In the Multi-Ethnic Study of Atherosclerosis (mean age, 62 y; mean CRP, 3.26 mg/L) (11) and among women with type 2 diabetes in the Nurses’ Health Study (mean age, 58 y; mean CRP, 5.75 mg/L) (10), significant inverse associations between whole grain intake and CRP remained consistent even after adjustment for several other factors. In a recent randomized clinical trial of 50 obese adults with metabolic syndrome (mean age, 45 y; mean initial CRP, 6.0 mg/L), a 12-wk intervention of 100% grain servings from whole grains resulted in a 38% decrease in CRP concentrations, independent of weight loss (30).
The potential antiinflammatory effect of whole grains among young women is biologically plausible. Whole grains are a good source of vitamins (i.e. vitamins B and E), dietary fiber, and minerals (i.e. iron, selenium, and magnesium) (31). These components may work synergistically to protect against inflammation and other disease processes. The antioxidants in whole grains are thought to lower the activation of inflammatory signals from reactive oxygen species. Selenium, e.g., may inhibit the activation of nuclear factor-κB (a protein that regulates immune response) by modulating selenoprotein gene expression and may increase selenoprotein biosynthesis leading to suppressed CRP production (32). It has also been proposed that the dietary fiber in whole grains accounts for some of this antiinflammatory action by decreasing lipid oxidation, which in turn is associated with decreased inflammation, and reducing other substances that cause inflammation (i.e. the inhibition of hyperglycemia and its effects on lipids, particularly LDL cholesterol) (33). It should be noted though that in the present study, the inverse association of whole grains with hs-CRP was not affected by adjustment for nutrients commonly found in whole grains, such as dietary fiber, magnesium, and vitamin E, suggesting that the reduced levels of inflammation could be due to other nutrients beyond these or synergy between them. Additionally, the association persisted even after adjustment for energy and/or fat (specifically MUFA and SFA) consumption despite the significant differences in intake by whole grain category. Finally, after adjustment for various metabolic factors, the association of whole grain intake with CRP was not attenuated, suggesting that in contrast with findings among older women (12), the association in our population of healthy women was not mediated by these factors.
The BioCycle study had a number of strengths. First, previous studies on the association of whole grain intake with CRP largely relied on a single measurement. In our study, the 8 measurements/cycle, standardized to the menstrual cycle, helped lower the variability in hs-CRP measurements that could have been due to endogenous hormone fluctuations (19). No previous study to our knowledge has had such detailed information on potential effect mediators, including up to 16 standardized measures of insulin resistance, glucose, homocysteine, cholesterol, estradiol, and blood pressure. The estimation of dietary intake with multiple 24-h recalls (up to 4 per cycle) decreased the probability of dietary misclassification. The assessment of illness up to 4 times/cycle and NSAID use daily across the study is a unique strength of this study and greatly improved our ability to adjust for confounding (34). Finally, the exclusion criteria at baseline strengthen the ability to draw inference, having reduced the potential for bias from known risk factors for inflammation.
Although this study expands on previous research, it does have several limitations. Like other observational studies, misclassification of dietary intake is possible. Although participants completed 4 diet assessments per cycle, it is plausible that some women classified as nonconsumers actually consumed a small amount of whole grains. However, when the effect of misclassification was assessed through a sensitivity analysis employing the NCI method for usual intake, the inverse association between whole grain intake and hs-CRP remained consistent. Also, given that in a nationally representative sample (NHANES 1999–2000) almost 30% of women consumed no whole grains, the high percentage of nonconsumers in this study is plausible (24). Finally, although the whole grain intake of this sample was characteristic of the American population, we were limited to evaluating the association of whole grains and hs-CRP at modest levels of consumption due to the small number of high consumers.
In conclusion, moderate whole grain consumption (≥1 serving/d) among young, healthy women was significantly associated with decreased concentrations of hs-CRP and lower odds of moving to a more severe AHA CVD risk categorization. This relationship was independent of various metabolic variables and dietary factors. Given that elevated concentrations of hs-CRP have been associated with adverse reproductive outcomes and risk of various chronic diseases, increased consumption of whole grains may offer a potential intervention for young women to improve not only short-term but also long-term health outcomes. Further studies are needed to confirm these findings and extend these results to assess the impact of higher levels of whole grain intake (i.e. USDA-recommended levels).
Acknowledgments
E.F.S. and J.W.W. formulated the study concept and design and supervised the study. E.F.S. and J.W.W. assisted in the acquisition of data. A.J.G., S.L.M., A.J.R., and E.F.S. analyzed and interpreted the data. A.J.G., S.L.M., A.J.R., C.Z., and E.F.S. drafted the manuscript. L.C., C.Z., E.F.S., J.W.W., and N.J.P. critically revised the manuscript for important intellectual content. A.J.G., S.L.M., N.J.P., and E.F.S. performed the statistical analyses. A.J.G. and E.F.S. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and approved the final version of the paper.
Footnotes
Supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH.
In addition to the listed authors, the BioCycle Study Group members include M. Hediger, R. Browne, M. Trevisan, B. Whitcomb, A. Liu, A. Gollenberg, E. Yeung, A. Pollack, M. Danaher, Y. Malinovsky, C. Liu, K. Hovey, C. Rudra, K. Lynch, K. Schliep, M. Bloom, A. Ye, P. Albert, O. Harel, M. Wilchesky, and P. Howards.
Abbreviations used: CRP, C-reactive protein; CVD, cardiovascular disease; hs-CRP, high sensitivity C-reactive protein; MUFA, monounsaturated fatty acid; NCI, National Cancer Institute; NSAID, nonsteroidal antiinflammatory drug.
Literature Cited
- 1.Rifai N, Ridker PM. High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clin Chem. 2001;47:403–11 [PubMed] [Google Scholar]
- 2.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3 [DOI] [PubMed] [Google Scholar]
- 3.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34 [DOI] [PubMed] [Google Scholar]
- 4.Heikkila K, Harris R, Lowe G, Rumley A, Yarnell J, Gallacher J, Ben-Shlomo Y, Ebrahim S, Lawlor DA. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20:15–26 [DOI] [PubMed] [Google Scholar]
- 5.Qiu C, Luthy DA, Zhang C, Walsh SW, Leisenring WM, Williams MA. A prospective study of maternal serum C-reactive protein concentrations and risk of preeclampsia. Am J Hypertens. 2004;17:154–60 [DOI] [PubMed] [Google Scholar]
- 6.Qiu C, Sorensen TK, Luthy DA, Williams MA. A prospective study of maternal serum C-reactive protein (CRP) concentrations and risk of gestational diabetes mellitus. Paediatr Perinat Epidemiol. 2004;18:377–84 [DOI] [PubMed] [Google Scholar]
- 7.Lohsoonthorn V, Qiu C, Williams MA. Maternal serum C-reactive protein concentrations in early pregnancy and subsequent risk of preterm delivery. Clin Biochem. 2007;40:330–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin I, Gamzu R, Mashiach R, Lessing JB, Amit A, Almog B. Higher C-reactive protein levels during IVF stimulation are associated with ART failure. J Reprod Immunol. 2007;75:141–4 [DOI] [PubMed] [Google Scholar]
- 9.Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2001;86:2453–5 [DOI] [PubMed] [Google Scholar]
- 10.Qi L, van Dam RM, Liu S, Franz M, Mantzoros C, Hu FB. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care. 2006;29:207–11 [DOI] [PubMed] [Google Scholar]
- 11.Lutsey PL, Jacobs DR, Jr, Kori S, Mayer-Davis E, Shea S, Steffen LM, Szklo M, Tracy R. Whole grain intake and its cross-sectional association with obesity, insulin resistance, inflammation, diabetes and subclinical CVD: The MESA Study. Br J Nutr. 2007;98:397–405 [DOI] [PubMed] [Google Scholar]
- 12.Jensen MK, Koh-Banerjee P, Franz M, Sampson L, Gronbaek M, Rimm EB. Whole grains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation 1. Am J Clin Nutr. 2006;83:275–83 [DOI] [PubMed] [Google Scholar]
- 13.Jones JM. Mining whole grains for functional components. Food Science & Technology Bulletin: Functional Foods. 2007;4:67–86 [Google Scholar]
- 14.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, et al. Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006. Arterioscler Thromb Vasc Biol. 2006;26:2186–91 [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services and USDA Dietary guidelines for Americans. 6th ed Washington (DC): U.S. Government Printing Office; 2005; [Google Scholar]
- 16.American Diabetes Association Nutrition Recommendations and Interventions for Diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2007;30Suppl 1:S48–65 [DOI] [PubMed] [Google Scholar]
- 17.Wactawski-Wende J, Schisterman EF, Hovey KM, Howards PP, Browne RW, Hediger M, Liu A, Trevisan M. BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23:171–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol. 2009;169:105–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wander K, Brindle E, O'Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136:138–46 [DOI] [PubMed] [Google Scholar]
- 20.Rifai N, Tracy RP, Ridker PM. Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin Chem. 1999;45:2136–41 [PubMed] [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502 [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9 [DOI] [PubMed] [Google Scholar]
- 23.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95 [DOI] [PubMed] [Google Scholar]
- 24.Good CK, Holschuh N, Albertson AM, Eldridge AL. Whole grain consumption and body mass index in adult women: an analysis of NHANES 1999–2000 and the USDA pyramid servings database. J Am Coll Nutr. 2008;27:80–7 [DOI] [PubMed] [Google Scholar]
- 25.Little RJA, Rubin DB. Statistical analysis with missing data. Hoboken (NJ): John Wiley & Sons, Inc; 1987 [Google Scholar]
- 26.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, Fadl YY, Fortmann SP, Hong Y, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511 [DOI] [PubMed] [Google Scholar]
- 27.Witte JS, Greenland S, Kim LL, Arab L. Multilevel modeling in epidemiology with GLIMMIX. Epidemiology. 2000;11:684–8 [DOI] [PubMed] [Google Scholar]
- 28.Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169:1182–90 [DOI] [PubMed] [Google Scholar]
- 29.Tooze JA, Midthune D, Dodd KW, Freedman LS, Krebs-Smith SM, Subar AF, Guenther PM, Carroll RJ, Kipnis V. A new statistical method for estimating the usual intake of episodically consumed foods with application to their distribution. J Am Diet Assoc. 2006;106:1575–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katcher HI, Legro RS, Kunselman AR, Gillies PJ, Demers LM, Bagshaw DM, Kris-Etherton PM. The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr. 2008;87:79–90 [DOI] [PubMed] [Google Scholar]
- 31.Seal CJ. Whole grains and CVD risk. Proc Nutr Soc. 2006;65:24–34 [DOI] [PubMed] [Google Scholar]
- 32.Duntas LH. Selenium and inflammation: underlying anti-inflammatory mechanisms. Horm Metab Res. 2009;41:443–7 [DOI] [PubMed] [Google Scholar]
- 33.King DE. Dietary fiber, inflammation, and cardiovascular disease. Mol Nutr Food Res. 2005;49:594–600 [DOI] [PubMed] [Google Scholar]
- 34.Beaton GH, Milner J, Corey P, McGuire V, Cousins M, Stewart E, de Ramos M, Hewitt D, Grambsch PV, et al. Sources of variance in 24-hour dietary recall data: implications for nutrition study design and interpretation. Am J Clin Nutr. 1979;32:2546–59 [DOI] [PubMed] [Google Scholar]