Abstract
The lung is constantly exposed to potentially pathogenic particles and microorganisms. It has become evident recently that not only innate but also adaptive immune responses to particulates, such as SiO2 entering the respiratory tract, are complex and dynamic events. Although the cellular mechanisms and anatomical consequences involved in the development of silicosis have been studied extensively, they still remain poorly understood. Based on their capacity for immune regulation, lymphocytes may play a key role in the respiratory response to environmental challenge by SiO2. The objective of this study was to characterize the impact of SiO2 exposure on respiratory immune processes, with particular emphasis on evaluating the importance of lymphocytes in the murine silicosis model. Therefore, lymphopenic mice, including NK-deficient, Rag1−/−, or a combination (Rag1−/− NK-depleted), were used and demonstrated that SiO2-induced fibrosis and inflammation can occur independently of T, B, NK T, and NK cells. Studies in Rag1−/− mice suggest further that lymphocytes may participate in the regulation of SiO2-induced inflammation through modulation of the Nalp3 inflammasome. This observation may have clinical relevance in the treatment of inflammatory and fibrotic lung diseases that are refractory or respond suboptimally to current therapeutics.
Keywords: lung, inflammation, fibrosis, lymphocyte, cytokine, inflammasome
Introduction
Prolonged exposure to SiO2 in occupational and environmental settings induces chronic lung inflammation, which may progress to fibrosis, i.e., silicosis [1, 2]. Despite existing standards in the workplace, silicosis remains a prevalent health problem throughout the world, particularly in developing nations. Insufficient information about the pathophysiological mechanisms that drive the expansion of inflammatory cells and collagen accumulation in silicosis has severely limited the development of effective therapeutic strategies. As various Th1- and Th2-associated cytokines have been implicated in inflammation and fibrosis, many investigators considered T cells a key participant in the initiation and progression of silicosis in rodent models [3,4,5,6,7]. Indeed, an influx of T cells into the lungs is a prominent component of the immune response to SiO2.
In contrast to the predictions noted above, previous studies demonstrated that nude mice genetically deficient in T cells exhibit inflammation, lung injury, and an enhanced neutrophil response to SiO2 and show similar collagen deposition and pulmonary fibrosis compared with their T cell-sufficient counterparts [4]. In much the same way, Helene et al. [8] demonstrated using SCID mice that T cells are not required for bleomycin-induced fibrosis. Taken together, these studies suggest that T cells do not play a pivotal part in the pathogenesis of fibrosis.
Similarly, the belief that Th1 and Th2 types of immune responses are simply mediated by T cells has become less rigid, as other cell types, including NK cells, have been identified, which produce these cytokines and may play a role in regulating an inflammatory response. NK cells resemble T cells: both arise from an immediate common progenitor and share the expression of several surface molecules, yet NK cells have unique morphology, phenotype, and functional properties. NK cells can act as a bridge between innate and adaptive immunity [9, 10] and are constitutively located in human and murine lung, suggesting their possible involvement in pulmonary disorders [11, 12]. Therefore, we hypothesized that those lymphocytes that accumulate in response to SiO2 exposure influence lung injury through direct or indirect effects on innate immune cells and fibroblasts and that T cells or the NK cell may carry out these functions.
Recent advances in immunity have implicated the formation of multi-protein complexes, termed inflammasomes, in both key features of silicosis—inflammation and fibrosis. In particular, Nlrp3-mediated inflammasome formation is critical for caspase 1 activation and IL-1β and IL-18 processing and release in response to several microbial molecules, ATP, urate crystals, silica, or asbestos particles [13, 14]. Although normal activation of the Nlrp3 inflammasome contributes to host defense, excessive activation leads to autoinflammatory diseases [15, 16]. Recent studies implicated T cells in limiting the innate immune response through inhibition of Nlrp3 inflammasome in vitro [17]; however, it is not known whether lymphocytes dampen SiO2-induced activation of the Nlrp3 inflammasome in vivo.
The objective of the current study was twofold: to determine whether lymphocytes are fundamentally important to the development of silicosis and to examine whether lymphocytes modulate SiO2-induced inflammation by influencing the termination of the inflammasome response. We tested this hypothesis by examining the progression of inflammation and fibrosis in response to SiO2 exposure in genetically modified strains of mice (all on C57Bl/6 background) that lack T and B cells but have NK cells (Rag1−/− mice), mice that have T and B lymphocytes but lack NK cells (NK-deficient mice), or mice that lack T, B, NK T, and NK cell populations (Rag1−/− NK-depleted mice).
MATERIALS AND METHODS
Mice
Breeding pairs of C57Bl/6 and Rag1−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). NK-deficient mice were generated through the introduction of a transgene encoding anti-NK1.1, which results in chronic depletion of NK cells [18]. All mice were maintained in the University of Montana specific pathogen-free laboratory animal facility (Missoula, MT, USA). Cages, bedding, and food were sterilized by autoclaving, and mice were handled with aseptic gloves at all times. Mice were allowed food and water ad libitum and were used experimentally at 6–8 weeks of age. All animal use procedures were in accordance with NIH and University of Montana Institutional Animal Care and Use Committee guidelines.
Experimental instillations
Silica (Min-U-Sil-5, with an average particle size of 1.5–2 μm), obtained from Pennsylvania Glass Sand Corp. (Pittsburgh, PA, USA), was acid-washed, dried, and determined to be free of endotoxin by Limulus assay (data not shown; Cambrex, Walkersville, MD, USA). Particulates were suspended in sterile saline and sonicated for 30 s immediately prior to instillation. For “acute” exposure studies (<28 days), mice were anesthetized with ketamine (80 mg/kg) and instilled i.n. with 25 μl sterile saline, 1 mg SiO2, or 0.5 mg TiO2 (nonfibrogenic particle control at the same surface area), suspended in 25 μl sterile saline. Mice were returned to their cages and monitored until mobility returned. At 1, 3, 7, and 28 days following the initial instillation, mice were killed and tissues collected for experimental analysis. For “chronic” exposure studies, Rag1−/−, NK-deficient, Rag1−/− NK-depleted, and C57Bl/6 wild-type mice were anesthetized with ketamine (80 mg/kg) and instilled i.n. with 25 μl sterile saline, 1 mg SiO2, or 0.5 mg TiO2, suspended in 25 μl sterile saline once/week for 4 weeks. Mice were returned to their cages and monitored until mobility returned. At 4 and 16 weeks following the initial instillation, mice were assayed for experimental end-points as indicated.
Antibody purification and depletion of NK cells from Rag1−/− mice
Anti-NK1.1 mAb was purified from hybridoma supernatants (PK136 clone, American Type Culture Collection, Manassas, VA, USA) using ImmunoPure immobilized protein A/G gel (Pierce, Rockford, IL, USA). Antibody specificity was determined by performing competitive binding studies against commercial PE-conjugated anti-NK1.1 mAb (PK136 clone; BD Biosciences, San Jose, CA, USA). To optimize the treatment regimen, pilot studies using 100, 300, or 500 μg purified mAb were performed. Efficient depletion of NK cells in vivo was determined by flow cytometry (FACSAria, BD Biosciences) of the leukoctye populations from lungs and spleens. In untreated C57Bl/6 mice, NK cells averaged 2.9% in spleens and 4.5% in lungs. Mice treated with all 3 concentrations of mAb resulted in essentially a complete loss of NK cells in the lungs (0.2–0.3%) and spleens (0.1–0.2%; data not shown). Similar analyses were performed on organs from random mice within the chronic exposure studies with the same results (data not shown). For the chronic exposure studies, Rag1−/− mice were injected i.p. with 300 μg anti-NK1.1 mAb on Days –6, –3, and –1 prior to the first i.n. instillation (Day 0). NK cell depletion was maintained via weekly injections of 300 μg anti-NK1.1 mAb throughout the course of the study, which was administered 2 days prior to particle exposure. Studies using anti-NK1.1 antibody-based depletion also resulted in the elimination of NK T cells.
Biochemical quantification of collagen content
Total collagen of the left lobe of chronic exposure mice was quantified by analysis of hydroxyproline, an amino acid unique to collagen. Briefly, lung tissue from the left lobe was excised, weighed, and frozen immediately in liquid nitrogen. The lung tissue was homogenized using a Tissue Tearor in sterile water. An aliquot of lung homogenate was hydrolyzed in 12 N HCl at 110°C for 24 h. The mixture was reacted with chloramine T and Ehrlich’s reagent to produce a hydroxyproline-chromophore that was quantified by 550 nm spectrophotometry. Hydroxyproline content for each lobe was determined by triplicate analysis of the sample to provide a mean value.
Histopathological evaluation of inflammation and fibrosis
The right lobe of the lung of chronic exposure mice was inflated with Histochoice and fixed for 24 h at 4°C. Routine histological procedures were used to paraffin-embed the lobe. As described previously, 5 μm sections were cut, mounted on Superfrost+ VWR slides (VWR International, West Chester, PA, USA), and stained with Gomori’s Trichrome (EMD Chemicals, Gibbstown, NJ, USA) [19, 20]. Five to 6 mice/group were examined microscopically and representative images captured with a Nikon E-800 microscope and Nikon DXM 1200 digital color camera connected to a Dell computer. Alternate sections were stained for LY (0.1 mg/ml; Molecular Probes Eugene, OR, USA) [19, 21, 22]. This fluorescent dye selectively stains connective tissue matrix macromolecules, permitting visualization as well as quantification of collagen matrix using LSC in a manner that yields comparable fibrosis values to the traditional hydroxyproline assay [21,22,23].
Image analysis
LSC (CompuCyte, Cambridge, MA, USA) was used in conjunction with LY to quantify the extent of lung fibrosis based on collagen deposition in intact tissue sections. Using WinCyte software, the mean pixel intensity/square μ was determined based on the assessment of 9 random areas within nonsequential tissue sections for each mouse.
Preparation of lung cell suspensions
At 1, 3, 7, 14, 21, and 28 days and 16 weeks post-instillation, mice were killed and the pulmonary cavities opened. The lungs were removed asceptically, cut away from the heart, and placed in ice-cold sterile PBS (pH 7.4). Thereafter, the lungs were minced into small pieces and incubated in complete RPMI-1640 culture medium (Life Technologies, Gaithersburg, MD, USA), supplemented with 10% FCS, antibiotic/antimycotic solution, β-ME, and sodium pyruvate containing 1 mg/ml collagenase IA (Sigma Chemical Co., St. Louis, MO, USA) with 1 μl DNAase I (Gibco, Carlsbad, CA, USA) during 2 h at 37°C. Digested lungs were disrupted further by gently pushing the tissue through a 70-μm cell strainer (BD Biosciences). Enzymatic action was terminated by adding excess RPMI medium and pelleting by centrifugation at 1500 rpm for 5 min at 4°C. Leukocytes were isolated by centrifugation over a 40–70% Percol gradient. Cells were enumerated using a Coulter counter and stored on ice prior to experimental use or analysis.
Antibodies
mAb, specific to CD3 (FITC, clone #17A2), NK1.1 (PE, clone #PK136), CD11b (PerCp Cy5.5, clone #M1/70), CD25 (allophycocyanin Cy7, clone #PC61), CD27 (allophycocyanin, clone #LG.7F9), and CD69 (PE Cy7, clone #H12.F3), were purchased from BD PharMingen (San Diego, CA, USA) or eBioscience (San Diego, CA, USA) for the analysis of NK and NK T cells. mAb specific to CD3 (FITC, clone #17A2), CD4 (allophycocyanin-Cy7, clone #GK1.5), CD8α (PE, clone #53-6.7), CD25 (PerCP Cy5.5, clone #PC61), and CD44 (allophycocyanin, clone #IM7) were purchased from BD PharMingen or eBioscience for the analysis of T cells. mAb specific to CD11c (allophycocyanin, clone #HL3), CD11b (PerCp Cy5.5, clone #M1/70), Gr-1/Ly-6C (allophycocyanin-Cy7, clone #RB6-8C5), and MHC class II (PE, clone #M5/114.15.2) were purchased from BD PharMingen or eBioscience for the analysis of polymorphonuclear neutrophils and APCs.
Immunophenotyping
Single-cell suspensions, obtained as indicated above, were washed in complete RPMI and resuspended in 50 μl purified rat anti-mouse CD16/CD32, diluted with PAB for 15 min on ice to block nonspecific antibody binding. Each antibody (1 μg) was added to 106 total cells and allowed to incubate for 30 min in the dark on ice with agitation 2–3 times. Finally, cells were washed twice with PBS and resuspended in 0.4 ml PAB. Cell acquisition and analysis were performed on a FACSAria flow cytometer using FACSDiva software (Version 4.1.2, BD Biosciences). Compensation of the spectral overlap for each fluorochrome was done by gating using anti-rat/hamster Ig compensation beads (BD Biosciences).
Purification of NK cells and cytokine ELISAs
The lungs of saline- and SiO2-exposed mice were digested enzymatically and single-cell suspensions obtained as indicated above 7 days following i.n. instillation. NK1.1+ cells were sterilely isolated from exposed lungs using MACS bead technology (Miltenyi Biotec, Auburn, CA, USA) into cells of 86.5% purity (saline) and 77.8% purity (SiO2). NK1.1+ cells (0.2×106) were then plated into 96-well microplates with 200 μl complete RPMI medium and incubated at 37°C for 24 h. Tissue-culture plates were centrifuged at 2000 rpm for 5 min and the resulting supernatants collected and analyzed for IFN-γ, TNF-α, IL-10, and IL-13 using murine cytokine ELISA kits, according to the manufacturer’s instructions and assay procedure (R&D Systems, Minneapolis, MN, USA). Color development was assessed at 450 nm on a plate reader.
Whole lung homogenates
Lung tissue was collected from saline controls and at 1, 3, 7, 14, 21, and 28 days after SiO2 exposure. The entire lung was rinsed quickly in ice-cold PBS and then homogenized using a Tissue Tearor in 1 ml radioimmunoprecipitation assay lysis buffer (10 mM HEPES, 150 mM NaCl2, 1 mM EDTA, 0.6% Nonidet P-40, 5 mM PMSF, with HALT protease inhibitor cocktail, according to the manufacturer’s instructions) on ice. After homogenization, the samples were centrifuged at 14,000 g for 30 min and the clarified supernatants stored at –20°C until assayed for cytokine profiling of IFN-γ, TNF-α, IL-10, and IL-13.
Whole lung lavage
Mice were killed and whole lung lavage performed by cannulating the trachea and infusing the lungs with 1 ml sterile 0.5 mM EDTA/PBS. The acellular lavage fluid was collected and frozen at –20°C until further analysis using murine cytokine ELISA kits, according to the manufacturer’s instructions and assay procedure (R&D Systems).
Preparation of lung cell suspensions, RNA isolation, and RT-PCR
The total leukocyte fraction was collected as indicated above, washed twice with ice-cold sterile PBS, pooled (8–10 lungs/sample; n=3), and resuspended in a small volume (<100 μl) of PBS. Trizol reagent (1 ml; Invitrogen Corp., Carlsbad, CA, USA)/1 × 107 cells was used to extract RNA from the samples. Additional purification was performed according to the Qiagen RNeasy manual. RNA quantity was determined using a NanoDrop ND1000 spectrophotometer (NanoDrop Technologies, LLC, Wilmington, DE, USA). RNA quality was assessed using an Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) with the RNA 6000 Nano LabChip and reagents to obtain RNA Integrity Numbers and 28S/18S ratios. RNA samples were reverse-transcribed using qScript Supermix (Quanta Biosciences, Gaithersburg, MD, USA) and subjected to real-time PCR using PerfeCTa SYBR Green Supermix (Quanta Biosciences) in a Stratagene Mx3005p instrument (Agilent Technologies).
Microarray analysis
RNA amplification and labeling were performed according to the two-color microarray-based gene expression analysis protocol (Version 5.5, Agilent Technologies). Arrays used were the whole genome mouse oligo microarrays, which are provided in a 4 × 44k format. A two-color system was used with saline- or SiO2-exposed samples being hybridized against a Universal Mouse Reference RNA sample (Stratagene, La Jolla, CA, USA). On each 4 × 44k array, two saline samples and two silica samples were hybridized. Hybridization was performed overnight at 65°C in a Robbins Scientific (SciGene, Sunnyvale, CA, USA) hybridization oven with a rotor designed to fit Agilent hybridization chambers. Arrays were washed according to the Agilent protocol and scanned using an Axon GenePix 4000B scanner (Molecular Devices, Sunnyvale, CA, USA). Resulting data files were normalized using a LOWESS algorithm. Normalized data were analyzed further using Microsoft Excel (Microsoft, Seattle, WA, USA), GoMiner (NCBI), and PathwayStudio (Ariadne Genomics, Rockville, MD, USA). The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus [24] and are accessible through GEO Series Accession Number GSE19563 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE19563).
Statistical analysis
For each parameter, the values for individual mice were averaged and the sd and se calculated. The significance of the differences between the exposure groups was determined by t test, one-way, two-way, or three-way ANOVA in conjunction with Tukey’s test for variance where appropriate. All ANOVA models were performed with Prism software, Version 4. A P value of <0.05 was considered significant.
Online supplemental material
Supplemental Fig. 1 provides representative FACS plots to illustrate NK cell-gating strategies used to identify the 3 subsets of respiratory NK cells, as well as information about the expression profiles and quantification of the cell surface markers CD25 and CD69 on each of the 3 subsets of NK cells 7 days following saline or silica exposure.
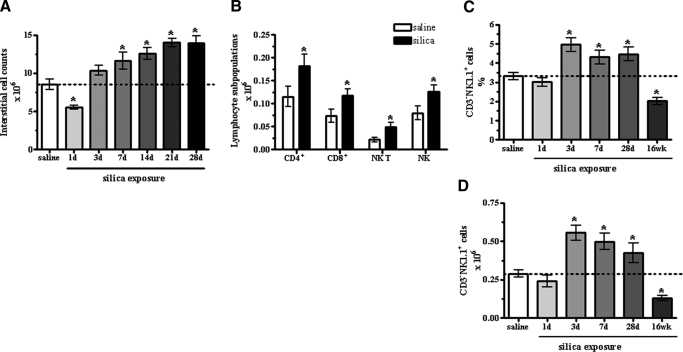
Figure 1.
Interstitial cell changes over time following instillation with SiO2. Following an initial loss in cell number, exposure to SiO2 increased the number of interstitial leukocytes recovered from the lungs over time compared with saline control (A). Within the interstitial immune cells recovered at 14 days post- SiO2 exposure, each of the four subpopulations of lymphocytes examined was augmented compared with saline control, although the ration amongst the cell populations remained unchanged (B). Values are means ± sem; n = 5 mice/group; *P < 0.05 compared with saline control. The percent (C) and absolute number (D) of NK1.1+ CD3– NK cells increased at 3, 7, and 28 days but decreased at 16 weeks post-SiO2 exposure compared with saline. No change was observed at Day 1 in the percent or the absolute number. Values are means ± sem; n = 8–13 mice/group; *P < 0.05 compared with saline control.
RESULTS
Silica triggers lymphocyte activation
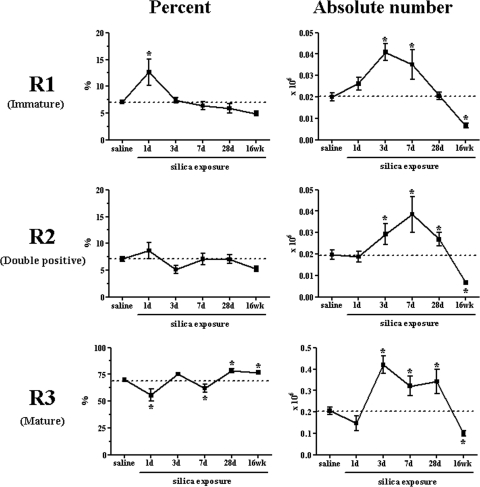
Our i.n. exposure paradigm resulted in increased cellularity within the lung, indicative of a significant influx of inflammatory cells into the parenchyma from 7 days post-SiO2 exposure onward. An initial decrease in the absolute number of cells recovered from the interstitial spaces after SiO2 exposure was no longer apparent at 3 days, when the number of recovered cells appears to have rebounded and even slightly increased over saline control (Fig. 1A). Fourteen days post-SiO2 exposure, an influx of all lymphocyte subtypes examined was observed compared with saline-exposed mice (Fig. 1B). As previous studies using SCID [8] and nude [4] mice suggested T cells do not play a pivotal part in the pathogenesis of fibrosis, we investigated whether NK cells respond to SiO2 through changes in the percent, absolute number, or maturation/activation state from Days 1 to 28 (acute) and at 16 weeks (chronic). The percent (Fig. 1C) and absolute number (Fig. 1D) of NK1.1+ CD3– NK cells increased at 3, 7, and 28 days but decreased at 16 weeks post-SiO2 exposure compared with saline. No change was observed at Day 1 in the percent or the absolute number. Further analysis confirmed the presence of 3 subsets of respiratory NK cells by gating on the expression of CD11b and CD27, yielding CD27hiCD11blo (R1, immature), CD27hiCD11bhi (R2, double-positive), and CD27loCD11bhi (R3, mature) populations [11, 25]. Noteworthy changes were observed in the kinetics of mature NK cells, and more modest effects were observed in the immature and double-positive NK subsets (Fig. 2). These effects were most significant at 16 weeks post-SiO2 exposure when the absolute numbers of all subsets decreased, yet the frequency of mature NK cells remained elevated (Fig. 2). Over time, changes in the absolute number resulted in fluctuating ratios of mature to immature NK cells (1 day=5, 3 days=10.5, 7 days=8, 28 days=17, 16 weeks=14.3) compared with saline controls (10.5). Differentiation of the three NK cell subsets, according to surface expression of CD25 and CD69, assessed activation state [26] and showed no change (Supplemental Fig. 1). These results show significant changes in NK subsets associated with SiO2-induced pathology.
Figure 2.
SiO2-induced changes in pulmonary NK subsets over time. Analysis of the NK cell subsets demonstrates changes in the percent and absolute number of immature (R1), double-positive (R2), and mature (R3) NK cell subsets in response to SiO2 exposure over time. Values are means ± sem; n = 8–13 mice/group; *P < 0.05 compared with cumulative saline control average.
Inflammatory mediators and other factors
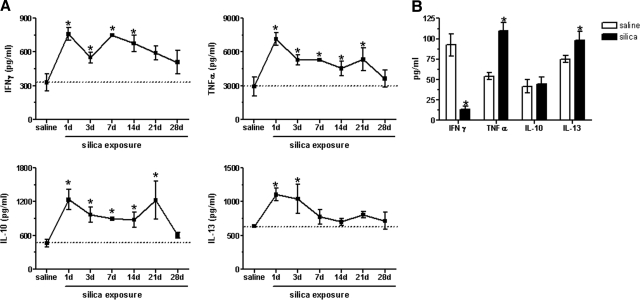
To establish a time course of cytokine expression following SiO2 exposure, clarified supernatants from homogenized whole lungs were analyzed by ELISA for IFN-γ, TNF-α, IL-10, and IL-13. Peak cytokine expression occurred at 1 day postexposure, gradually declining over time, although expression levels remained elevated at Day 28 compared with baseline (Fig. 3A), indicating that the immune polarization status remained in flux [27]. To clarify the contribution of NK cells to the observed cytokine levels, sterile-sorted NK1.1+ cells were isolated from saline- and SiO2-exposed mice at 7 days postexposure and cultured for 24 h. Although NK1.1+ cells isolated from SiO2-exposed mice decreased production of IFN-γ, TNF-α and IL-13 were concomitantly increased compared with NK1.1+ cells isolated from saline-exposed mice (Fig. 3B). No changes were observed in IL-10 production (Fig. 3) or lactate dehydrogenase release, indicative of the viability of the cultures (data not shown). As the majority of respiratory NK cells is of the mature subset [11, 25] (data not shown; Supplemental Fig. 1), cytokine production is assumed to be the result of mature NK cells. Together, these results indicate a significant level of NK activation following SiO2 exposure.
Figure 3.
Cytokine expression following SiO2 exposure. SiO2 increased the levels of IFN-γ, TNF-α, IL-10, and IL-13, present in whole lung homogenates over time, compared with saline control (dashed line; n=5 mice/group; *P < 0.05 compared with saline control; A). Sterile-sorted NK1.1+ cells from 7 days post-saline- or -SiO2-exposed mice contributed to increased levels of TNF-α and IL-13, present in the whole lung homogenates. In contrast, SiO2 exposure resulted in decreased production of IFN-γ and no change in IL-10 production by NK1.1+ cells (B). Values are means ± sem; n = 6–12 wells/group; *P < 0.05 compared with saline control.
NK cells in lymphocyte-deficient mice
In mice where T cell development is genetically blocked, a relatively high number of NK cells develop in the thymus [28]. To establish if this same phenomenon occurs in other tissues, we examined the distribution of respiratory NK cells in lymphopenic mice and analyzed the resulting NK cell response to SiO2 at 16 weeks post-exposure. Untreated (data not shown) and saline-exposed Rag1−/− mice showed increased levels of NK cells compared with C57Bl/6 mice; whereas in response to SiO2, the percent and absolute number of NK cells decreased (Table 1). These data contrast with our earlier findings in C57Bl/6 mice, which showed an increase in the percent and absolute number of NK cells following SiO2 exposure. As anticipated, NK-deficient and Rag1−/−NK-depleted mice showed significantly reduced NK cells, confirming the natural depletion of NK cells (NK-deficient mice) and the antibody-based elimination of NK cells (Rag1−/−NK-depleted mice). Background levels of NK cells within NK-deficient and Rag1−/−NK-depleted mice are not altered in a meaningful way by SiO2 exposure (Table 1). These data suggest that the increase in NK cell numbers following SiO2 exposure may somehow depend on the presence of the adaptive immune system.
Table 1.
Percent and Absolute Number of NK Cells in Lymphocyte-Deficient Mice Following SiO2 Exposure
| Strain | Saline | SiO2 | |
|---|---|---|---|
| C57Bl/6 | % | 5.81 ± 0.37 | 7.74 ± 0.56a |
| ×106 | 0.188 ± 0.03 | 0.257 ± 0.04a | |
| NK-deficient | % | 0.80 ± 0.09b | 0.87 ± 0.15 |
| ×106 | 0.043 ± 0.01 | 0.045 ± 0.01 | |
| Rag1–/– | % | 8.94 ± 0.92b | 5.87 ± 0.94a |
| ×106 | 0.370 ± 0.06 | 0.301 ± 0.06 | |
| Rag1–/– NK-depleted | % | 0.33 ± 0.03b | 0.53 ± 0.03a |
| ×106 | 0.014 ± 0.00 | 0.037 ± 0.00a |
Interstitial lung cells isolated from the indicated mouse strains exposed to 25 μl saline or 1 mg SiO2 resuspended in 25 μl saline were stained for CD3, NK1.1, CD11b, and CD27 expression and analyzed by flow cytometry, as indicated in Figure 2. Results are shown as the percent-positive (upper value) and the absolute number (lower value) of each NK subset. These values were calculated from the total number of interstitial cells recovered following exposure; n = 3–5 mice/exposure group; repeated 2–3 times.
P < 0.05 compared with relative saline control value;
P < 0.05 compared with C57Bl/6 wild-type control.
T, B, and NK cells are not necessary for SiO2-induced fibrosis
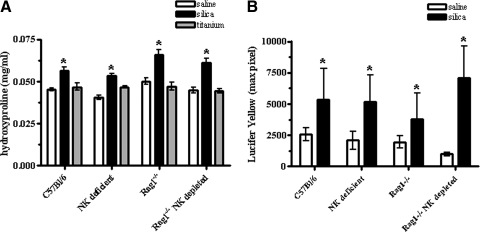
To determine whether lymphocytes play a role in the development of SiO2-induced fibrosis, collagen deposition was measured biochemically using the hydroxyproline assay at 16 weeks post-SiO2 exposure. C57Bl/6 mice exhibited a 24.4% increase, NK-deficient mice a 29.3% increase, Rag1−/− mice a 32% increase, and Rag1−/− NK-depleted mice a 35.5% increase in hydroxyproline content compared with their respective saline controls. In contrast, TiO2-exposed mice showed no change from saline controls (Fig. 4A). Fluorescence microscopy, in conjunction with LSC, was also used to study the deposition of the connective tissue matrix and to quantify the fibrotic response to SiO2 exposure in tissue sections. Analysis of the intensity of LY staining confirmed that SiO2-exposed mice exhibited increased collagen deposition (Fig. 4B), comparable with the observed changes in hydroxyproline values. All saline-exposed mice showed similar patterns and intensity of LY staining, indicating that lymphocytes are not necessary for the deposition of collagen in the lung (data not shown). In response to SiO2 exposure, a clear accumulation of extracellular matrix components was visible in all mice (data not shown). These findings suggest that lymphocytes are not necessary for collagen deposition in the silicosis model.
Figure 4.
Collagen deposition following instillation with SiO2. SiO2 exposure results in fibrosis in lymphopenic mice. All SiO2-exposed mice, regardless of strain, have significantly higher levels of hydroxyproline compared with their respective saline controls. In contrast, TiO2 (nonfibrogenic control particle) showed no change from saline control values (A); n = 7–17 mice/group. Values are means ± sem; *P < 0.05 compared with saline controls. Lung sections from C57Bl/6 and lymphocyte-deficient mice exposed to SiO2 show increased collagen deposition, as measured by quantification of LY fluorescence using LSC techniques (B); n = 5–6 mice/group. Values are means ± sem; *P < 0.05 compared with saline controls.
T, B, and NK cells are not necessary for SiO2-induced inflammation
As we observed increased frequencies and numbers of lymphocytes in the lungs of SiO2-exposed wild-type mice, we investigated their relative importance to SiO2-induced inflammation. All SiO2-exposed mice showed significant increases in tissue wet weight, indicative of inflammation and edema within the lungs (Table 2). Relative increases in the wet weight were 58.0% (C57Bl/6), 65.9% (NK-deficient), 63.3% (Rag1−/−), and 103.6% (Rag1−/− NK-depleted). In contrast, exposure to TiO2 resulted in no change (Table 2). These data suggest that lymphocyte deficiency results in an exacerbated inflammatory response following SiO2 exposure.
Table 2.
Wet Weight of Whole Lungs Isolated from Saline-, SiO2-, or TiO2-Exposed Lymphocyte-Deficient Mice
| Wet weight (mg)
|
|||
|---|---|---|---|
| Saline | SiO2 | TiO2 | |
| C57Bl/6 | 35.92 ± 3.6 | 56.77 ± 4.6a | 45.84 ± 3.2 |
| NK-deficient | 36.19 ± 2.9 | 60.04 ± 3.7a | 38.41 ± 2.8 |
| Rag1–/– | 50.31 ± 4.4 | 82.18 ± 5.3a | 41.31 ± 2.0 |
| Rag1–/– (NK-depleted) | 39.96 ± 3.9 | 81.34 ± 5.5a | 43.00 ± 2.2 |
The left lobe was isolated from the lungs of C57Bl/6, NK-deficient, Rag1–/–, and Rag1–/– NK-depleted mice exposed to 25 μl saline, 1 mg SiO2, or 0.5 mg TiO2 (nonfibrogenic particle control), resuspended in 25 μl saline, and weighed as an indication of inflammation and edema. Results are shown as the mean ± SEM (n = 7–17 mice/exposure group).
P < 0.05 compared with the respective saline control.
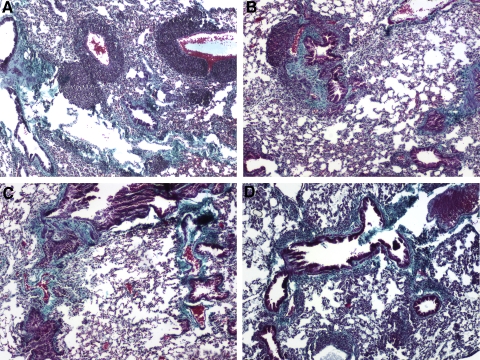
Histological analysis of Gomori’s trichome staining from SiO2-exposed C57Bl/6 lung sections showed classical signs of silicosis, including inflammation and collagen deposition. Fibrosis was detected in the perivascular regions, as well as the lung pleura. Inflammatory cell infiltration was also observed throughout the lung and in many of the fibrotic regions as diffuse infiltrates and granulomas (Fig. 5A). SiO2-exposed, NK-deficient mice (Fig. 5B) exhibited a pathology similar to C57Bl/6 mice; however, SiO2-exposed Rag1−/− (Fig. 5C) and Rag1−/− NK-depleted mice (Fig. 5D) showed diffuse inflammation throughout the lung. Furthermore, no granuloma formation was detected in Rag1−/− and Rag1−/− NK-depleted mice, implicating lymphocytes in the formation of SiO2-induced granulomas. A comparison of the gross architecture of the lung sections from saline-exposed mice reveals no difference between lymphocyte-deficient strains and C57Bl/6 mice, indicating that lymphocytes are not crucial to the normal development of the lung (data not shown). These observations suggest a role of lymphocytes in the regulation of SiO2-induced inflammation, but interestingly, granuloma formation appears to require cells of the adaptive immune system.
Figure 5.
Histological comparison of lymphocyte-deficient mice compared with controls following instillation with SiO2. Lung sections from C57Bl/6 and lymphopenic mice 16 weeks following exposure were subjected to histochemical analysis for Gomori’s trichrome. A comparison of the gross architecture of the lung sections from saline-exposed mice revealed no difference between lymphocyte-deficient strains and C57Bl/6 mice (data not shown). In contrast, SiO2-exposed mice develop features of pulmonary inflammation and fibrosis, characteristic of murine silicosis. Representative images from C57Bl/6 mice show increased staining for collagen (green), as well as increased inflammation and granuloma formation typical of chronic silicosis (A). Similar results were obtained from NK-deficient mice (B). In contrast, although Rag1−/− (C) and Rag1−/− NK-depleted (D) mice showed increased collagen deposition, inflammation was diffuse throughout the parenchyma, and granulomas were not visible; n = 5–6 mice/group; 20× original magnification.
Lymphocytes limit SiO2-induced inflammasome activation
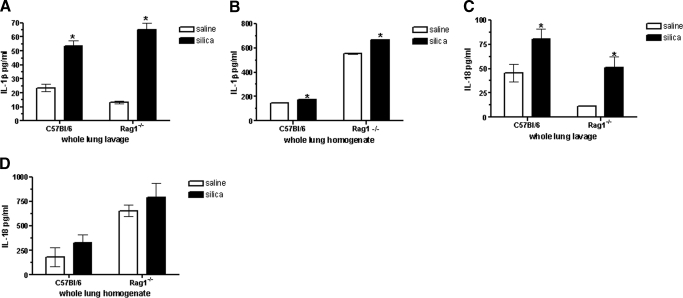
Recent evidence suggests that IL-1β and IL-18, resulting from activation of the Nalp3 inflammasome, play critical roles in SiO2-induced inflammation and fibrosis. Considering the strong proinflammatory actions of these cytokines, their processing and secretion must be tightly regulated. Therefore, we investigated whether the differences in silicosis observed between Rag1−/− and C57Bl/6 mice may be explained by altered expression of IL-1β and IL-18, as well as components of the inflammasome. Increased IL-1β production was detected in whole lung lavages (Fig. 6A), as well as whole lung homogenates (Fig. 6B) 4 weeks following exposure, although this induction was more dramatic in Rag1−/− than in C57Bl/6 mice. IL-18 was markedly up-regulated within the whole lung lavage of Rag1−/− mice (335.2% increase) compared with C57Bl/6 (76.1% increase; Fig. 6C), as well as increased modestly in whole lung homogenates (Fig. 6D) after SiO2 exposure relative to their respective saline controls (Fig. 6D). Therefore, we examined the possibility that lymphocytes dampen inflammatory responses in Rag1−/− mice through modulation of the Nalp3 inflammasome using microarray and real-time PCR techniques [13, 29, 30]. Microarrays generated a comprehensive assessment of the expression of SiO2-responsive genes in interstitial leukocytes from C57Bl/6 versus Rag1−/− NK-depleted mice. A select listing of genes known to function in inflammasome activation is shown in Table 3, along with their SiO2-induced fold changes relative to their saline control. Microarray analysis demonstrated that the expression levels of Nalp3, cathepsin, caspase 1, Stat2, and Serpinb2 were up-regulated following SiO2 exposure in Rag1−/− NK-depleted mice compared with saline control. In contrast, the expression levels of Nalp3, cathepsin B, caspase 1, Stat2, and Serpinb2 showed no change compared with saline control in C57Bl/6 mice. Interestingly, in C57Bl/6 mice, IL-18 and IL-33 were up-regulated significantly in response to SiO2 exposure, whereas the expression levels were not altered in Rag1−/− NK-depleted mice. Real-time PCR confirmed that Nalp3, cathepsin B, caspase 1, Stat2, and Serpinb2 RNA expression is up-regulated significantly following SiO2 exposure relative to saline control values in Rag1−/− NK-depleted mice. In addition, real-time PCR indicated a significant induction in IL-1β expression in Rag1−/− NK-depleted mice. In contrast, in C57Bl/6 mice, there was no change observed in Nalp3, caspase 1, or Serpinb2, although expression of cathepsin B, Stat2, and IL-1β appeared increased following SiO2 exposure relative to saline control. These results show differential inflammasome activation between lymphocyte-sufficient (C57Bl/6) and -deficient (Rag1−/− NK-depleted) mice, suggesting a role for this pathway in SiO2-induced inflammation.
Figure 6.
IL-1β and IL-18 expression in the whole lung lavage and lung homogenates of SiO2-exposed mice. C57Bl/6 and Rag1−/− mice were exposed to saline or SiO2 and whole lung lavage (A and C) and lung homogenates (B and D) analyzed for the expression of IL-1β and IL-18. SiO2-exposed C57Bl/6 and Rag1−/− mice showed elevated levels of IL-1β and IL-18 at Day 28, although Rag1−/− mice showed greater increases in cytokine expression relative to saline control values; n = 7–17 mice/group. Values are means ± sem; *P < 0.05 compared with saline controls.
Table 3.
Microarray and Real-Time PCR Analysis of Gene Expression Changes from Interstitial Leukocytes Isolated from C57Bl/6 and Rag1–/– NK-Depleted Mice
| Gene Symbol | Genbank | Description | Microarray
|
Real-time PCR
|
||
|---|---|---|---|---|---|---|
| Rag1–/– NK-depl.-fold change | C57Bl/6-fold change | Rag1–/– NK-depl.-fold change | C57Bl/6 fold-change | |||
| Ctsb | NM_007798 | Cathepsin B | 2.39a | 1.24 | 1.52a | 1.31a |
| Nlrp3 | NM_145827 | NLR family, pyrin domain-containing 3 | 1.31b | 0.95 | 1.32a | 0.96 |
| Casp 1 | NM_009807 | Caspase 1 | 1.65b | 0.79 | 1.74a | 0.20 |
| IL-1β | NM_008361 | Interleukin-1β | 0.94 | 0.76 | 2.64a | 0.69a |
| IL-18 | NM_008360 | Interleukin-18 | 1.11 | 1.45a | 0.95 | 0.39 |
| IL-33 | NM_133775 | Interleukin-33 | 0.99 | 2.09a | 0.95 | 0.65 |
| Stat2 | NM_019963 | Signal transducer and activator of transcription 2 | 2.96a | 1.43 | 1.28 | 4.20a |
| Bcl-2 | NM_009741 | B cell leukemia/lymphoma 2 | 1.36 | 0.91 | 0.67a | 0.83 |
| Serpinb2 | NM_011111 | Plasminogen activator inhibitor 2 | 3.41a | 1.07 | 3.78a | 0.42 |
The lungs of saline- and SiO2-exposed C57Bl/6 and Rag1–/– NK-depleted mice were digested enzymatically and single-cell suspensions obtained 28 days following instillation. RNA was then analyzed and microarrays and real-time PCR reactions performed as indicated in Materials and Methods. Results are shown as fold-change relative to saline control values.
P < 0.05 compared with the respective saline control;
P < 0.06.
DISCUSSION
Although the cellular mechanisms and physiological consequences involved in the development of silicosis have been studied extensively, they still remain poorly understood. To evaluate the importance of lymphocytes and NK cells, in particular, to silicosis in the mouse model, we examined development of silicosis in strains of mice lacking NK, T, and B cells or all three. Similarly, T cells have been implicated in restricting the innate immune response through inhibition of Nlrp3 inflammasome in vitro [17]; however, it is not known whether lymphocytes dampen SiO2-induced activation of the Nlrp3 inflammasome in vivo. Therefore, we also evaluated activation of the Nlrp3 inflammasome using the lymphocyte-deficient mice mentioned above.
An influx of lymphocytes into the lungs is considered a major component of the immune response to SiO2 in rodent models [3,4,5,6,7]. After an initial loss in cell number, SiO2 exposure resulted in increasing numbers of immune cells recovered from the interstitial spaces from 3 to 28 days, indicative of a persistent inflammatory state (Fig. 1A). The initial decrease in cell number observed at Day 1 following SiO2 exposure may be a result of acute migration, cell death, or both. Although this phenomenon has also been observed with other particulates such as wood smoke (unpublished results), further studies are necessary to determine the mechanisms responsible for this cell loss. Subsequent analysis showed an increase in each of the four lymphocyte subtypes examined, although the ratio amongst the different lymphocyte populations remained the same following SiO2 exposure (Fig. 1B). As previous studies had found T cells to be nonessential to the development of silicosis [4, 8], it was of interest to pursue the potential involvement of NK cells in SiO2-induced inflammation and fibrosis.
To date, there has been no systematic analysis of NK cell kinetics and activity in rodent models of respiratory disease. Our results demonstrated a transient increase in the percent (Fig. 1C) and the absolute number (Fig. 1D) of NK cells following SiO2 exposure. These data show for the first time that the distribution of NK cells within the lungs is responsive to particulate exposures such as SiO2. An increased presence of NK cells in vivo was observed in response to i.n. instillation of SiO2-exposed bone marrow-derived macrophages, suggesting that an initial interaction between SiO2 and phagocytic cells initiates subsequent effects on NK cells (data not shown). Although these results suggest that alterations in NK cells may be occurring indirectly as a result of SiO2-induced changes in macrophage functions, it remained of interest to pursue the role of NK cells in silicosis.
The kinetics of each NK cell subset within the lungs [11, 25] was then examined to determine which were affected specifically and temporally by SiO2. An initial increase in the percent of immature NK cells was observed, although these levels corresponded to no significant change in cell number. However, at later time-points, the percent of immature NK cells remained unchanged, and the absolute number was increased significantly. This trend in the immature subset was mirrored inversely by mature NK cells, where an initial decrease was followed by a gradual increase in percent-positive cells. No significant changes were observed in the percent of double-positive NK cells, although there was a significant increase in the absolute number between 3 and 28 days compared with saline control (Fig. 2). These data suggest that the lung may be replenished by circulating NK cells, which later gain the more mature phenotype of respiratory NK cells through interactions with inflammatory cytokines such as IL-1, IL-12, IL-18, or type I IFNs [25, 28]. Although the relative contribution of factors that might regulate tissue-homing and egress of NK cells in response to SiO2 also remains to be addressed, chemokines play an important role in NK recruitment to the site of inflammation. NK cells express a wide range of chemokine receptors that may attract them to different types of immune microenvironments, such as the SiO2-exposed lung [25, 31, 32]. Moreover, the study of gene-targeted mice has identified several transcription factors, in which the ablation results in incomplete or aberrant NK cell differentiation and maturation, including T-bet and IFN regulatory factor-2 [33,34,35].
Numerous cytokines participate in the development and progression of inflammation and fibrosis [36,37,38,39]. Of these, IFN-γ, TNF-α, IL-10, and IL-13 play a prominent role in the development and regulation of silicosis [40]. To determine the role of soluble factors in silicosis, whole lung homogenates from C57Bl/6 mice were assessed for the expression levels of four key inflammatory and fibrotic cytokines (Fig. 3A). Results indicate a dynamic interplay amongst the four cytokines, and significantly elevated levels of these four cytokines occur simultaneously, which highlights the uncertainty that exists regarding the respective roles of Th1- and Th2-associated cytokines in silicosis. Although analysis of the cytokine environment was not performed at 16 weeks post-SiO2 instillation, future studies, to provide a more comprehensive assessment of the timing of cytokine expression, may yield important information about the role of cytokines in silicosis. Our data imply that attempts to manipulate single cytokines therapeutically may be overly simplistic.
NK cells express constitutive levels of various cytokines and respond to diverse pathogens by increasing cytokine production in vitro and in vivo [26]. Therefore, we sought to determine the contribution of NK cells to the observed cytokine levels measured in the whole lung. NK cells sterilely isolated from SiO2-exposed mice showed decreased levels of IFN-γ and increased levels of TNF-α and IL-13 yet no change in IL-10 (Fig. 3B). These data indicate that NK cells are contributing to the observed increases in lung TNF-α and IL-13 but not IFN-γ. Similarly, increases in IL-10 production in lavage fluid (data not shown) and whole lung lysates from SiO2-exposed mice may not be attributed to NK cells. Macrophages are believed to be the primary source of IL-10 in response to SiO2 [40,41,42,43], which may in turn negatively regulate the amount of IFN-γ produced by NK cells [44].
Excessive numbers of NK cells develop in the thymus of mice, where T cell development is genetically blocked, indicating the ability of T cell precursors to develop into NK cells under abnormal conditions [28]. This phenomenon also occurs in the lungs of Rag1−/− mice, which exhibited higher numbers of baseline NK cells than C57Bl/6 mice (Table 1). Although C57Bl/6 mice exhibit an increased presence of NK cells in response to SiO2 exposure, the opposite response was observed in Rag1−/− mice. At the current time, it is not known what the cytokine milieu is in the lungs of each strain examined and whether this might have an effect on the distribution of NK cells in lymphocyte-deficient mice and their response to SiO2 exposure.
Fibrosis is characterized by proliferation and progressive accumulation of connective tissue replacing normal functional parenchyma. All mice increased hydroxyproline content, a component of collagen, in response to SiO2 exposure relative to saline control, although Rag1−/− NK-depleted mice exhibited the greatest increase (35.5%; Fig. 4A), which was confirmed using LSC (Fig. 4B) [23]. Although these findings are consistent with the work of Helene et al. [8], who demonstrated that lymphocytes are not required for bleomycin-induced fibrosis, they imply further that lymphocytes may limit the extent of SiO2-induced fibrosis rather than playing a ’nonrequired role’ during the development of the fibrotic process. In addition, our data suggest that mediators typically associated with T cells, which have been linked with fibrosis, could derive from nontraditional sources, supporting the theme of “redundancy” often observed in the immune system.
Lung weight increases with fibrosis, as well as accompanying inflammation and edema [23]. Increases in tissue wet weight were observed following SiO2 exposure (Table 2). The relative increase in wet weight following SiO2 exposure was the most dramatic in Rag1−/− NK-depleted mice, suggesting that lymphopenia results in an exacerbated inflammatory response. This hypothesis is supported by our histological findings, which demonstrated that Rag1−/− (Fig. 5C) and Rag1−/− NK-depleted mice (Fig. 5D) developed an exacerbated inflammatory response compared with C57Bl/6 mice, whereby no granulomas were formed, and the inflammatory component was diffused throughout the lungs (Fig. 5A). Together, these results suggest that whereas lymphocytes play a role in regulating SiO2-induced pulmonary inflammation, formation of granulomas may require B or T cells but not NK cells. These data implicate lymphocytes in the regulation of granuloma formation and inflammation in response to SiO2.
Recent evidence points to a prominent role for IL-1β and IL-18 in SiO2-induced inflammation and fibrosis [13, 29, 30]. The more robust and persistent inflammatory response in Rag1−/− mice may be related to increased expression of inflammatory mediators such as IL-1β and IL-18 and further suggests that lymphocytes may negatively regulate innate immune responses. Our results demonstrated a significant increase in IL-1β and IL-18 production in the whole lung lavage and homogenized whole lung, which was exacerbated in Rag1−/− mice compared with C57Bl/6 (Fig. 6). Furthermore, microarray analysis of interstitial leukocytes from SiO2-exposed mice, confirmed by real-time PCR, showed that in the absence of lymphocytes, expression of genes important to the activation of the Nlrp3 inflammasome and the innate immune response was up-regulated compared with lymphocyte-sufficient control mice (Table 3). These data suggest that lymphocytes dampen the innate immune response by regulating inflammasome activation.
As a whole, our data demonstrated that although a robust lymphocyte response was observed following SiO2 instillation, it was not necessary for disease development, indicating a marked predominance of innate immunity in the pathogenesis of silicosis. However, as mediators normally attributed to lymphocytes have been found to play a key role in fibrosis, and in normal conditions, lymphocytes are present, one cannot ignore their potential role. Our results suggest multiple explanations: redundancy in the immune system overcomes the loss of lymphocytes; lymphopenic mice develop silicosis via a different pathway/mechanism than their wild-type counterparts; or lymphocytes play a protective role in SiO2-induced inflammation and fibrosis. Although the data demonstrate an immune-active environment, lymphopenic mice appear to be at a much higher level of activation, perhaps as a result of the absence of an efficient regulatory component provided by lymphocytes in the form of a specific cell subset (e.g., CD4+ Forkhead box p3+ regulatory T cells) or their subsequent production of regulatory cytokines (e.g., IL-10 and TGF-β) [45]. Pulmonary fibrosis is an end-stage, lethal lung disorder, for which efficacious therapeutics are not readily available. The information presented herein should facilitate further investigations into the physiology and contributions of specific immune cells to regulation of inflammation and the development of fibrosis within the respiratory system. These findings point further to innate immune cells as potential targets for therapeutic intervention in the modulation of lung fibrosis.
AUTHORSHIP
C.A.B. designed and executed the experiments and wrote the manuscript. C.T.M. contributed to the experimental design and execution of the experiments. F.J. isolated the PK136 antibody and aided in completing the in vivo exposures. M.T. performed histology and biochemical analysis. D.Y. provided breeding pairs of NK-deficient mice and numerous editorial comments. A.H. was the principal investigator and contributed numerous editorial comments to the preparation of the manuscript.
ACKNOWLEDGMENTS
This work is supported by NIH grants ES-015294 (A.H.) and COBRE grants P20 RR17670 and P20 RR15583 from NCRR. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. We thank Pam Shaw from the Fluorescence Core, Lou Herritt from the Histology Core, and Corbin Schwanke from the Molecular Biology Core for their expert technical assistance with various aspects of this manuscript.
Supplementary Material
Footnotes
Abbreviations: i.n.=intranasally, LSC=laser-scanning cytometry, LY=Lucifer yellow, Nalp3=Nacht domain-, leucine-rich repeat-, and pyrin domain-containing protein 3, NCBI=National Center for Biotechnology Information, NCRR=National Center for Research Resources, NIH=National Institutes of Health, Nlrp3=nucleotide-binding oligomerization domain-like receptor family, pyrin domain-containing 3 (also called cryopyrin and Nalp3), PAB= PBS with 1% BSA and 0.1% sodium azide, SiO2=crystalline silica, TiO2=titanium dioxide
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
References
- Parks C G, Cooper G S, Nylander-French L A, Sanderson W T, Dement J M, Cohen P L, Dooley M A, Treadwell E L, St Clair E W, Gilkeson G S, Hoppin J A, Savitz D A. Occupational exposure to crystalline silica and risk of systemic lupus erythematosus: a population-based, case-control study in the southeastern United States. Arthritis Rheum. 2002;46:1840–1850. doi: 10.1002/art.10368. [DOI] [PubMed] [Google Scholar]
- Ding M, Chen F, Shi X, Yucesoy B, Mossman B, Vallyathan V. Diseases caused by silica: mechanisms of injury and disease development. Int Immunopharmacol. 2002;2:173–182. doi: 10.1016/s1567-5769(01)00170-9. [DOI] [PubMed] [Google Scholar]
- Arras M, Louahed J, Simoen V, Barbarin V, Misson P, van den Brule S, Delos M, Knoops L, Renauld J C, Lison D, Huaux F. B lymphocytes are critical for lung fibrosis control and prostaglandin E2 regulation in IL-9 transgenic mice. Am J Respir Cell Mol Biol. 2006;34:573–580. doi: 10.1165/rcmb.2004-0383OC. [DOI] [PubMed] [Google Scholar]
- Hubbard A K. Role for T lymphocytes in silica-induced pulmonary inflammation. Lab Invest. 1989;61:46–52. [PubMed] [Google Scholar]
- Davis G S, Holmes C E, Pfeiffer L M, Hemenway D R. Lymphocytes, lymphokines, and silicosis. J Environ Pathol Toxicol Oncol. 2001;20 (Suppl. 1):53–65. [PubMed] [Google Scholar]
- Suzuki N, Ohta K, Horiuchi T, Takizawa H, Ueda T, Kuwabara M, Shiga J, Ito K. T lymphocytes and silica-induced pulmonary inflammation and fibrosis in mice. Thorax. 1996;51:1036–1042. doi: 10.1136/thx.51.10.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R K, Li W, O'Grady R. Activation of lymphocytes in the pulmonary inflammatory response to silica. Immunol Invest. 1990;19:363–372. doi: 10.3109/08820139009050776. [DOI] [PubMed] [Google Scholar]
- Helene M, Lake-Bullock V, Zhu J, Hao H, Cohen D A, Kaplan A M. T cell independence of bleomycin-induced pulmonary fibrosis. J Leukoc Biol. 1999;65:187–195. doi: 10.1002/jlb.65.2.187. [DOI] [PubMed] [Google Scholar]
- Orange J S, Ballas Z K. Natural killer cells in human health and disease. Clin Immunol. 2006;118:1–10. doi: 10.1016/j.clim.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Seaman W E, Sleisenger M, Eriksson E, Koo G C. Depletion of natural killer cells in mice by monoclonal antibody to NK-1.1. Reduction in host defense against malignancy without loss of cellular or humoral immunity. J Immunol. 1987;138:4539–4544. [PubMed] [Google Scholar]
- Hayakawa Y, Smyth M J. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- Weissler J C, Nicod L P, Lipscomb M F, Toews G B. Natural killer cell function in human lung is compartmentalized. Am Rev Respir Dis. 1987;135:941–949. doi: 10.1164/arrd.1987.135.4.941. [DOI] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad E O, Kono H, Rock K L, Fitzgerald K A, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman B T, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30:860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C A. Mutations in cryopyrin: bypassing roadblocks in the caspase 1 inflammasome for interleukin-1β secretion and disease activity. Arthritis Rheum. 2007;56:2817–2822. doi: 10.1002/art.22841. [DOI] [PubMed] [Google Scholar]
- Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, Schneider P, Tschopp J. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009;460:269–273. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- Yuan D, Bibi R, Dang T. The role of adjuvant on the regulatory effects of NK cells on B cell responses as revealed by a new model of NK cell deficiency. Int Immunol. 2004;16:707–716. doi: 10.1093/intimm/dxh071. [DOI] [PubMed] [Google Scholar]
- Beamer C A, Holian A. Scavenger receptor class A type I/II (CD204) null mice fail to develop fibrosis following silica exposure. Am J Physiol Lung Cell Mol Physiol. 2005;289:L186–L195. doi: 10.1152/ajplung.00474.2004. [DOI] [PubMed] [Google Scholar]
- Thakur S A, Beamer C A, Migliaccio C T, Holian A. Critical role of marco in crystalline silica-induced pulmonary inflammation. Toxicol Sci. 2009;108:462–471. doi: 10.1093/toxsci/kfp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini J M, Charron T G, Roberts J R, Lai J, Blake T L, Rogers R A. Application of laser scanning confocal microscopy in the analysis of particle-induced pulmonary fibrosis. Toxicol Sci. 1999;51:126–134. doi: 10.1093/toxsci/51.1.126. [DOI] [PubMed] [Google Scholar]
- Antonini J M, Hemenway D R, Davis G S. Quantitative image analysis of lung connective tissue in murine silicosis. Exp Lung Res. 2000;26:71–88. doi: 10.1080/019021400269880. [DOI] [PubMed] [Google Scholar]
- Taylor M D, Roberts J R, Hubbs A F, Reasor M J, Antonini J M. Quantitative image analysis of drug-induced lung fibrosis using laser scanning confocal microscopy. Toxicol Sci. 2002;67:295–302. doi: 10.1093/toxsci/67.2.295. [DOI] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash A E. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J C, Beilke J N, Lanier L L. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M S, Wynn T A. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich C A, Garcia-Ojeda M E, Samson-Villeger S I, Pasqualetto V, Enault L, Richard-Le Goff O, Corcuff E, Guy-Grand D, Rocha B, Cumano A, Rogge L, Ezine S, Di Santo J P. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-α mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel S L, Eisenbarth S C, Iyer S S, Sadler J J, Colegio O R, Tephly L A, Carter A B, Rothman P B, Flavell R A, Sutterwala F S. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Screpanti V, Yagita H, Grandien A, Ljunggren H G, Smyth M J, Chambers B J. NK cell TRAIL eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J Immunol. 2004;172:123–129. doi: 10.4049/jimmunol.172.1.123. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y, Huntington N D, Nutt S L, Smyth M J. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- Townsend M J, Weinmann A S, Matsuda J L, Salomon R, Farnham P J, Biron C A, Gapin L, Glimcher L H. T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- Taki S, Nakajima S, Ichikawa E, Saito T, Hida S. IFN regulatory factor-2 deficiency revealed a novel checkpoint critical for the generation of peripheral NK cells. J Immunol. 2005;174:6005–6012. doi: 10.4049/jimmunol.174.10.6005. [DOI] [PubMed] [Google Scholar]
- Di Santo J P. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- Castranova V, Porter D, Millecchia L, Ma J Y, Hubbs A F, Teass A. Effect of inhaled crystalline silica in a rat model: time course of pulmonary reactions. Mol Cell Biochem. 2002;234-235:177–184. [PubMed] [Google Scholar]
- Sumida A, Hasegawa Y, Okamoto M, Hashimoto N, Imaizumi K, Yatsuya H, Yokoi T, Takagi K, Shimokata K, Kawabe T. TH1/TH2 immune response in lung fibroblasts in interstitial lung disease. Arch Med Res. 2008;39:503–510. doi: 10.1016/j.arcmed.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Saffiotti U. Silicosis and lung cancer: a fifty-year perspective. Acta Biomed. 2005;76:30–37. [PubMed] [Google Scholar]
- Rimal B, Greenberg A K, Rom W N. Basic pathogenetic mechanisms in silicosis: current understanding. Curr Opin Pulm Med. 2005;11:169–173. doi: 10.1097/01.mcp.0000152998.11335.24. [DOI] [PubMed] [Google Scholar]
- Barbarin V, Nihoul A, Misson P, Arras M, Delos M, Leclercq I, Lison D, Huaux F. The role of pro- and anti-inflammatory responses in silica-induced lung fibrosis. Respir Res. 2005;6:112. doi: 10.1186/1465-9921-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarin V, Xing Z, Delos M, Lison D, Huaux F. Pulmonary overexpression of IL-10 augments lung fibrosis and Th2 responses induced by silica particles. Am J Physiol Lung Cell Mol Physiol. 2005;288:L841–L848. doi: 10.1152/ajplung.00329.2004. [DOI] [PubMed] [Google Scholar]
- Driscoll K E, Carter J M, Howard B W, Hassenbein D, Burdick M, Kunkel S L, Strieter R M. Interleukin-10 regulates quartz-induced pulmonary inflammation in rats. Am J Physiol. 1998;275:L887–L894. doi: 10.1152/ajplung.1998.275.5.L887. [DOI] [PubMed] [Google Scholar]
- Huaux F, Louahed J, Hudspith B, Meredith C, Delos M, Renauld J C, Lison D. Role of interleukin-10 in the lung response to silica in mice. Am J Respir Cell Mol Biol. 1998;18:51–59. doi: 10.1165/ajrcmb.18.1.2911. [DOI] [PubMed] [Google Scholar]
- Chiu B C, Stolberg V R, Chensue S W. Mononuclear phagocyte-derived IL-10 suppresses the innate IL-12/IFN-γ axis in lung-challenged aged mice. J Immunol. 2008;181:3156–3166. doi: 10.4049/jimmunol.181.5.3156. [DOI] [PubMed] [Google Scholar]
- D'Alessio F R, Tsushima K, Aggarwal N R, West E E, Willett M H, Britos M F, Pipeling M R, Brower R G, Tuder R M, McDyer J F, King L S. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.