Abstract
Circulating monocytes exhibit an apoptotic resistance phenotype during HIV viremia in association with increased MT expression. MTs are known to play an important role in zinc metabolism and immune function. We now show, in a cross-sectional study using peripheral monocytes, that expression of MT1 isoforms E, G, H, and X is increased significantly in circulating monocyte cells from HIV+ subjects during chronic viremic episodes as compared with uninfected subjects. This increase in expression is also observed during acute viremia following interruption of suppressive ART. Circulating monocytes from HIV+ donors were also found to have elevated zinc importer gene Zip8 expression in conjunction with elevated intracellular zinc levels in contrast to CD4+T-lymphocytes. In vitro HIV-1 infection studies with elutriated MDM confirm a direct relation between HIV-1 infection and increased MDM MT1 (isoform G) gene expression and increased intracellular zinc levels. A direct link between elevated zinc levels and apoptosis resistance was established using a cell-permeable zinc chelator TPEN, which reversed apoptosis resistance effectively in monocytes from HIV-infected to levels comparable with uninfected controls. Taken together, increases in MT gene expression and intracellular zinc levels may contribute directly to maintenance of an immune-activated monocyte by mediating an increased resistance to apoptosis during active HIV-1 viremia.
Keywords: AIDS, macrophages, MT1
Introduction
HIV-1 targets CD4+ T cells and monocytes, yet the CD4 T cells are the main subset that is depleted during chronic infection via various mechanisms including activation-induced apoptosis [1,2,3,4,5,6]. In addition to increased expression levels of proapoptotic factors FasL and TRAIL on cell surfaces and in plasma during HIV-1 replication in vivo, HIV envelope interactions have been associated with direct apoptosis induction on T cells, while the same interactions can lead to a potential mechanism of apoptosis resistance in monocytes [7,8,9]. Likewise, several studies have reported that viral-derived products, including gp120, tat, vpr, and Nef, can have proapoptotic effects on lymphocytes but not monocytes. The transactivating protein (Tat) of HIV increases the expression of FasL on the surface of APC and induces apoptosis in CD4 T cells [10]. Although several studies have also indicated an increased resistance to apoptosis in circulating monocytes and MDM during HIV infection [8, 10,11,12], there is little understanding of the cellular changes within in vivo monocytes that can mediate apoptosis resistance during HIV infection and/or exposure.
Studies of circulating monocytes in HIV-infected subjects and in vitro HIV infection of MDM have shown an increased expression of MT genes, raising the possibility that these genes may contribute to monocyte activation and survival. MT genes encode for MTs and cysteine-rich low molecular weight metal-binding proteins with multiple functions and are encoded in four MT isoforms. MT1 and MT2 are found in most tissues, MT3 is found primarily in the brain [13,14,15], and MT4 is found predominately in stratified tissues [16]. MT1 and MT2 protect the cell against oxidative stress and heavy metal toxicity as well regulate the balance of essential metals (Cu and Zn) [17,18,19]. They are inducible by a number of cellular stressors and compounds, such as cytokines, reactive oxygen species, glucocorticoids, and metals, and MT3 and MT4 are nonresponsive to these inducers. In murine studies, MT1 and MT2 gene expression was also demonstrated to increase after microbial infections, indicating that these genes may be intrinsic to immune activation and monocyte response to infection [20,21,22]. Compared with other leukocytes, monocytes exhibit the highest levels of MT protein and mRNA as well as the ability to bind metallic zinc [23, 24]. Several studies have shown the importance of zinc in immunity and the role of MT in the regulation of zinc homeostasis (reviewed in refs. [25,26,27]). Apoptosis regulation in immune cells can also be regulated by zinc, as demonstrated in studies using zinc-deficient mammalian cell lines and animal models, where zinc was shown to inhibit apoptosis [24, 28, 29]. Therefore, we hypothesized that MT gene expression during HIV-1 infection/exposure could be associated with higher expression of several MT isoforms in the presence of increased zinc within circulating monocytes and that monocytes would exhibit a zinc-dependent apoptosis resistance.
Here, we report the isoforms of MT that are increased in circulating monocytes from HIV-infected subjects in vivo and document that resistance to apoptosis results from elevated intracellular zinc levels. This is confirmed by the reversal of apoptosis resistance in monocytes from HIV-infected subjects upon zinc chelation at higher concentrations than those required by monocytes from uninfected subjects. We conclude that MT expression and associated increases in zinc content are integral to the antiapoptotic phenotype exhibited by monocytes during HIV viremia.
MATERIALS AND METHODS
Subjects
Chronically, HIV-seropositive viremic patients from the Jonathan Lax Immune Disorder Clinic (Philadelphia FIGHT, Philadelphia, PA, USA) with a mean age of 44 years and not on therapy served as our donor population for monocyte characterization and apoptosis-induction assays. For inclusion, CD4 T cell counts were required to be >200 cells/mm3 (mean of 490 cells/mm3) and viral load >10,000 copies/ml (mean of 39,294 copies/ml). HIV-infected donors were asymptomatic with no clinical evidence of active comorbidities. Age- and gender-matched, healthy, uninfected donors from the Wistar Institute Blood Donor Program (Philadelphia, PA, USA) were included as control subjects. Institutional Review Board approval from the Wistar Institute and Philadelphia FIGHT and informed consent were obtained before blood donation. As with HIV-infected donors, uninfected donors with an abnormal temperature or abnormal hematocrit or reporting any symptoms were excluded. Blood was processed within 2–3 h from being drawn.
ISOLATION OF PRIMARY CELLS
All reagents used were selected for their low levels of endotoxin contamination. PBMCs were separated by Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden) density gradient separation. Monocytes were isolated from PBMCs by plastic adherence. Adherent monocytes were detached from culture by use of cold media and pipette stream detachment (not scraping). CD4+ T cells were isolated from nonadherent PBMCs by negative selection using magnetic beads (Miltenyi Biotec, Auburn, CA, USA), according to the manufacturer’s protocol. Purity of cell subsets was confirmed by flow cytometry as described below. For ART interruption samples, sequential, cryopreserved PBMCs were used from subjects with documented ART interruption and viral-rebound time-points, where CBC, CD4, and monocyte counts were measured. Adherent CD14 cell subsets were isolated from PBMCs as described above and processed immediately for gene expression analysis (described below).
Flow cytometric analysis
Following isolation, surface expression of CD14 (monocytes), along with CD4 and CD3 (T cells), was determined. Cells were washed with FACS wash buffer (PBS, 0.1% BSA, 0.01% sodium azide) and then incubated with appropriate antibody or labeled isotype control for 30 min at 4°C. Monocytes were incubated with CD14-allophycocyanin (BD PharMingen, San Diego, CA, USA), and CD4+ T cells were incubated with CD3-FITC and CD4-PE (BD PharMingen). After 30 min, cells were washed with FACS wash buffer, fixed with 1% paraformaldehyde, and analyzed using a FACSCalibur flow cytometer and FlowJo software (Tree Star, Inc., Ashland, OR, USA). To be used in the study, cell preparations required a minimum of >85% of CD14+ cell expression with a mean purity of CD14+ monocyte preparations used in the study of 92% (±5%). For CD4+ T cells, >95% of cells expressed CD4 and CD3 [mean 96% (±3%)] The distribution of total percent CD14 cells and percent CD4+ T-lymphocytes between HIV-1 and control subjects tested was found not to be different.
Gene expression analysis
Gene expression was assessed pre- and post-treatment with apoptosis inducers. Cells were lysed and total RNA extracted within 2 h of isolation. Total RNA was isolated from cells using RNeasy (Qiagen, Valencia, CA, USA) and cDNA-generated using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA), according to the manufacturer’s instructions, and was used in real-time PCR reactions performed on an ABI Prism 7000. Briefly, 100 ng cDNA and 800 nm specific primers were added to SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA). All real-time PCR was performed using 95°C for 5 min, followed by 40 cycles of 95°C for 30 s, 58°C for 30 s, 72°C for 30 s, and then finally, extension for 10 min at 72°C. Primers used for real-time PCR were as follows: MT1E: 5′-GCT TGT TCG TCT CAC TGG TG-3′ (forward); 5′-CAG GTT GTG CAG GTT GTT CTA-3′ (reverse). MT1G: 5′-CTT CTC GCT TGG GAA CTC TA-3′ (forward); 5′-AGG GGT CAA GAT TGT AGC AAA-3′ (reverse). MT1H: 5′-CCT CTT CTC TTC TCG CTT GG-3′ (forward); 5′-GCA AAT GAG TCG GAG TTG TAG-3′ (reverse). MT1F: 5′-ACC TGC CCC ACT GCT TCT T-3′ (forward); 5′-TTG CAA GCC GAG GAG AGA CT-3′ (reverse). MT1X: 5′-TCT CCT TGC CTC GAA ATG GAC-3′ (forward); 5′-GGG CAC ACT TGG CAC AGC-3′ (reverse). β-Actin: 5′-TTC CTG GGG ATG GAG TC-3′ (forward); 5′-CAG GTC TTT GCG GAT GTC-3′ (reverse). Data collected were analyzed using the SDS software (Applied Biosystems). CT experiments (ΔΔCT method) were used to determine the relative fold differences in cell subsets from uninfected and HIV+ donors. Gene expression within all samples was normalized to β-actin levels by subtracting CT values of β-actin RT-PCR reactions from CT values of target gene (i.e., MT1E, MT1G, etc.) reactions (ΔCT). The median ΔCT value among the untreated/control group was then subtracted from normalized samples (ΔΔCT). Standard curves using cDNA derived from spleen total RNA (Ambion, Austin, TX, USA) were generated to determine reaction efficiency (slope) for the housekeeping gene and MT isoform.
Apoptosis induction
Apoptosis was induced by exposure to sFasL or cadmium. sFasL was purchased from Peprotech (Rocky Hill, NJ, USA), and cadmium was obtained from Sigma-Aldrich (St. Louis, MO, USA). Monocytes and CD4+ T-lymphocytes exposed for 6 h to cadmium (100 μM) or sFasL (100 ng/ml) at 37°C were harvested and apoptosis induction assessed by measuring activated caspase-3 by flow cytometry. Intracellular caspase-3 staining was performed in human cells, according to the manufacturer’s protocol (BD Cytofix/Cytoperm permeabilization kit) provided by BD Biosciences (San Jose, CA, USA). After incubation in the presence or absence of 20 μM cadmium chloride (Sigma-Aldrich), an established apoptosis inducer [30, 31], for 6 h or with 100 ng/ml FasL (Peprotech) for 6 h, one million cells were stained with surface antibodies (CD14 for monocytes), lineage cocktail (CD56, CD3, CD19, and CD20), and CD4 and CD3 for CD4 T cells (from BD Biosciences) and 7AAD as described above. Cells were then fixed/permeabilized and stained with intracellular FITC caspase-3 antibody for 30 min at 4°C and measured for active caspase-3 expression using a FACSCalibur flow cytometer. A total of 100,000 events was collected and analyzed in control and HIV-1 samples. Analysis of acquired data was performed using FlowJo software (Tree Star, Inc.).
Zinc measurements
Intracellular zinc levels were determined in monocytes isolated from uninfected and HIV+ donors with Newport Green DCF diacetate (Invitrogen/Molecular Probes, Eugene, OR, USA), a cell-permeable, fluorescent, zinc-specific probe. Cells (∼106) were incubated for 45 min with Newport Green (10 μM) at room temperature and subsequently stained with CD14-allophycocyanin (BD PharMingen) for an additional 15 min at room temperature. Cells were washed, resuspended in FACS wash buffer, and analyzed by flow cytometry. Zinc depletion was achieved by treating cells with increasing doses of TPEN (5, 10, and 20 μM) for 12 h at 37°C. Following TPEN exposure, cells were subsequently treated with an apoptotic inducer (sFasL or cadmium chloride) for an additional 6 h. Intracellular zinc and apoptosis were measured as described above.
HIV infection
A macrophage-tropic, laboratory-adapted strain (Ba-L) and a clinical isolate (JAGO) of HIV-1 were used to infect 3-day-old elutriated monocytes in vitro. Both infectious isolates were obtained from the University of Pennsylvania Center for AIDS Research (Philadelphia, PA, USA). Human PBMCs, obtained by leukopheresis of healthy donors, were enriched for monocytes by elutriation and seeded in six-well plates (Corning, Corning, NY, USA) at 5 × 106 cells/well. Monocytes were infected/exposed to HIV-1 (10 ng/l × 106) for 96 h. Change in gene expression and zinc content was monitored by real-time PCR and flow cytometry, respectively.
Statistical analysis
Statistical analysis between unrelated groups was performed using the Mann-Whitney or Wilcoxon test. All tests with P values ≤0.05 were considered significant and were performed using JMP (SAS Institute, Cary, NC, USA) statistical software.
RESULTS AND DISCUSSION
HIV-1 replication in vivo induces MT gene expression in circulating monocytes
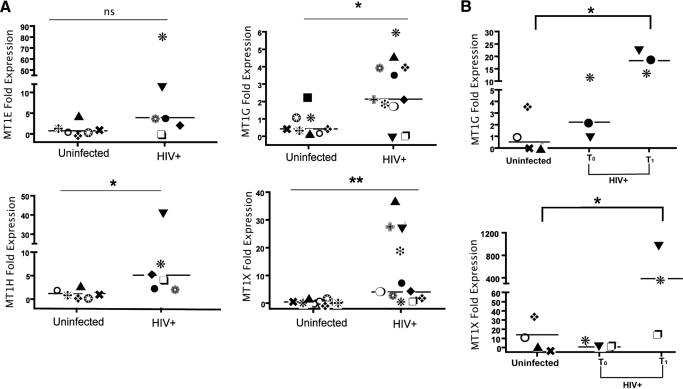
To investigate the MT gene family and its expression levels in circulating monocytes during HIV-1 viremia in vivo, we analyzed isolated monocyte expression of isoforms MT1E, -G, -H, and -X. Results showed basal expression of MT1H, -G, and -X isoforms to be increased three- to tenfold, as measured by quantitative RT-PCR in viremic donors when compared with uninfected controls (Fig. 1A). To determine whether the observed, constitutive, increased level of monocyte MT1 gene isoform expression was subject to change upon changes in viral replication, we examined MT1 gene expression in isolated CD14 monocytes from a subset of HIV+ patients with acute viremia following treatment interruption. As expected, analysis of cell subset changes by cell differential analysis (CBC) and flow cytometry following therapy interruption showed a clear decline in CD4 T cell count upon 6 weeks of viral replication. Of interest, absolute monocyte numbers increased rather than decreased during the same time interval (data not shown). Analysis of gene expression within CD14 cells showed MT1G and MT1X expression in cell subsets isolated from suppressed and therapy interruption viremic time-points, showing that rebounding viremia was associated with an enhanced expression of MT1G and -X isoforms (Fig. 1B). Taken together, results indicate a positive relationship between viral replication and induction of MT gene expression in circulating monocytes.
Figure 1.
Increased MT gene expression in circulating monocytes derived from HIV+ individuals with chronic or acute viremia. (A) Differential expression of MT1 isoforms in CD14+ monocytes derived from uninfected and HIV+ subjects during HIV viremia confirmed by real-time PCR [MT1E and MT1H, n=6 for uninfected and HIV+ donors; MT1G and MT1X results consisted of uninfected (n=6) and HIV+ donors (n=11)]. In t-test comparisons, ns indicates nonsignificant P; *, P < 0.05; **, P < 0.01. (B) Real-time PCR results using RNA isolated from CD14+ monocytes derived from cryopreserved PBMCs collected pre- and post-treatment interruption (n=3). *, P < 0.05, in ANOVA comparisons with monocytes from cryopreserved, uninfected PBMCs. Each symbol in group identifies same donors between panels.
Elevated MT gene expression in HIV-1 infection in vivo correlates with an increase in monocyte intracellular zinc content and expression of zinc transporter gene Zip8
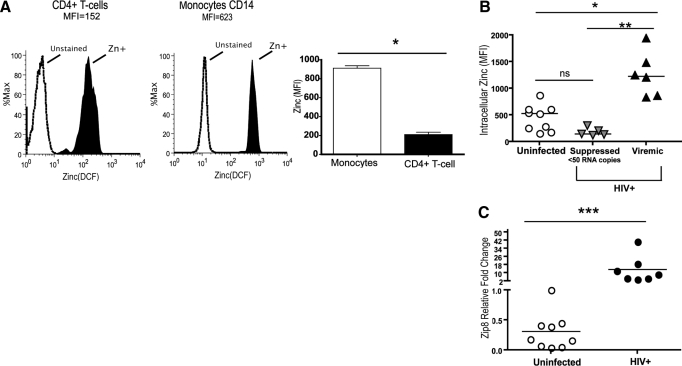
Based on the increased MT1 gene expression in circulating monocytes, we asked whether MT1 expression would impact zinc retention and homeostasis in circulating monocytes. Zinc levels were found to be threefold higher in circulating monocytes when compared with circulating CD4+ T cells from the same HIV-negative donor using the cell-permeable zinc probe DCF (Fig. 2A), illustrating greater zinc levels in steady-state within CD14+ cells. In HIV-1 viremia, intracellular zinc levels were observed to be significantly higher in circulating monocytes from viremic subjects when compared with circulating monocytes from ART-suppressed (<50 copies/ml) or uninfected subject monocytes (Fig. 2B). In vitro evidence supporting a direct link between HIV-1 exposure and intracellular zinc was tested further in MDM infected/exposed to HIV-1-bronchoalveolar lavage for a period of 4 days, showing that MT1G (and not MT1H, MT1X, or MT2 expression) and intracellular zinc were elevated significantly in MDM exposed/infected with an R5 HIV-1 isolate (Supplemental Fig. 1). In addition to elevated in vivo MT gene expression and elevated zinc levels, an increase in zinc transporter gene Zip8 was observed in circulating monocytes, further supporting the ability of monocytes to sustain higher levels of intracellular zinc in the presence of viral replication (Fig. 2C). Taken together, the data support a direct link among sustained HIV-1 replication, elevated MT gene expression, and increased zinc levels in circulating monocytes.
Figure 2.
Intracellular zinc is increased in monocytes during chronic HIV viremia. (A) Intracellular zinc levels, measured via DCF uptake, were compared in monocytes and CD4+ T cells from three viremic HIV+ donors. Histograms of DCF flow cytometric reading, along with a composite of the three donors, are shown. MFI, Mean fluorescence intensity. (B) Intracellular zinc in CD14+ monocytes from uninfected (n=9) and HIV+-suppressed (n=5) or -viremic (n=6) donors measured by DCF uptake shows that active HIV replication increases intracellular zinc in monocytes. (C) Expression of zinc importer gene Zip8 assessed by real-time PCR. Groups (uninfected, n=9; HIV+, n=7) were analyzed using ANOVA or paired comparison tests. ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001. Subject data for each group represented by circles and triangles.
Monocyte resistance to FasL-induced apoptosis linked to high intracellular zinc levels during HIV viremia
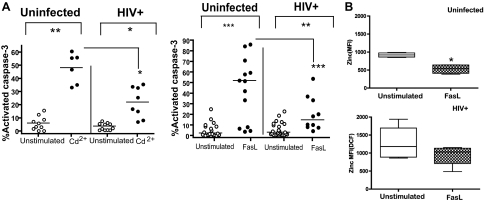
During HIV infection, elevated levels of cell-free FasL are detected in the plasma as well as the presence of an increased cellular oxidative stress, which together, may impact apoptosis [4, 5, 32, 33]. To re-confirm monocyte sensitivity to induced apoptosis in this study as a prelude to measuring zinc levels, we exposed monocytes from uninfected and HIV+ donors to sFasL or cadmium (heavy metal-inducing oxidative stress), both known inducers of apoptosis. As expected, monocytes from HIV+ patients were found to be less sensitive to cadmium or FasL-induced apoptosis (Fig. 3A). Of interest, monocyte activation by FasL results in retention of higher levels of intracellular zinc after activation when compared with a twofold decrease in control-activated monocytes (Fig. 3B). These data document that elevated zinc levels may remain in monocytes from HIV-infected subjects in spite of apoptotic induction. This is consistent with the interpretation of a sustained apoptosis resistance in monocytes during chronic HIV viremia.
Figure 3.
Monocyte apoptosis resistance in HIV viremic subjects associated with retention of higher intracellular zinc levels. (A) Monocytes derived from uninfected donors (•; n=10) or viremic HIV+ patients (○; n=8) were exposed to cadmium chloride (Cd2+; 100 μM) and FasL [100 ng/ml; uninfected (○; n=15) and HIV+ (•; n=10)] for 6 h. Cells were harvested, apoptosis was measured by staining for activated caspase-3, and samples were analyzed by flow cytometry. Totals shown were derived from CD14+ cells that were 7AAD-negative/caspase-3-positive and double-positive 7AAD/caspase-3 cells. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Intracellular zinc was measured in monocytes from HIV+-infected (checkered bar; n=10) and uninfected (open bar; n=15) donors following apoptosis induction of FasL (100 ng/ml). *, P < 0.05, in ANOVA comparisons with monocytes from the untreated group.
Intracellular zinc levels determine susceptibility to activation-induced apoptosis in circulating monocytes
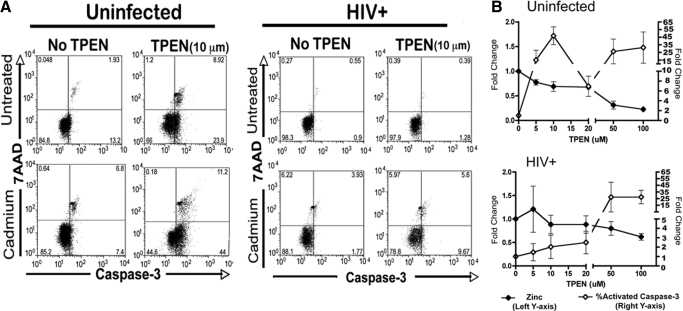
As we observed elevated intracellular zinc content, MT gene expression, and increased resistance to apoptosis in circulating monocytes from HIV-infected subjects (Fig. 3A), we tested directly whether the increase in intracellular zinc may act to augment monocyte resistance to induced apoptosis. We assessed apoptosis induction in circulating monocytes from uninfected as well as viremic, HIV-infected subjects in the presence of the zinc chelator TPEN. As predicted, lowering intracellular zinc sensitized the constitutive apoptosis of monocytes from HIV-uninfected donors to cadmium at a 10-μm concentration, whereas constitutive apoptosis was not changed in monocytes from HIV-infected persons (Fig. 4A). Although depletion of zinc content in cells is expected to increase apoptosis induction eventually [20], we tested whether the threshold for apoptosis would be different between HIV-infected and uninfected circulating monocytes with increasing doses of TPEN. Indeed, TPEN pretreatment lowered intracellular zinc and increased caspase-3 activation in uninfected controls at lower concentrations than those required to elicit similar apoptosis induction in monocytes from HIV-infected subjects (Fig. 4B). These functional data support that higher zinc stores are present in circulating monocytes from HIV-1 viremic subjects and that these zinc stores can affect susceptibility toward constitutive and induced apoptosis.
Figure 4.
Higher threshold of monocyte apoptosis resistance in HIV infection upon an induced decrease of intracellular zinc levels. (A) Monocytes from uninfected (n=5) or HIV+ donors (n=5) were exposed to cadmium (100 μM) for 6 h in the presence or absence of TPEN. Cells were harvested and apoptosis assessed by staining for activated caspase-3 and 7AAD uptake. Shown is a representative cadmium chloride-induced apoptosis in an uninfected (left) and HIV donor (right). (B) Shown is a dose response of TPEN concentration and the induction of caspase-3 activation or intracellular zinc level in circulating monocytes from uninfected (n=5) or HIV+ subjects (n=5). Monocytes from HIV+ individuals appear to be less sensitive to TPEN treatment.
Taken all data sections above together, we demonstrate for the first time that monocytes from HIV+ donors express increased levels of MT gene expression in association with increased intracellular zinc levels mediating resistance against monocyte activation-induced apoptosis. Expression of MT genes in association with changes in zinc metabolism may represent a common host response to inflammation and viral pathogens. Coxsackievirus Type B infection increases MT expression in association with redistribution of kidney zinc levels [20], and influenza virus infection also mediates increased MT gene and protein expression [34]. We now extend these observations to HIV-1 infection by providing supporting data about the relation between MT expression and increased intracellular zinc in monocytes from HIV-1 viremic subjects from cross-sectional (Fig. 1A) and longitudinal treatment-interruption studies (Fig. 1B) and induced changes by HIV-1 binding and replication in MDM (Supplemental Fig. 1). Interestingly, endotoxin, shown to be elevated in plasma of HIV+ patients [35], has also been implicated in the induction of MT gene expression [36, 37], yet the association of increases in MT gene expression and zinc following in vitro HIV-1 infection of MDM suggests that viral replication alone could account for a major part of these changes. With regard to the mechanism of action for apoptosis resistance, total zinc, bound in part by MT proteins, can influence key signaling pathways including apoptosis regulation [13, 38, 39]. For example, zinc is known to be a potent inhibitor of the apoptotic protease caspase-3 [30]. This is consistent with our data showing that monocytes with increased zinc levels are more resistant to apoptosis induction by cadmium chloride (Fig. 3A, left) or FasL (Fig. 3A, right). Future investigation will need to determine if changes in intracellular zinc are also related to altered monocyte function. Aside from the response to apoptosis, zinc has been shown to be a positive regulator in LPS-mediated activation of myeloid dendritic cells [25, 40, 41]. The latter raises the possibility that a decrease in monocyte activation-induced apoptosis may occur at the expense of zinc-dependent alterations on immune functionality.
The observation that zinc chelation via TPEN in monocytes from HIV+ individuals resulted in a retained apoptosis resistance when compared with uninfected controls provides support to the interpretation that zinc levels may contribute directly to monocyte apoptosis susceptibility. We infer that elevated levels of the chelating agent were needed to counteract the increased expression of Zip8 and MT isoforms in monocytes from HIV-infected subjects. In addition to the role for LPS in HIV pathogenesis noted above, several studies have shown that LPS can modulate zinc levels via regulation of transporter genes including Zip8 [41, 42], further supporting the interpretation that modulation of Zip8 by viral-binding or by circulating LPS in vivo could account for changes in monocyte zinc import during HIV-1 viremia. Evidence for monocyte retention of elevated zinc levels after in vitro apoptosis induction as compared with monocytes of uninfected subjects supports the hypothesis that monocytes are able to retain higher levels of zinc during viral replication despite the presence of elevated, apoptotic-inducing host factors, such as plasma FasL, confirmed to be higher in viremic subjects (data not shown). Lastly, the observed rises in monocyte counts associated with increased MT gene expression following therapy interruption (data not shown), at a time when CD4 T cells counts were declining, are consistent with a viral replication-dependent modulation against cell death in the monocyte compartment.
Although we find increased zinc levels in monocytes during viremia, zinc deficiency is common in HIV-infected subjects. A reduction in available zinc and a lower intracellular zinc level are known to negatively affect T-lymphocyte immune responses in geriatric subjects and may in part account for their lowered immunity or immunosenesence [43,44,45]. Our data suggest that a lowering of available zinc levels occurs concurrently with higher zinc uptake and retention in monocytes/macrophages, which may affect T cell functionality indirectly during HIV viremia. Zinc supplementation in HIV-1 viremia may combat this aspect of HIV immunopathogenesis by supporting available zinc levels in spite of the increased uptake by the monocyte/macrophage pool. Of interest, zinc supplementation has been used to reduce diarrhea-related morbidity in HIV-infected children, but it remains to be determined whether these supplements can also impact immune function and/or T cell function [46,47,48,49,50]. Conversely, our study also suggests that lowering HIV-induced increases in zinc content within the monocyte/macrophage from HIV+ donors should sensitize these cells to apoptosis and thereby, decrease available targets for long-term reservoirs and/or replication. It is of interest to view our data in light of recent reports documenting a higher level of monocyte turnover at end-stage disease, as it remains to be determined whether increased intracellular zinc levels decline within monocytes, with disease progression accounting for greater monocyte loss/turnover [51]. Regulation of the MT gene family coupled with the associated increased intracellular zinc level and apoptosis resistance may provide a mechanism of monocyte retention and infected macrophage persistence. Zinc may therefore be a major intracellular factor mediating the differential response of monocytes/macrophage to apoptosis when compared with T cells during HIV infection.
AUTHORSHIP
A.R. performed ex vivo and in vitro experiments with assistance from B.G., M.S.G., A.H., and J.C.; and L.S. and E.P. collected the therapy-interruption cohort. HIV-infected cohorts were provided by K.M., J.S., and J.K. L.J.M. assisted in the design and the interpretation of data. A.R. and L.J.M. wrote the paper.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health AI047760, the Philadelphia Foundation, and Wistar funds from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health. We thank D. Davis, Philadelphia FIGHT staff, C. Gallo, A. Hancock, S. Creer, J. Faust, C. Nichols, and M. Farabaugh for their help with experiments.
Supplementary Material
Footnotes
Abbreviations: 7AAD=7-amino-actinomycin D, ART=antiretroviral therapy, CBC=complete blood count, CT=comparative threshold, DCF= dichlorofluorescein, FasL=Fas ligand, FIGHT=Field Initiation Group for HIV Trials, MDM=monocyte-derived macrophage(s), MT=metallothionein, sFasL=soluble Fas ligand, TPEN=(N,N,N′,N′-tetrakis 2-pyridylmethyl) ethylenediamine
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
References
- Lichtner M, Maranon C, Vidalain P O, Azocar O, Hanau D, Lebon P, Burgard M, Rouzioux C, Vullo V, Yagita H, Rabourdin-Combe C, Servet C, Hosmalin A. HIV type 1-infected dendritic cells induce apoptotic death in infected and uninfected primary CD4 T lymphocytes. AIDS Res Hum Retroviruses. 2004;20:175–182. doi: 10.1089/088922204773004897. [DOI] [PubMed] [Google Scholar]
- Herbeuval J P, Boasso A, Grivel J C, Hardy A W, Anderson S A, Dolan M J, Chougnet C, Lifson J D, Shearer G M. TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood. 2005;105:2458–2464. doi: 10.1182/blood-2004-08-3058. [DOI] [PubMed] [Google Scholar]
- Dockrell D H, Badley A D, Villacian J S, Heppelmann C J, Algeciras A, Ziesmer S, Yagita H, Lynch D H, Roche P C, Leibson P J, Paya C V. The expression of Fas ligand by macrophages and its upregulation by human immunodeficiency virus infection. J Clin Invest. 1998;101:2394–2405. doi: 10.1172/JCI1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badley A D, Dockrell D H, Algeciras A, Ziesmer S, Landay A, Lederman M M, Connick E, Kessler H, Kuritzkes D, Lynch D H, Roche P, Yagita H, Paya C V. In vivo analysis of Fas/FasL interactions in HIV-infected patients. J Clin Invest. 1998;102:79–87. doi: 10.1172/JCI2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badley A D, McElhinny J A, Leibson P J, Lynch D H, Alderson M R, Paya C V. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol. 1996;70:199–206. doi: 10.1128/jvi.70.1.199-206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm G H, Gabuzda D. Distinct mechanisms of CD4+ and CD8+ T-cell activation and bystander apoptosis induced by human immunodeficiency virus type 1 virions. J Virol. 2005;79:6299–6311. doi: 10.1128/JVI.79.10.6299-6311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri M S, Nebozhyn M, Showe L, Montaner L J. Microarray data on gene modulation by HIV-1 in immune cells: 2000–2006. J Leukoc Biol. 2006;80:1031–1043. doi: 10.1189/jlb.0306157. [DOI] [PubMed] [Google Scholar]
- Swingler S, Mann A M, Zhou J, Swingler C, Stevenson M. Apoptotic killing of HIV-1-infected macrophages is subverted by the viral envelope glycoprotein. PLoS Pathog. 2007;3:1281–1290. doi: 10.1371/journal.ppat.0030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. Basic science highlights. Top HIV Med. 2007;15:21–25. [PubMed] [Google Scholar]
- Kiener P A, Davis P M, Rankin B M, Klebanoff S J, Ledbetter J A, Starling G C, Liles W C. Human monocytic cells contain high levels of intracellular Fas ligand: rapid release following cellular activation. J Immunol. 1997;159:1594–1598. [PubMed] [Google Scholar]
- Busca A, Saxena M, Kryworuchko M, Kumar A. Anti-apoptotic genes in the survival of monocytic cells during infection. Curr Genomics. 2009;10:306–317. doi: 10.2174/138920209788920967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman H, Pagliari L J, Georganas C, Mano T, Walsh K, Pope R M. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J Exp Med. 1999;190:1679–1688. doi: 10.1084/jem.190.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt P, Denizeau F. Metallothionein in physiological and physiopathological processes. Drug Metab Rev. 1997;29:261–307. doi: 10.3109/03602539709037585. [DOI] [PubMed] [Google Scholar]
- Kagi J H. Overview of metallothionein. Methods Enzymol. 1991;205:613–626. doi: 10.1016/0076-6879(91)05145-l. [DOI] [PubMed] [Google Scholar]
- Zeng J, Heuchel R, Schaffner W, Kagi J H. Thionein (apometallothionein) can modulate DNA binding and transcription activation by zinc finger containing factor Sp1. FEBS Lett. 1991;279:310–312. doi: 10.1016/0014-5793(91)80175-3. [DOI] [PubMed] [Google Scholar]
- Quaife C J, Findley S D, Erickson J C, Froelick G J, Kelly E J, Zambrowicz B P, Palmiter R D. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry. 1994;33:7250–7259. doi: 10.1021/bi00189a029. [DOI] [PubMed] [Google Scholar]
- Kagi J H, Schaffer A. Biochemistry of metallothionein. Biochemistry. 1988;27:8509–8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- Park J D, Liu Y, Klaassen C D. Protective effect of metallothionein against the toxicity of cadmium and other metals(1) Toxicology. 2001;163:93–100. doi: 10.1016/s0300-483x(01)00375-4. [DOI] [PubMed] [Google Scholar]
- Sato M, Kondoh M. Recent studies on metallothionein: protection against toxicity of heavy metals and oxygen free radicals. Tohoku J Exp Med. 2002;196:9–22. doi: 10.1620/tjem.196.9. [DOI] [PubMed] [Google Scholar]
- Ilback N G, Glynn A W, Wikberg L, Netzel E, Lindh U. Metallothionein is induced and trace element balance changed in target organs of a common viral infection. Toxicology. 2004;199:241–250. doi: 10.1016/j.tox.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Kimura T, Itoh N, Takehara M, Oguro I, Ishizaki J I, Nakanishi T, Tanaka K. Sensitivity of metallothionein-null mice to LPS/D-galactosamine-induced lethality. Biochem Biophys Res Commun. 2001;280:358–362. doi: 10.1006/bbrc.2000.4085. [DOI] [PubMed] [Google Scholar]
- Mita M, Satoh M, Shimada A, Okajima M, Azuma S, Suzuki J S, Sakabe K, Hara S, Himeno S. Metallothionein is a crucial protective factor against Helicobacter pylori-induced gastric erosive lesions in a mouse model. Am J Physiol Gastrointest Liver Physiol. 2008;294:G877–G884. doi: 10.1152/ajpgi.00251.2007. [DOI] [PubMed] [Google Scholar]
- Harley C B, Menon C R, Rachubinski R A, Nieboer E. Metallothionein mRNA and protein induction by cadmium in peripheral-blood leucocytes. Biochem J. 1989;262:873–879. doi: 10.1042/bj2620873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode H F, Kelleher J, Walker B E. Zinc concentrations in pure populations of peripheral blood neutrophils, lymphocytes and monocytes. Ann Clin Biochem. 1989;26:89–95. doi: 10.1177/000456328902600114. [DOI] [PubMed] [Google Scholar]
- Rink L, Haase H. Zinc homeostasis and immunity. Trends Immunol. 2007;28:1–4. doi: 10.1016/j.it.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Keen C L, Gershwin M E. Zinc deficiency and immune function. Annu Rev Nutr. 1990;10:415–431. doi: 10.1146/annurev.nu.10.070190.002215. [DOI] [PubMed] [Google Scholar]
- Pauwels M, van Weyenbergh J, Soumillion A, Proost P, De Ley M. Induction by zinc of specific metallothionein isoforms in human monocytes. Eur J Biochem. 1994;220:105–110. doi: 10.1111/j.1432-1033.1994.tb18603.x. [DOI] [PubMed] [Google Scholar]
- Nodera M, Yanagisawa H, Wada O. Increased apoptosis in a variety of tissues of zinc-deficient rats. Life Sci. 2001;69:1639–1649. doi: 10.1016/s0024-3205(01)01252-8. [DOI] [PubMed] [Google Scholar]
- Truong-Tran A Q, Ho L H, Chai F, Zalewski P D. Cellular zinc fluxes and the regulation of apoptosis/gene-directed cell death. J Nutr. 2000;130:1459S–1466S. doi: 10.1093/jn/130.5.1459S. [DOI] [PubMed] [Google Scholar]
- Perry D K, Smyth M J, Stennicke H R, Salvesen G S, Duriez P, Poirier G G, Hannun Y A. Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J Biol Chem. 1997;272:18530–18533. doi: 10.1074/jbc.272.30.18530. [DOI] [PubMed] [Google Scholar]
- Fukamachi Y, Karasaki Y, Sugiura T, Itoh H, Abe T, Yamamura K, Higashi K. Zinc suppresses apoptosis of U937 cells induced by hydrogen peroxide through an increase of the Bcl-2/Bax ratio. Biochem Biophys Res Commun. 1998;246:364–369. doi: 10.1006/bbrc.1998.8621. [DOI] [PubMed] [Google Scholar]
- Wanchu A, Rana S V, Pallikkuth S, Sachedeva R K. Short communication: oxidative stress in HIV-infected individuals: a cross-secional study. AIDS Res Hum Retroviruses. 2009;25:1307–1311. doi: 10.1089/aid.2009.0062. [DOI] [PubMed] [Google Scholar]
- Hulgan T, Morrow J, D'Aquila R T, Raffanti S, Morgan M, Rebeiro P, Haas D W. Oxidant stress is increased during treatment of human immunodeficiency virus infection. Clin Infect Dis. 2003;37:1711–1717. doi: 10.1086/379776. [DOI] [PubMed] [Google Scholar]
- Ghoshal K, Majumder S, Zhu Q, Hunzeker J, Datta J, Shah M, Sheridan J F, Jacob S T. Influenza virus infection induces metallothionein gene expression in the mouse liver and lung by overlapping but distinct molecular mechanisms. Mol Cell Biol. 2001;21:8301–8317. doi: 10.1128/MCB.21.24.8301-8317.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J M, Price D A, Schacker T W, Asher T E, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar B R, Rodriguez B, Teixeira-Johnson L, Landay A, Martin J N, Hecht F M, Picker L J, Lederman M M, Deeks S G, Douek D C. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Haase H, Hebel S, Engelhardt G, Rink L. Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells. Anal Biochem. 2006;352:222–230. doi: 10.1016/j.ab.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Ibs K H, Rink L. Zinc-altered immune function. J Nutr. 2003;133:1452S–1456S. doi: 10.1093/jn/133.5.1452S. [DOI] [PubMed] [Google Scholar]
- Sakurai A, Hara S, Okano N, Kondo Y, Inoue J, Imura N. Regulatory role of metallothionein in NF-κB activation. FEBS Lett. 1999;455:55–58. doi: 10.1016/s0014-5793(99)00839-x. [DOI] [PubMed] [Google Scholar]
- Youn J, Borghesi L A, Olson E A, Lynes M A. Immunomodulatory activities of extracellular metallothionein. II. Effects on macrophage functions. J Toxicol Environ Health. 1995;45:397–413. doi: 10.1080/15287399509532004. [DOI] [PubMed] [Google Scholar]
- Von Bulow V, Dubben S, Engelhardt G, Hebel S, Plumakers B, Heine H, Rink L, Haase H. Zinc-dependent suppression of TNF-{α} production is mediated by protein kinase A-induced inhibition of Raf-1, I{κ}B kinase β, and NF-{κ}B. J Immunol. 2007;179:4180–4186. doi: 10.4049/jimmunol.179.6.4180. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, Yamashita S, Kaisho T, Akira S, Murakami M, Hirano T. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–977. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- Haase H, Ober-Blobaum J L, Engelhardt G, Hebel S, Heit A, Heine H, Rink L. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J Immunol. 2008;181:6491–6502. doi: 10.4049/jimmunol.181.9.6491. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Costarelli L, Giacconi R, Cipriano C, Muti E, Malavolta M. Zinc-binding proteins (metallothionein and α-2 macroglobulin) and immunosenescence. Exp Gerontol. 2006;41:1094–1107. doi: 10.1016/j.exger.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Giacconi R, Cipriano C, Malavolta M. NK and NKT cells in aging and longevity: role of zinc and metallothioneins. J Clin Immunol. 2009;29:416–425. doi: 10.1007/s10875-009-9298-4. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Muzzioli M, Cipriano C, Giacconi R. Zinc, T-cell pathways, aging: role of metallothioneins. Mech Ageing Dev. 1998;106:183–204. doi: 10.1016/s0047-6374(98)00115-8. [DOI] [PubMed] [Google Scholar]
- Green J A, Paton N I. Zinc supplementation in children with HIV-1 infection. Lancet. 2006;367:814–815. doi: 10.1016/S0140-6736(06)68332-3. author reply 815–816. [DOI] [PubMed] [Google Scholar]
- Bobat R, Coovadia H, Stephen C, Naidoo K L, McKerrow N, Black R E, Moss W J. Safety and efficacy of zinc supplementation for children with HIV-1 infection in South Africa: a randomized double-blind placebo-controlled trial. Lancet. 2005;366:1862–1867. doi: 10.1016/S0140-6736(05)67756-2. [DOI] [PubMed] [Google Scholar]
- Reich E N, Church J A. Oral zinc supplementation in the treatment of HIV-infected children. Pediatr AIDS HIV Infect. 1994;5:357–360. [PubMed] [Google Scholar]
- Canani R B, Ruotolo S, Buccigrossi V, Passariello A, Porcaro F, Siani M C, Guarino A. Zinc fights diarrhoea in HIV-1-infected children: in-vitro evidence to link clinical data and pathophysiological mechanism. AIDS. 2007;21:108–110. doi: 10.1097/QAD.0b013e328011849a. [DOI] [PubMed] [Google Scholar]
- Carcamo C, Hooton T, Weiss N S, Gilman R, Wener M H, Chavez V, Meneses R, Echevarria J, Vidal M, Holmes K K. Randomized controlled trial of zinc supplementation for persistent diarrhea in adults with HIV-1 infection. J Acquir Immune Defic Syndr. 2006;43:197–201. doi: 10.1097/01.qai.0000242446.44285.b5. [DOI] [PubMed] [Google Scholar]
- Kuroda M J. Macrophages: do they impact AIDS progression more than CD4 T cells? J Leukoc Biol. 2010;87:569–573. doi: 10.1189/jlb.0909626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.