Abstract
Keratocan and lumican are small, leucine-rich repeat KSPGs in the extracellular matrix (ECM) of the mammalian cornea, whose primary role is to maintain corneal transparency. In the current study, we examined the role of these proteoglycans in the breakdown of the chemokine gradient and resolution of corneal inflammation. LPS was injected into the corneal stroma of C57BL/6 mice, and corneal extracts were examined by immunoblot analysis. We found reduced expression of the 52-kD keratocan protein after 6 h and conversely, increased expression of 34/37 kD immunoreactive products. Further, appearance of the 34/37-kD proteins was dependent on neutrophil infiltration to the cornea, as the appearance of these products was coincident with neutrophil infiltration, and the 34/37-kD products were not detected in explanted corneas or in CXCR2−/− corneas with deficient neutrophil recruitment. Furthermore, the 34/37-kD products and CXCL1/KC were detected in the anterior chamber, into which the corneal stroma drains; and CXCL1/KC was elevated significantly in keratocan−/− and lumican−/− mice. Together, these findings indicate that the inflammatory response in the cornea is regulated by proteoglycan/CXCL1 complexes, and their diffusion into the anterior chamber is consistent with release of a chemokine gradient and resolution of inflammation.
Keywords: keratitis, extracellular matrix, matrix metalloproteinase
Introduction
Neutrophils have an essential role in controlling microbial pathogens, in part, as these cells are recruited rapidly to infected tissues, including the cornea, within hours of exposure to bacteria or to bacterial products such as LPS [1, 2]. Neutrophils also secrete proteolytic enzymes into the ECM, causing degradation of the tissue, which serves to trap bacteria at the site and restrict their dissemination through the body [3]. Although neutrophil activation can result in damage to any tissue, in the transparent cornea, local tissue damage results in loss of corneal clarity and visual impairment. Severe or chronic inflammation also results in scarification and blindness. The role of soluble mediators, such as chemotactic cytokines (chemokines) CXCL1, CXCL2, and CXCL5 in neutrophil recruitment to murine tissues, has been characterized extensively [1,2,3]; however, the role of ECM in the inflammatory response is less well understood.
Bacterial and fungal infections of the cornea are major worldwide causes of blindness and visual impairment; however, the cornea is also a unique tissue in which to dissect molecular pathways of inflammation, as it is avascular, transparent, and readily accessible to examination. Cells are recruited to the injured cornea from peripheral vessels and travel several millimeters through a dense ECM comprised of mostly type I collagen fibrils separated by keratan sulfate proteoglycans, primarily lumican and keratocan. Whereas lumican is present in the lungs, skin, and on blood vessels, in addition to the cornea, keratocan is expressed solely in the corneal stroma in adult mammals [4,5,6,7,8,9]. However, lumican and keratocan are essential for corneal transparency, as corneas of lumican null mice are thinner and more opaque than normal corneas, with severely disrupted collagen interfibrillar spacing and diameter [10, 11]. Corneas from keratocan null mouse also have disrupted fibrils, although the phenotype is less severe than in lumican null mice [9].
We demonstrated recently that neutrophil recruitment to corneas of LPS-treated lumican- and keratocan-deficient mice is impaired, and that keratocan binds the neutrophil chemokine CXCL1/KC and facilitates neutrophil migration into the corneal stroma [12]. In the current study, we demonstrate that: 1) the 52-kD keratocan protein is degraded to a 34/37-kD protein during corneal inflammation; 2) degradation is dependent on the presence of neutrophils; 3) the 34/37-kD protein diffuses into the underlying anterior chamber along with CXCL1/KC; and 4) CXCL1/KC in the anterior chamber is elevated in keratocan- and lumican-deficient mice. These findings extend our understanding of the inflammatory response by demonstrating that neutrophil activation at the site of inflammation results in KSPG degradation and diffusion into the underlying anterior chamber, disrupts the KSPG/CXC chemokine gradient, and contributes to resolution of the inflammatory response.
MATERIALS AND METHODS
Animals
Six- to 8-week-old C57BL/6 lumican−/− and keratocan−/− mice on a C57BL/6 background (generated by W. W-Y. K. [9, 11]) were used for these experiments. CXCR2−/− mice on a BALB/c background were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Pre-experimental examination of the cornea was performed to eliminate any animals with ocular surface disease or injury. Animals were treated in accordance with the guidelines provided in the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Ophthalmic and Vision Research.
Induction of LPS keratitis
Ultrapure Escherichia coli LPS (Invivogen, San Diego, CA, USA) was dissolved in PBS, and 750 ng/3 μl was injected into the corneal stroma of sedated animals as described previously [1]. Briefly, a small tunnel from the corneal epithelium to the anterior stroma was created using a 33-gauge needle (Hamilton Co., Reno, NV, USA). Another 33-gauge needle attached to a 10-μl Hamilton syringe was passed through the tunnel, at which time, the contents of the syringe were injected into the corneal stroma.
Western blot
Mice were euthanized, and corneas were immediately dissected and placed in 150 μl 6 M urea, 0.1 M Tris-acetate, pH 6.0. Corneas were then homogenized with a 5-mm stainless-steel ball using a TissueLyser (Qiagen, Valencia, CA, USA) for 4 min at 30 cycles/sec. Following homogenization, the samples were centrifuged, supernatants were collected, and protein concentration was measured using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Protein (10 μg) was loaded into each well of the SDS-PAGE gel, and Western blots were performed using rabbit IgG to mouse keratocan (generated by Drs. Kao and Liu and co-workers [11, 13]) or to mouse lumican (R&D Systems, Minneapolis, MN, USA), and developed using an ECL development kit (ECL Plus, Amersham, Buckinghamshire, UK), according to the manufacturer’s recommendations. Band intensities were quantified using NIH Image J (National Institutes of Health, Bethesda, MD, USA).
ELISA
Corneas were homogenized as described above and soluble fractions used for ELISA. Aqueous humor was collected from the anterior chamber using a 33-gauge needle with a 10-μl Hamilton syringe. Samples were diluted in HBSS, and CXCL1/KC chemokine levels assessed by a sandwich ELISA, according to the manufacturer’s protocol (R&D Systems). The lower limit of detection was 15 pg/ml.
Immunostaining of corneal sections to detect neutrophils
Eyes were enucleated after injection of LPS, fixed in 10% paraformaldehyde, and processed for histology. To detect neutrophils, 5 μm sections were incubated for 2 h with rat anti-neutrophil antibody NIMP-R14 (Abcam Inc., Cambridge, MA, USA), diluted 1:100 in 1% FCS/TBS (1% FCS/PBS) as described [12]. After washing, corneal sections were incubated with FITC-conjugated rabbit anti-rat antibody (Vector Laboratories, Burlingame, CA, USA), diluted 1:200 in 1% FCS/PBS. Slides were mounted with Vectashield containing DAPI (Vector Laboratories) to detect total cell nuclei, and the number of neutrophils in each section was determined by direct counting.
Statistics
ANOVA with a Tukey post-hoc analysis (Prism, GraphPad Software, Cupertino, CA, USA) was used to determine statistical significance (P<0.05).
RESULTS AND DISCUSSION
Keratocan is cleaved after LPS injection and recruitment of neutrophils to the corneal stroma
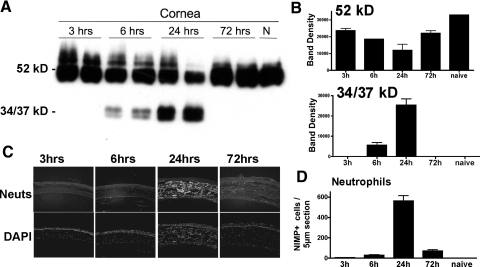
In normal corneas, keratocytes constitutively produce KSPGs, including keratocan, which is secreted into the ECM and functions to maintain corneal transparency. The importance of these proteoglycans in maintaining corneal clarity was demonstrated by Kao and co-workers [11] and Chakravarti et al. [10], who showed that lumican null corneas have irregular collagen fibril arrangements in the stroma and that Lum−/− corneas are thinner and opaque compared with wild-type littermates. Kao and co-workers [9] also reported that Kera−/− mice have thinner, more opaque corneas, although the phenotype is less pronounced than Lum−/− mice. Our recent study [12] showed that during inflammation, keratocan regulates neutrophil infiltration to the corneal stroma by binding CXC chemokines, which is consistent with formation of a chemokine gradient in vivo. Corneal inflammation, independent of etiology, can result in corneal opacity and subsequent vision loss. To examine the fate of keratocan during inflammation, LPS was injected into the corneal stroma of C57BL/6 mice, corneal proteins were extracted after 3, 6, 24, and 72 h, and keratocan was detected by Western blot analysis. As shown in Figure 1A, the 52-kD keratocan core protein was expressed in naïve mice 3 h after injection; however, at 6 h and 24 h, the 52-kD protein was decreased, and a 34-kD/37-kD doublet was detected using the α keratocan antibody. These lower molecular weight products were not present in LPS-treated corneas prior to 6 h—at 72 h or in extracts of naïve corneas (Fig. 1A). Relative band intensity (Fig. 1B) shows an inverse relationship between the 52-kD protein and the 34/37-kD bands, suggesting that the lower molecular weight bands are breakdown products of the mature 52-kD protein.
Figure 1.
Keratocan cleavage in LPS-injected corneas. LPS was injected into the corneal stroma of C57BL/6 mice. At indicated time-points, corneas were dissected, and 10 μg protein from each cornea (as quantified by the Bio-Rad protein assay) was processed for SDS-PAGE and Western blot analysis using rabbit anti-mouse keratocan sera. (A) Representative duplicate blots (each lane is from a separate cornea) showing the 52-kD core protein and 34 kD and 37 kD bands that react with these antibodies. (B) Band densitometry shows the reduction of the core protein and the increase in lower molecular weight fragments at 6 and 24 h. N, Naïve, normal cornea (P<0.05 for band density of lower molecular weight products at 6 h and 24 h compared with naïve control). (C and D) At indicated time-points, eyes were fixed in paraformaldehyde and processed for histology, and 5 μm sections were immunostained with NIMP-R14+ to detect neutrophils. (C) Representative corneal sections after staining with NIMP to identify neutrophils (Neuts; abundant in the corneal stroma at 24 h) or with DAPI to detect total nuclei in the stroma and epithelium. (D) Total number of NIMP+ cells/cornea; P < 0.05 at 6 h, 24 h, and 72 h compared with naïve control. Note peak neutrophil numbers at 24 h after LPS injection, coincident with keratocan degradation. These experiments were repeated five times with similar results.
To determine if there is a correlation between the time course of neutrophil infiltration to the corneal stroma and keratocan degradation, eyes were enucleated after injection of LPS, fixed in paraformaldehyde, and 5 μm sections were immunostained using antimurine neutrophil antibody NIMP-R14. As shown in Figure 1, C and D, neutrophil numbers in the corneal stroma were maximal at 24 h after LPS injection and diminished significantly at 72 h, which is coincident with appearance of the 34/37-kD bands.
Neutrophils are required for keratocan cleavage
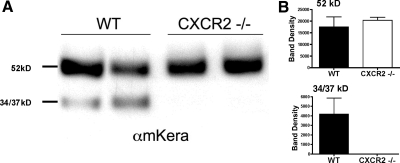
We reported CXC chemokines that mediate neutrophil recruitment are elevated in the first 24 h after LPS injection [1, 2, 12]. Furthermore, we and others [14, 15] showed that neutrophil infiltration is diminished in mice where the CXC receptor for these chemokines is absent (CXCR2−/− mice). Although there is a strain difference in the response to corneal infection with Pseudomonas aeruginosa [16], we found no significant difference in neutrophil infiltration to the corneal stroma of LPS-injected BALB/c compared with C57BL/6 mice [15]. To determine if keratocan cleavage is dependent on infiltrating neutrophils, LPS was injected into the corneal stroma of CXCR2−/− and BALB/c mice. Corneas were excised at 24 h and processed for SDS-PAGE and Western blot analysis. Figure 2A shows that the 52-kD parent protein is expressed in wild-type and in CXCR2−/− corneas; however, the lower molecular weight cleavage products were detected in wild-type mice but were absent completely in CXCR2−/− corneal extracts at 24 h (Fig. 2, A and B).
Figure 2.
Keratocan expression in CXCR2−/− mice. CXCR2−/− and wild-type (WT) mice were injected with LPS, corneas were dissected 24 h later, and a Western blot for keratocan (αmKera) was performed on 10 μg corneal extracts as described in the legend to Figure 1. (A) Representative blots of two individual corneas from wild-type BALB/c and CXCR2−/− mice. (B) Densitometry results are shown for the 52-kD core protein and for the 34-kD and 37-kD bands. Data represent the mean and sem of three corneas/group. P < 0.05 for band density of 34/37 kD products in wild-type compared with CXCR2−/− corneas. The experiment is representative of two similar studies.
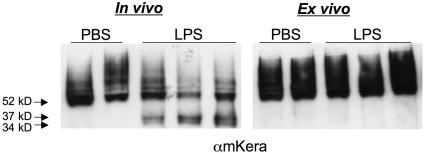
As a second experimental approach, corneas of wild-type mice were injected with LPS, and either dissected after 24 h as before (in vivo), or excised immediately and maintained in culture at 37°C for 24 h (ex vivo). Western blots for keratocan were performed, and as shown in Figure 3, keratocan cleavage was detected in the in vivo samples as before; however, no lower molecular weight products were detected in the ex vivo LPS or PBS-injected corneas, consistent with the absence of neutrophils. This was not a result of cell apoptosis, as explanted corneas continued transcription of alcohol dehydrogenase 3A1 and G3PDH (data not shown).
Figure 3.
Keratocan expression in LPS-injected and excised corneas. LPS was injected into the corneal stroma of C57BL/6 mice, and corneas were dissected after 24 h (A) or were dissected immediately after injection and cultured (ex vivo) for 24 h (B). Corneas were homogenized, and 10 μg protein from each cornea was processed for SDS-PAGE and Western blot analysis.
These data support the results of studies with CXCR2−/− mice (Fig. 2) which together, demonstrate that keratocan cleavage is dependent on the infiltration of neutrophils into the corneal stroma.
Keratocan cleavage products and CXCL1/KC are detected in the anterior chamber
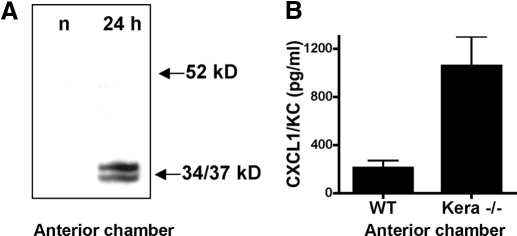
Given that the lower molecular weight keratocan bands were not detected after 24 h, we examined the possibility that the 34/37-kD products diffused out of the cornea into the anterior chamber, which is a well-defined, limited region between the cornea and the lens. To determine if keratocan full-length protein or related products are present at this site during inflammation, LPS was injected into the corneal stroma as before, and 2 μl aqueous humor was removed from the anterior chamber using a 33-gauge needle. This was processed for SDS-PAGE, and keratocan was detected by Western blot analysis. As shown in Figure 4A, there was no detectable keratocan in anterior chamber fluid from naïve mice; however, in LPS-injected corneas, the 37/34-kD bands were clearly detected, whereas the 52-kD keratocan protein was absent. This observation indicates that these lower molecular weight keratocan products are released from the corneal stroma and diffuse into the anterior chamber.
Figure 4.
Keratocan and CXCL1 in the anterior chamber of LPS-injected corneas. LPS was injected into the corneal stroma of C57BL/6 mice, as described in the legend to Figure 1. After 24 h, 2 μl aqueous humor was recovered from the anterior chamber of LPS-injected mice or from naïve mice (n) and processed for Western blot analysis to detect keratocan (A), or CXCL1/KC production was detected by ELISA (B). P < 0.05 for CXCL1 in keratocan−/− compared with wild-type samples.
Our previous studies demonstrated that the time course of CXCL1/KC production in the LPS-treated corneal stroma is maximal at 24 h and reduced at 72 h [2], which is similar to the time course of the appearance of the 37/34-kD bands in the stroma (Fig. 1). To determine if CXCL1 is also detected in the anterior chamber, mice were injected intrastromally with LPS as before, aqueous humor was extracted from the anterior chamber at 24 h, and CXCL1/KC was measured by ELISA. As shown in Figure 4B, CXCL1/KC was detected in the anterior chamber of LPS-injected wild-type mice; furthermore, the concentration of CXCL1 at this site was elevated significantly in keratocan null mice. Our recent study showed that CXCL1 interacts with keratocan in the corneal stroma, as shown by coimmunoprecipitation, although stromal CXCL1 levels are similar in wild-type and keratocan null mice [12]. Therefore, the current finding that CXCL1 in the anterior chamber of keratocan null mice is elevated supports the concept that keratocan maintains a chemokine gradient of CXCL1 in the corneal stroma. Moreover, the current study indicates that cleavage of keratocan during corneal inflammation results in release of CXCL1 from the corneal stroma, where it diffuses into the anterior chamber.
Our previous study showed that keratanase treatment did not inhibit CXCL1 binding to keratocan [12], indicating that the chemokine does not bind to the keratan sulfate moiety of this proteoglycan. However, we cannot completely eliminate the possibility that keratanase digestion was incomplete or that a functional role for carbohydrate moieties occurs in this process. Future studies will examine the effect of removing all post-translation modifications of the core protein to determine if CXCL1 binds to the keratan sulfate or to the core region of keratan. If CXCL1 binds to the protein moiety, it is likely that endogenous proteinases cleave the parent protein to the smaller molecular weight fragments and release CXCL1.
Lumican cleavage and CXCL1 production during inflammation
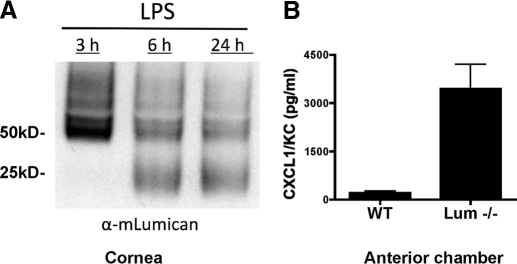
Lumican is also a major KSPG in the corneal stroma, and we had demonstrated previously that lumican regulates expression of keratocan [13]. We also showed that neutrophil recruitment to the corneal stroma is impaired in lumican null mice to an even greater extent than in keratocan null mice [12], we examined if lumican is degraded during corneal inflammation. Wild-type and lumican null mice were injected intrastromally with LPS as before, and corneas were dissected after 3 h, 6 h, or 24 h and subjected to SDS-PAGE and Western blot analysis using anti-lumican antibody. As shown in Figure 5A, lumican protein was detected at 3 h, but intensity was reduced over time. Conversely, lower molecular weight products were detected at 6 h and 24 h but not at 3 h, thereby paralleling the keratocan results.
Figure 5.
Lumican expression and CXCL1 after LPS injection. LPS was injected into the corneal stroma of C57BL/6 mice as described in the legend to Figure 1. After 24 h, whole corneas were dissected and processed for Western blot analysis (A), and the anterior chamber fluid was examined by ELISA to detect CXCL1/KC (B). Data are mean ± sem of four mice; P < 0.05 for CXCL1 in lumican−/− compared with wild-type samples. Experiments were repeated twice with similar results.
To determine if lumican regulates CXCL1 sequestration in the corneal stroma, anterior chamber fluid from wild-type and lumican null mice was examined 24 h after LPS injection. As shown in Figure 5B, CXCL1 production was elevated in the absence of lumican, indicating that as with keratocan, lumican appears to bind CXCL1 during corneal inflammation. In addition, in lumican null mice, there is a loss of keratocan as reported previously [13]. Therefore, increased diffusion of unbound CXCL1 to the anterior chamber as a result of a loss of lumican and a significant reduction in keratocan expression.
CXCL1/KC also binds syndecan and mediates neutrophil migration into the alveolar space, forming a transendothelial chemokine gradient. This complex is disrupted by MMP-7 (matrilysin) [17], which also regulates neutrophil migration and dispersion through the gut mucosa and submucosa [18]. Although we have yet to identify a role for specific MMPs in KSPG degradation, we showed that MMP-8 mediates neutrophil recruitment to the corneal stroma by production of the collagen degradation tripeptide Pro-Gly-Pro [15], which binds CXCR2 and has neutrophil chemotactic activity [19, 20].
In agreement with our findings, Chakravarti and co-workers [21] reported that neutrophil infiltration to the corneal stroma of LPS-stimulated Lum−/− mice is impaired; however, these investigators proposed alternative roles for lumican in this process, including lumican binding of the Fas ligand to induce apoptosis. Chakravarti and others [22] also reported that lumican null macrophages are hyporesponsive to LPS and conversely, that lumican protein binds LPS; therefore, they also propose [22] a direct role for lumican in LPS activation of TLR4 on macrophages, in part, by binding CD14. More recently, Chakravarti and co-workers [23] reported that lumican has an additional role in neutrophil migration (to the peritoneal cavity) by binding β1 and β2 integrins on the cell surface. We agree that there is a role for macrophages in neutrophil recruitment to the corneal stroma, as we recently used chimeric mice to demonstrate a role for resident corneal stromal macrophages and dendritic cells in TLR4 responses [24]; however, as we also showed that these cells are essential for production of proinflammatory and chemotactic cytokines such as CXCL1 [24], we propose that the role of TLR4 on macrophages is to initiate LPS activation and chemokine production in the corneal stroma and that the role of lumican and keratocan is subsequent binding of CXCL1 and other chemokines to form the gradient that is essential for neutrophil infiltration.
In summary, results from the current study, taken together with those from our recent report [12], demonstrate that in addition to having an important structural role, these proteoglycans have a role in regulating corneal inflammation. First, keratocan has an essential role in the early stage of the inflammatory process by actively binding the major neutrophil chemokine CXCL1/KC, thereby forming a chemokine gradient that mediates neutrophil recruitment [12]; and second, our present study shows a role for keratocan and lumican in resolution of the inflammatory response, as neutrophils are essential for cleavage of these KSPGs and release of cleavage products and chemokines are detected in the anterior chamber, resulting in loss of the chemokine gradient and cessation of neutrophil infiltration. The observation that CXCL1/KC levels in the anterior chamber of Kera−/− and Lum−/− mice were several-fold higher than that of wild-type littermates further supports the role for these KSPGs in binding chemokines.
Given these observations, we propose a sequence of events outlining the role of KSPGs in corneal inflammation: 1) Microbial infection of the corneal stroma (or exposure to bacterial products such as LPS) induces TLR signaling in resident macrophages and dendritic cells, resulting in production of CXC chemokines, including CXCL1/KC. 2) These positively charged chemokines bind the highly negatively charged proteoglycans, with the highest chemokine concentration likely at the site of injury and generating a chemokine gradient. 3) Neutrophils are recruited from peripheral vessels and migrate along the gradient to the site of infection, where 4) neutrophils secrete MMPs or stimulate activation of endogenous MMPs in the cornea. 5) Keratocan and lumican are cleaved, resulting in release of lower molecular weight KSPG products and CXCL1 that diffuse into the anterior chamber. Our findings using Kera−/− and Lum−/− mice support this potential sequence of events, as these mice show impaired neutrophil infiltration to the corneal stroma, which is consistent with impaired chemokine gradient formation. Furthermore, our observation that CXCL1 levels are elevated in the anterior chamber but not in the corneal stroma of Kera−/− and Lum−/− mice compared with wild-type littermates indicates that the regulatory role of these KSPGs is not in CXCL1 secretion but rather, in the formation and maintenance of a chemokine gradient.
AUTHORSHIP
Eric C. Carlson designed and performed experiments, analyzed data, and wrote the manuscript. Yan Sun performed experiments shown in the final manuscript. Jeffrey Auletta helped support data analysis and manuscript preparation. Winston W.-Y. Kao and Chia-Yang Liu provided reagents, support in data analysis, and manuscript preparation. Victor L. Perez helped with experimental design, data analysis, and manuscript preparation. Eric Pearlman designed experiments, analyzed data, and co-wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported by National Institute of Health grant EY10320 and EY11373 (to E. P.), EY11845 (to W. W-Y. K.), EY12486 (to C-Y. L.), and K08 EY014912 (to V. L. P.). These studies also received funding from the Research to Prevent Blindness Foundation and Ohio Lions Eye Research Foundation.
Footnotes
Abbreviations: DAPI=4′,6-diamidino-phenylindole, ECM=extracellular matrix, KC=keratinocyte-derived chemokine, KSPG=keratan sulfate proteoglycan, MMP=matrix metalloproteinases, MPO=myeloperoxidase
References
- Carlson E C, Drazba J, Yang X, Perez V L. Visualization and characterization of inflammatory cell recruitment and migration through the corneal stroma in endotoxin-induced keratitis. Invest Ophthalmol Vis Sci. 2006;47:241–248. doi: 10.1167/iovs.04-0741. [DOI] [PubMed] [Google Scholar]
- Lin M, Carlson E, Diaconu E, Pearlman E. CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J Leukoc Biol. 2007;81:786–792. doi: 10.1189/jlb.0806502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Magnuson T, Lass J H, Jepsen K J, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburgh J L, Funderburgh M L, Mann M M, Conrad G W. Arterial lumican. Properties of a corneal-type keratan sulfate proteoglycan from bovine aorta. J Biol Chem. 1991;266:24773–24777. [PubMed] [Google Scholar]
- Funderburgh J L, Funderburgh M L, Brown S J, Vergnes J P, Hassell J R, Mann M M, Conrad G W. Sequence and structural implications of a bovine corneal keratan sulfate proteoglycan core protein. Protein 37B represents bovine lumican and proteins 37A and 25 are unique. J Biol Chem. 1993;268:11874–11880. [PubMed] [Google Scholar]
- Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk D E. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Arar H, Kao C, Kao W W. Identification of a 3.2 kb 5′-flanking region of the murine keratocan gene that directs β-galactosidase expression in the adult corneal stroma of transgenic mice. Gene. 2000;250:85–96. doi: 10.1016/s0378-1119(00)00165-7. [DOI] [PubMed] [Google Scholar]
- Liu C Y, Birk D E, Hassell J R, Kane B, Kao W W. Keratocan-deficient mice display alterations in corneal structure. J Biol Chem. 2003;278:21672–21677. doi: 10.1074/jbc.M301169200. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Petroll W M, Hassell J R, Jester J V, Lass J H, Paul J, Birk D E. Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci. 2000;41:3365–3373. [PMC free article] [PubMed] [Google Scholar]
- Saika S, Shiraishi A, Liu C Y, Funderburgh J L, Kao C W, Converse R L, Kao W W. Role of lumican in the corneal epithelium during wound healing. J Biol Chem. 2000;275:2607–2612. doi: 10.1074/jbc.275.4.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson E C, Lin M, Liu C Y, Kao W W, Perez V L, Pearlman E. Keratocan and lumican regulate neutrophil infiltration and corneal clarity in lipopolysaccharide-induced keratitis by direct interaction with CXCL1. J Biol Chem. 2007;282:35502–35509. doi: 10.1074/jbc.M705823200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson E C, Liu C Y, Chikama T, Hayashi Y, Kao C W, Birk D E, Funderburgh J L, Jester J V, Kao W W. Keratocan, a cornea-specific keratan sulfate proteoglycan, is regulated by lumican. J Biol Chem. 2005;280:25541–25547. doi: 10.1074/jbc.M500249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Cole N, Hume E B, Garthwaite L, Conibear T C, Miles D H, Aliwaga Y, Krockenberger M B, Willcox M D. The role of CXC chemokine receptor 2 in Pseudomonas aeruginosa corneal infection. J Leukoc Biol. 2007;81:315–318. doi: 10.1189/jlb.0506344. [DOI] [PubMed] [Google Scholar]
- Lin M, Jackson P, Tester A M, Diaconu E, Overall C M, Blalock J E, Pearlman E. Matrix metalloproteinase-8 facilitates neutrophil migration through the corneal stromal matrix by collagen degradation and production of the chemotactic peptide Pro-Gly-Pro. Am J Pathol. 2008;173:144–153. doi: 10.2353/ajpath.2008.080081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett L D, McClellan S, Kwon B, Barrett R. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. Invest Ophthalmol Vis Sci. 2000;41:805–810. [PubMed] [Google Scholar]
- Li Q, Park P W, Wilson C L, Parks W C. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- Swee M, Wilson C L, Wang Y, McGuire J K, Parks W C. Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. J Leukoc Biol. 2008;83:1404–1412. doi: 10.1189/jlb.0108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddox J L, Pfister R R, Sommers C I, Blalock J E, Villain M. Inhibitory effect of a complementary peptide on ulceration in the alkali-injured rabbit cornea. Invest Ophthalmol Vis Sci. 2001;42:2769–2775. [PubMed] [Google Scholar]
- Weathington N M, van Houwelingen A H, Noerager B D, Jackson P L, Kraneveld A D, Galin F S, Folkerts G, Nijkamp F P, Blalock J E. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- Vij N, Roberts L, Joyce S, Chakravarti S. Lumican regulates corneal inflammatory responses by modulating Fas-Fas ligand signaling. Invest Ophthalmol Vis Sci. 2005;46:88–95. doi: 10.1167/iovs.04-0833. [DOI] [PubMed] [Google Scholar]
- Wu F, Vij N, Roberts L, Lopez-Briones S, Joyce S, Chakravarti S. A novel role of the lumican core protein in bacterial lipopolysaccharide-induced innate immune response. J Biol Chem. 2007;282:26409–26417. doi: 10.1074/jbc.M702402200. [DOI] [PubMed] [Google Scholar]
- Lee S, Bowrin K, Hamad AR, Chakravarti S. Extracellular matrix lumican deposited on the surface of neutrophils promotes migration by binding to β integrin. J Biol Chem. 2009;284:23662–23669. doi: 10.1074/jbc.M109.026229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery H R, Carlson E C, Sun Y, Lin M, Burnett S H, Perez V L, McMenamin P G, Pearlman E. Bone marrow chimeras and c-fms conditional ablation (Mafia) mice reveal an essential role for resident myeloid cells in lipopolysaccharide/TLR4-induced corneal inflammation. J Immunol. 2009;182:2738–2744. doi: 10.4049/jimmunol.0803505. [DOI] [PMC free article] [PubMed] [Google Scholar]