Abstract
The observation that depletion or inhibition of regulatory T cells (Tregs) unleashes efficient antitumor effector immune responses that can lead to tumor eradication in mice has opened new perspectives for the development of cancer immunotherapy. The quality and overall efficiency of the effector immune responses induced in the absence of Tregs seem to depend on multiple factors that determine the result of a battle involving effector T cells (Teffs), Tregs and tumor cells. In this study, we investigated the quality of tumor-associated antigens (TAAs) as one such factor. We show that the presence of a strong dominant antigen is required for the induction of effector responses capable of tumor eradication in the absence of Tregs. The sole addition of a dominant antigen on tumor cells does not change tumor growth in unmanipulated mice, but improves tumor eradication rate from a few to almost 100% in the absence of Tregs. This eradication can be shown to result from the recruitment and activation of specific Teffs recognizing this antigen. We also show that the presence of such dominant antigens has the side effect of restricting the breadth of the immune response to other TAAs, which could favor the generation of escape mutant by tumor editing. Taken together, our results highlight the potential, and some requirements for cancer immunotherapy based on Treg depletion. They also show that, ultimately, tumor fate depends on multiple factors that should all be taken into consideration for the design of more efficient immunotherapy.
Keywords: tumor immunity, tolerance, immunodominance, immunosurveillance
Introduction
It is now well documented that during immune responses against cancer, effector and regulatory T cells (Teffs and Tregs, respectively) coexist in the tumor environment and draining lymph nodes (dLNs).1, 2, 3 In vivo depletion/inhibition of Tregs by anti-CD25 antibody (Ab) injection during the very first days of tumor growth, leads to tumor-reactive Teff activation and induces a potent antitumor immune response that can lead to tumor eradication. It is noteworthy that this has been observed in most if not all tumor models, in various genetic backgrounds and with tumor cells of various origins, thus indicating that it is a general property of antitumor immune responses.4, 5, 6
Interestingly, tumor eradication on Treg control occurs with various frequencies according to the tumor models, from all animals being cured in some models to only a delay in tumor growth in others. For instance, the B16F10 melanoma growth in C57BL/6 mice is only delayed by Treg depletion,7, 8 whereas Treg depletion in BALB/c mice induces the rejection of the 4T1 adenocarcinoma in 50–80% of mice6, 9 and of AB1-HA mesothelioma in close to 100% of mice, as presented in this study.
It is striking that, for each tumor, only a proportion of the treated animals is able to reject the tumors, when considering that in such experiments, mice have the same genetic background, the same polyclonal immune cell repertoire, are kept in the same environmental conditions in animal facilities and receive the same batch of tumor cells. These results suggest that tumor fate is the uncertain result of a battle, in which the characteristic of the players—tumor cells, Tregs and Teffs—are key parameters. Even in the absence of Tregs, the efficacy of Teffs to eradicate tumor cells is ‘border line,' leading to either rejection or just a slower tumor growth.
In this study, we aimed at evaluating the importance of the nature of tumor-associated antigens (TAAs) in the significance of these observations. For this, we compared the growth of tumor cells differing only by the expression of a single TAA known to induce excellent cellular immune responses. We chose the hemagglutinin (HA) of Influenza virus because: (1) HA is a potent immunogen for both BALB/c and C57BL/6 mice; vaccination against HA is known to induce strong cytotoxic T-lymphocyte responses in these mice10 and (2) there exists a set of transgenic mice that are interesting tools for studying anti-HA immune responses.11, 12 SFE mice express a HA-specific transgenic T-cell receptor (TCR) and can serve as a source of anti-HA Teffs. Although the SFE-TCR is primarily major histocompatibility complex class-II restricted and expressed on effector CD4+ cells, it is also expressed and functional on cytotoxic CD8+ T cells of these mice.12 It is noteworthy that the transgenic TCR of SFE mice can be detected with both a specific monoclonal Ab and an even more sensitive quantitative PCR assay. Besides, InsHA transgenic mice express HA under the control of the insulin promoter; in these mice, HA is thus an autoantigen expressed in the pancreas. Using the insulin promoter for HA expression, it has been shown that HA is also expressed in medullary thymic epithelial cells13 by an autoimmune regulator-dependant mechanism;14 similarly, HA-specific Teffs are deleted,15, 16 whereas HA-specific Tregs are generated during thymocyte differentiation in InsHA mice.17 Accordingly, TCR-HA-specific Tregs, but not TCR-HA-specific Teffs, can be detected in the pancreatic lymph nodes (LNs) of InsHA mice.
We thus compared the growth of the malignant asbestos-induced AB1 mesothelioma, and its AB1-HA derivative, in different experimental conditions. Our results show that the expression of a potent TAA by tumor cells is essential for tumor eradication when Tregs are depleted. However, it also strongly biases the response toward this antigen, hampering the response to additional TAAs. This could be detrimental for an efficient antitumoral response as it could favor the emergence of immune escape mutant by tumor editing.
Materials and methods
Animals
Female BALB/c mice, 6–8-weeks of age, were obtained from Elevage Janvier (Le Genest St Isle, France). InsHA and SFE TCR-HA mice were bred in our animal facility. Mice were housed in filter-topped cages under specific pathogen-free conditions in our animal facilities. All mice were treated in accordance with the European Union guidelines for animal experimentation.
Cell lines and tumor assays
The BALB/c 4T1 mammary carcinoma and its 4T1-HA derivative (obtained in our laboratory) expressing the murine influenza HA were cultured in Iscove's modified Dulbecco's medium (IMDM) (Gibco–Invitrogen, Cergy Pontoise, France) supplemented with 10% fetal calf serum (Gibco–Invitrogen) and 1% gentamycin (Gibco–Invitrogen).
The BALB/c AB1 malignant mesothelioma,18 and its AB1-HA derivative,1 were cultured in RPMI (Gibco–Invitrogen) complemented with 10% fetal calf serum, 2 m-glutamine (Gibco–Invitrogen), 100 Units per ml of penicillin–streptomycin (Gibco–Invitrogen) and 50 μ 2-mercaptoethanol. These AB1 and AB1-HA cell lines were a generous gift from Dr Bernadette Scott (CFGHD, MIMR, Clayton, Victoria, Australia). All cell lines were mycoplasma free. For in vivo experiments, 1 × 105 (4T1 and 4T1-HA) or 5 × 105 (AB1 and AB1-HA) tumor cells were injected subcutaneously into the right flank of the mice. The right inguinal LN was used as the dLN. The contralateral brachial LN was used as the non-dLN. Tumor-inoculated mice were killed when average tumor diameters reached 15–20 mm. Tumor volume (mm3) was determined by measuring perpendicular tumor diameters using Vernier calipers (V=(L × l2)/2).
Antibodies and flow cytometry analysis
LN cells were obtained after a mechanical dissociation and were then stained with saturating amounts of combinations of the following mAbs: Alexa700-labeled anti-CD4, PerCp-labeled anti-CD8, PE (phycoerythrin)-labeled anti-CD62L, APC-labeled anti-CD25 and PE-Cy7-labeled CD44 (all from BD Biosciences, Pont de Claix, France). Labeling with the anti-clonotypic mAb (clone 6.5) specific to TCR-HA was revealed by a biotin anti-rat IgG2b mAb and streptavidin-PerCp (BD Pharmingen). Intracellular labeling of transcription factor Foxp3 (forkhead/winged-helix protein 3) by anti-Foxp3 conjugated to PE or Pacific Blue (FJK-16s, e-Bioscience, San Diego, CA) was performed according to the manufacturer's recommendations. Isotype-irrelevant mAbs were used as controls.
Lymphocytes were analyzed using a FACSCalibur (Pont de Claix, France) or LSR-II. Further analyses were performed with FlowJo (Tree Star, Ashland, OR) software.
In vivo depletion of CD4+ CD25+ T cells
Treg in vivo depletion was performed by intraperitoneal injection of 125 μg of an anti-CD25 monoclonal Ab (PC61) the day before tumor inoculation. This induces a >80% transient depletion of CD25high cells for ∼4 weeks in LNs of normal mice.
Adoptive transfer of HA-specific naive Teffs or antigen-experienced Teffs
Naive Teffs were magnetically depleted of CD25+ cells and then were sorted on the CD44low marker on a FacsARIA (Pont de Claix, France) cytometer, with a purity of >98%. Antigen-experienced HA-specific Teffs were generated in vivo by subcutaneous immunization of SFE mice on the right (50 μg) and left (50 μg) flanks and at the base of the tail (100 μg) with HA peptide in Incomplete Freund's Adjuvant (Sigma-Aldrich, Lyon, France). Mice were killed 2 months later and popliteal, inguinal LNs and spleen were harvested. Total cells are enriched in antigen-experienced Teffs of HA specificity (data not shown). These cells were then infused intravenously in BALB/c mice then challenged with AB1-HA tumor cells at day 0.
TCR-specific quantitative PCR assays
AB1, AB1-HA or 4T1, 4T1-HA tumor cells were injected subcutaneously in InsHA and BALB/c mice at day 0 with or without PC61 depletion of Tregs. Fifteen days later, mice were killed and tumor dLNs, non-dLNs and tumor intra lymphocytes were harvested as well as pancreatic LN from InsHA mice. Total mRNA was prepared from cells using TRIzol Reagent (Invitrogen) and phenol chloroform extraction. Reverse transcription PCR was performed to synthesize cDNA for PCR analysis. TCR-HA cDNA in each sample was quantified by real-time quantitative PCR (Applied Biosystems, Courtaboeuf, France) as described previously.19
Statistical methods
Statistical analyses of survival curves were performed using log-rank (Mantel–Cox) test. Comparisons of tumor growth curve were performed day per day using two-tailed t-test. We evaluated statistical significance using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, http://www.graphpad.com).
Results
HA behaves as a potent TAA that elicits efficient effector responses in the absence of Tregs
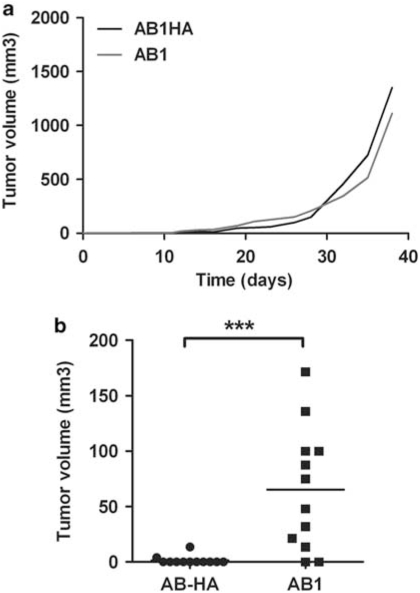
To investigate the importance of the quality of TAAs in mounting effector responses in the presence or absence of Tregs, we used the AB1 mesothelioma and its cloned AB1-HA derivative that express the HA of the Influenza virus.1, 18 When unmanipulated mice are subcutaneously inoculated with the same amount of AB1 or AB1-HA tumor cells (5 × 105 per mice), tumors grew in all the animals with similar kinetics for both tumors (Figure 1a). In contrast, Treg depletion has a markedly different effect on these tumors. A single injection of the PC61 anti-CD25 Ab 1 day before tumor challenge led to a rejection rate of 85% for AB1-HA tumor cells, compared with only 10% for AB1 tumor cells (Figure 1b). Thus, as it has been documented that Treg depletion unleashes antitumor Teffs, this suggests that the presence of HA led to the development of better effector responses.
Figure 1.
HA behaves as a potent tumor-associated antigen that elicits efficient effector responses in the absence of Tregs. (a) Six-week-old BALB/c mice were injected s.c. with 5 × 105 AB1-HA (black line) or AB1 (gray line) tumor cells at day 0 (n=5 in each group). Graph shows the tumor volume growth (mm3) of each tumor line. One representative of >5. (b) Six-week-old BALB/c mice were injected s.c. with 5 × 105 AB1-HA (n=12, circle) or AB1 (n=12, square) tumor cells at day 0 after Treg depletion by a single injection i.v. of 125 μg PC61 at day −1 (n=10 in each group). Graph shows the tumor volume growth (mm3) of each tumor line at day 25 in the absence of Tregs. AB1-HA tumor volumes were statistically different from AB1 tumor volumes (***P=0.0006, with an unpaired t-test). Five independent experiments were conducted.
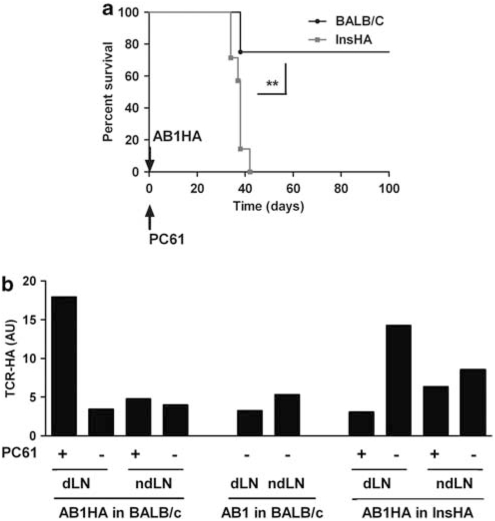
To actually show that anti-HA effector responses were responsible for AB1-HA rejection, and to rule out that the different tumor outcome was due to hidden differences between the two different tumor cell clones, we repeated these experiments in InsHA transgenic mice. These mice lack anti-HA Teffs that are deleted during thymic differentiation. When AB1-HA tumor cells are injected in Treg-depleted InsHA mice, the percentage of survival decreases from 75% in normal mice to 0% in InsHA mice (Figure 2a).
Figure 2.
Recruitment of HA-specific effector T cells in the draining lymph node of Treg-depleted AB1-HA bearing BALB/c mice. (a) Six-week-old BALB/c (n=8, black line, circle) or 2-month-old InsHA (n=7, gray line, square) mice were depleted of Tregs by a single injection of 125 μg PC61 at day −1 and subsequently injected s.c. with 5 × 105 AB1-HA or AB1 tumor cells at day 0. Graph shows the Kaplan–Meyer survival curves of mice in the absence of Tregs. The survival of BALB/c mice was statistically different from the survival of InsHA mice (**P=0.0017, with a log-rank (Mantel–Cox) test). (b) InsHA mice (n=4) of 6 weeks of age received 5 × 105 AB1-HA tumors s.c. Age-matched BALB/c mice (n=4) were injected s.c. with 5 × 105 AB1-HA or AB1 tumor cells at day 0. The mice injected with AB1-HA were separated into two groups and treated or not with a single injection of 125 μg PC61 at day −1 for Treg depletion. + or − refers to PC61 treatment or not, respectively. Normal BALB/c LN and InsHA pancreatic LN mice were used as negative and positive controls, respectively. At day 15, mice were killed and the tumor draining lymph nodes were harvested. mRNA was extracted, cDNA was synthesized and a TCR-HA TS-Q-PCR was performed. Graph shows the relative quantity of TCR-HA+ cells in each lymphoid organs at day 15. Data are representative of three experiments using two tumor lines AB1-HA and 4T1-HA.
Recruitment of HA-specific Teffs in the dLNs of Treg-depleted AB1-HA bearing mice
To further show that the difference in AB1/AB1-HA tumor rejection rates in Treg-depleted mice is due to the effector responses mounted against HA, we quantified HA-specific T cells in tumor-bearing mice using a novel TCR-specific quantitative PCR,19 which detects the immunodominant HA-specific TCR that was used to generate SFE TCR-transgenic mice.12 In SFE mice, the transgenic TCR is major histocompatibility complex-II restricted and mostly expressed on CD4+ Teffs, although functional major histocompatibility complex-II-restricted CD8+ killer T cells are also generated.12 BALB/c and InsHA mice, Treg-depleted or not, were challenged with AB1-HA or AB1 tumor cells. Tumor dLNs and non-dLNs were harvested, and the relative proportion of HA-specific T cells expressing the SFE TCR was determined by quantitative PCR (Figure 2b).
Compared with the basal levels of HA-specific cells that can be detected in controls, that is, LNs of AB1 tumor-bearing BALB/c mice, the frequency of HA-specific T cells is increased only in the dLNs of Treg-depleted BALB/c mice challenged with AB1-HA tumors. This indicates that HA-specific Teffs are indeed recruited by the HA expressing tumor, but only upon the relief of a Treg-mediated blockage.
In contrast, in InsHA mice in which HA is a self-antigen and the SFE TCR is thus expressed on Tregs,19 increased level of HA-specific T cells can be detected only in the dLNs of mice receiving AB1-HA tumor cells, and this detection is abolished in PC61-treated mice. In this setting, this reveals the recruitment of self-antigen-specific Tregs by the tumor. Similar results were observed using 4T1-HA tumor cells (data not shown).
Taken together, HA expression on tumor cells recruits Teffs in normal BALB/c mice, whereas it recruits Tregs in InsHA mice.
Only antigen-experienced anti-HA Teffs are capable of eradicating HA tumors in the presence of Tregs
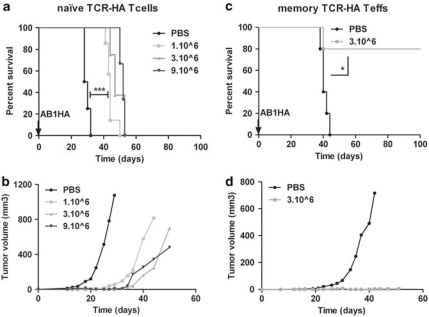
We asked whether the presence of a strong TAA such as HA could make the tumor cells good targets for adoptively transferred Teffs when Tregs are present, a usual setting of cancer immunotherapy protocols. For this, we first injected naive anti-HA-specific T cells obtained from TCR-HA SFE-transgenic mice to mice challenged with AB1-HA tumor cells. Treg-depleted cells from SFE mice were sorted on the basis of the CD44low expression and then injected at increasing doses 1 day before an AB1-HA challenge. This resulted in a delay in tumor growth, and similarly prolonged survival, indicating some contribution of the injected cells to the Treg/Teff battle. However, no tumor rejection could be observed, even after the injection of up to 9 × 106 naive HA-specific cells (Figures 3a and b).
Figure 3.
Only antigen-experienced anti-HA effector T cells are capable of eradicating HA tumors in the presence of Tregs. (a, b) Six-week-old BALB/c mice were injected i.v. with PBS (n=4, black line) or graded quantities of naive CD25− CD44low TCR-HA T cells from CMH-II restricted SFE transgenic mice: 1 × 106 (n=7, light gray line), 3 × 106 (n=8, gray line) or 9 × 106 (n=6, dark gray line) cells at day −1 and 5 × 105 AB1-HA (s.c.) at day 0. Graphs show (panel a), the Kaplan–Meyer survival curves and (panel b), the tumor volume growth curves (mm3). The treated groups were statistically different from the PBS-treated group (***P=0.0005, ***P=0.0002, ***P=0.0001, respectively, with a log-rank (Mantel–Cox) test). (c, d) TCR-HA transgenic mice were immunized in vivo s.c. with HA peptide in IFA. Two months later, mice were killed and the spleen and inguinal/political lymph nodes were harvested. Six-week-old BALB/c mice were injected s.c. with 5 × 105 AB1-HA at day 0 and i.v. with 15 × 106 effector T cells from immunized TCR-HA mice (n=5, gray line), containing ∼3 × 106 memory TCR-HA Teffs, or PBS (n=5, black line) at day 0. Graphs show (panel c), the Kaplan–Meyer survival curves and (panel d), the tumor volume growth curves (mm3). The treated group was statistically different from the PBS-treated group (*P=0.0157, with a log-rank (Mantel–Cox) test.
We next investigated whether activated/memory HA-specific Teffs could better reject HA tumor cells in the presence of Tregs. We thus collected Teffs from SFE TCR-HA mice that had been immunized with the HA peptide and were consequently enriched in HA-specific memory Teffs (data not shown and Darrasse-Jeze et al.19) and then injected them into AB1-HA tumor-bearing mice. We observed that antigen-experienced Teffs efficiently rejected AB1-HA (Figures 3c and d) but not AB1 tumor cells (data not shown).
Expression of the immunodominant HA prevents the development of a broader response to additional tumor antigens
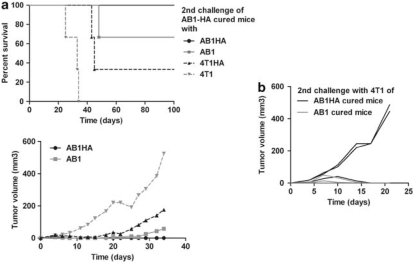
We next asked whether the effector response against HA-expressing tumor was essentially HA directed or also directed against other tumor antigens. To answer this question, Treg-depleted BALB/c mice were first challenged with AB1-HA tumor cells. Mice that rejected the first tumor and survived were rechallenged 2 months later with either AB1-HA or AB1 tumor cells, or with the 4T1 adenocarcinoma or its 4T1-HA derivative (Figure 4a, top and bottom). It is noteworthy that at the time of the second challenge, these mice have normal Treg number and function.20 We observed that all mice rejected the second AB1-HA tumor challenge (100%), whereas AB1 tumor cells were rejected less efficiently (66% survival). In addition, 4T1 tumor cells could not be rejected (0%), unless they expressed HA (33% survival). Thus, the immunization against AB1-HA tumor cells in the absence of Tregs drives not only a predominant HA-specific response but also a response to additional AB1-associated tumor antigen(s).
Figure 4.
Expression of the immunodominant HA prevents the development of a broader response to additional tumor antigens. (a) Six-week-old BALB/c mice were depleted of Tregs by a single injection of 125 μg PC61 and subsequently injected s.c. with 5 × 105 AB1-HA tumor cells as in Figure 1. Mice that had rejected the first tumor and survived were rechallenged s.c. 2 months later with 5 × 105 AB1 (gray line) or AB1-HA (black line) or 1 × 105 4T1 (hatched gray line) or 4T1-HA (hatched black line) tumor cells (n=3 in each group). Graphs show top, the Kaplan–Meyer survival curves and bottom, the tumor volume growth curves (mm3). (b) Six-week-old BALB/c mice were depleted of Tregs by a single injection of 125 μg PC61 at day −1 and subsequently injected s.c. with 5 × 105 AB1-HA (black line) or AB1 (gray line) tumor cells at day 0 (n=3 in each group). Mice that had rejected the first tumor and survived were rechallenged 2 months later with 105 4T1 tumor cells, as well as age-matched naive mice as a control (n=2, not shown). Graph shows the tumor volume growth curves (mm3).
We also compared the ability of BALB/c mice that survived a first challenge with AB1 or AB1-HA tumor cells performed after Treg depletion, to reject a second challenge with 4T1 tumor cells (Figure 4b). AB1-HA-cured mice are poorly able to reject 4T1 tumor (33% in Figure 4b and 0% in Figure 4a). In contrast, 4T1 tumor cells were rejected by 100% of AB1-cured mice. This result shows that 4T1 and AB1 express shared antigens, which could represent efficient targets of an antitumor response after immunization. In the presence of an immunodominant TAA such as HA, the immune response against the non-HA tumor antigens is masked or at least reduced. All these results are recapitulated in Table 1.
Table 1. Rejection rate of each tumor in a first or second challenge.
| First challenge | PC61 | % Rejection | Second challenge | % Rejection |
|---|---|---|---|---|
| AB1-HA | − | 0 (n=8) | ||
| AB1 | − | 0 (n=8) | ||
| 4T1HA | − | 0 (n=8) | ||
| 4T1 | − | 0 (n=8) | ||
| AB1-HA | + | 83.3 (n=12) | ||
| AB1 | + | 16.7 (n=12) | ||
| 4T1HA | + | ND | ||
| 4T1 | + | 60 (n=10) | ||
| AB1-HA | + | AB1-HA | 100 (n=6) | |
| AB1-HA | + | AB1 | 66 (n=3) | |
| AB1-HA | + | 4T1HA | 33 (n=3) | |
| AB1-HA | + | 4T1 | 16.7 (n=6) | |
| AB1 | + | 4T1 | 100 (n=3) |
ND, not defined.
− Indicates no PC61 treatment.
+ Indicates with PC61 treatment.
Numbers are expressed in percentages.
Data representative of one experiment among five.
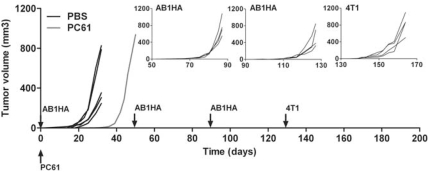
However, this masking of immune response against non-dominant TAA can be overcome by repeated immunization (Figure 5). BALB/c mice that survived a first AB1-HA challenge after Treg depletion were rechallenged twice with AB1-HA tumor cells. At each rechallenge, AB1-HA tumor cells were efficiently rejected without any intervention. When these mice were finally challenged with 4T1 tumor cells, they were all able to reject 4T1 tumor cells (Figure 5). This indicates that repeated immunizations in the presence of the immunodominant antigen nevertheless boosted the response to non-dominant tumor antigens.
Figure 5.
Repeated tumor challenges foster non-HA immune response. Six-week-old BALB/c mice were injected i.v. with 125 μg PC61 at day −1 to deplete Tregs (n=5, gray line) or PBS (n=5, black line) and subsequently injected s.c. with 5 × 105 AB1-HA tumor cells at day 0. Mice that had rejected the first tumor (4/5) were rechallenged s.c. 1.5 months later with 5 × 105 AB1-HA, as well as 4 naive age-matched BALB/c mice (left insert). Mice that rejected the second tumor (4/4) were again rechallenged 1.5 months later with 5 × 105 AB1-HA as well as 4 naive age-matched BALB/c mice (middle insert). Mice that rejected the third AB1-HA tumor (4/4) were rechallenged 1 month later with 1 × 105 4T1 as well as 4 naive age-matched BALB/c mice (right insert). Graphs show the tumor volume growth (mm3).
Discussion
Despite overall disappointing results so far, immunotherapy of cancer still holds strong rationale and great potential.21, 22, 23 Indeed, it remains very attractive to use the patients' own immune system to specifically attack and destroy cancer cells, a specific process that could be both very efficient and safer than chemotherapy.24, 25 However, the true potential of immunotherapy is likely depending on (1) the expression by tumor cells of enough antigens that could be the target of immune effector cells and (2) the existence and proper activation of a large enough repertoire of Teffs recognizing these antigens. The discovery of a large array of TAAs,26, 27 notably using cloned patients' Teffs,28 provided a rationale foundation for immunotherapy. However, the numerous attempts at generating therapeutic immune responses in cancer patients, generated only very few positive responses and rather pessimistic views on the real potential of cancer immunotherapy.23 Optimism renewed with the realization that, in mice, depletion of Tregs could unleash effector responses that could lead to tumor eradication. Incidentally, these experiments showed that in most settings there exist both and enough antigens on the tumor cells and specific Teffs to obtain tumor eradication. This opened a new era of immunotherapy, which incorporates strategies to block Treg responses.4, 5, 29, 30, 31, 32, 33, 34 However, Treg-depletion effects on tumor growth ranged from full eradication to only a slow down of tumor progression; this led us to investigate the importance of target antigens for effector immune responses in the absence of Tregs.
Antigen quality and the efficiency of antitumor immune responses in the absence of Tregs
Our results clearly indicate that the expression of a potent TAA is the key to induce effector responses capable of eradicating tumor cells. It is remarkable that the sole expression of HA turns from 0–15% to 80–100%, the frequency of tumor rejection in the absence of Tregs (Figure 1). We showed that this is clearly due to the sole presence of the HA antigen. Indeed, the 80–100% frequency of tumor rejection in the absence of Tregs in BALB/c mice is reverted back to 0–10% in InsHA mice that cannot mount anti-HA effector immune responses (Figure 2). We ruled out that this could be due to the fact that InsHA mice have a large pool of HA-specific Tregs, which is an autoantigen in this mouse background, and that could change the balance between Teffs and Tregs numbers in this context. Indeed, we showed that Treg number is not limiting in the Teffs/Tregs battle inasmuch that the injection of extremely high numbers of CD25− CD44low naive T cells does not significantly affect tumor growth in an otherwise nonmanipulated setting (Figure 3a). Thus, the poor tumor eradication rate in InsHA mice can be attributed to the lack of anti-HA Teffs.
Nevertheless, the quality of the antigen is not the only factor determining tumor fate in the absence of Tregs. HA-sensitized mice that have developed an excellent memory attested by their ability to eradicate AB1-HA tumor cells in 100% of the cases, could only eradicate 33% of 4T1-HA tumors (Figure 4a). Thus, the global efficiency of the immune responses is likely multiparametric, but requires potent antigens.
Antigen quality and the efficiency of antitumor immune responses in the presence of Tregs
The expression of a potent TAA that can trigger very efficient effector responses in the absence of Tregs is clearly not sufficient for gaining tumor eradication in their presence. AB1-HA tumor cells (as well as 4T1-HA) grow as well as their AB1 (or 4T1) parental cells in unmanipulated immunocompetent mice (Figure 1a). This is not due to an insufficient number of Teffs that could not counteract the natural Treg response. Indeed, the injection of up to 9 × 106 HA-specific effectors, which is >103 times the frequency of highly represented specificity,35, 36, 37 can only slightly delay but cannot eradicate AB1-HA tumor cells (Figure 3a). The rejection of AB1-HA tumor cells in the presence of Tregs could only be achieved with the injection of antigen-experienced T cells (Figure 3b). The capacity of memory Teffs to escape Treg-mediated suppression is mostly a question of kinetics of responses. We indeed recently showed that tumor emergence leads to a brisk recruitment and activation of memory Tregs19 that respond to self-antigens with a memory type kinetic; in contrast, antitumor Teffs that have never seen their cognate antigen beforehand are recruited more slowly with a naive type kinetic of the response. If memory Teffs are present, they are recruited with the same memory kinetics than Tregs, which are unable to control them. Besides the kinetics of Treg vs Teff responses, memory effectors appear to be somehow resistant to Treg-mediated suppression as previously shown in auto-immune and transplantation settings.38, 39
Thus, altogether, Treg manipulation will likely be always required to generate efficient antitumor immune responses, even in the presence of a potent antigen.
Immunodominance of tumor antigens restricts the breadth of antitumor immune responses in the absence of Tregs
Although the presence of a potent antigen is advantageous for the development of efficient antitumor immune response in the absence of Tregs, it may also be disadvantageous.40 Indeed, we show that the presence of a ‘too' immunodominant TAA can strongly bias the immune response toward this antigen, preventing the development of a broader one. Although only a few mice survived a challenge with the mesothelioma AB1 tumors after Treg depletion, they could all resist to a second challenge not only with the same tumor, but also to a challenge with mammary carcinoma 4T1 tumor cells (Figure 4b). In contrast, only 16.7% of the numerous mice that survived an initial challenge with AB1-HA tumor cells could resist a 4T1 challenge (Figure 4b). This indicates that (1) the breadth of the anti-AB1 immune responses covers some antigens that are shared by the 4T1 tumors and (2) the presence of HA onto the tumor deviates the immune response against this dominant antigen. However, subsequent challenges with HA-expressing tumors could increase immune response to nondominant tumor antigens (Figure 5).
Cryptic/subdominant antigens are defined as self-hindered epitopes, due to indolent or excessive processing of protein. Therefore, T cells recognizing such epitopes are not submitted to clonal deletion, which allow the existence of reactive T-cell clones in the periphery. Recognition of these epitopes by T-cell clones can lead to their activation.41 An ‘epitope spreading' revealed by the generation of immune responses to such cryptic/subdominant antigens has indeed been shown (1) in cancer models, with T-cell responses directed toward distinct p53 peptides depending on the stage of tumorigenesis42, 43 or with peptide immunizations that yield the generation of cytotoxic T cells against different protein44, 45 or (2) in autoimmune models such as experimental autoimmune encephalomyelitis or insulin-dependent diabetes mellitus, under prolonged stimulation with myelin basic protein or glutamic acid decarboxylase, respectively.46, 47
Thus, a too important restriction of the antitumor immune response would not allow the development of responses toward existing cryptic/subdominant antigens; this could favor immunoediting of the tumor, leading to the appearance of escape variants by mutation of this immunodominant antigen,48, 49, 50 a process that would be less likely to occur if the immune response breadth is broader. Further analyses of antigens and major histocompatibility complex expression in tumors that grow out despite previous successful response to a tumor challenge would substantiate the occurrence of tumor escape variants and point to its relevance for future therapies.
Implications for immunotherapy
Taken together, our results highlight that successful immunotherapies will most likely be combinatorial, controlling Tregs while stimulating Teffs. However, we anticipate that such strategies should lead to improved clinical responses only in a fraction of the patients. Indeed, in treated inbred mice injected with the same tumors, there is already heterogeneity of the tumor fate; this indicates that the outcome of the battle between the tumor cells and the immune system is multiparametric and uncertain, and thus likely to much vary between heterogeneous patients. Ultimately, combination treatments using the whole panoply of treatments—surgery, chemotherapy, gene therapy, immunotherapy—are likely to be required to treat cancer patients.
In this line, we have previously reported the results of a clinical trial based on vaccination of patients with renal cell carcinoma with autologous bone-marrow derived dendritic cells sensitized with tumor lysates.51 The outcome was poor, in large part because we co-treated the patients with interleukin-2 to attempt to better activate effector responses,52 while this cytokine is now known as a Treg inducer. We also have an undergoing clinical trial for patients with metastatic colorectal cancer in which patients receive a lymphoablation, followed by the reinjection of Treg-depleted T cells (STARTREK ClinicalTrials.gov NCT00986518). We believe that on the basis of our results, these two approaches could be combined in the future, but with sensitizing dendritic cells with tumor extract as well as with a strong colorectal cancer tumor antigen such as carcinoembryonic antigen.53
In conclusion, we believe that the manipulation of Tregs should become an almost mandatory part of multimodal cancer immunotherapy.
Acknowledgments
We thank Bernadette Scott, from the Centre for Functional Genomics and Human Disease, Monash Institute of Medical Research, at the Monash University, Clayton, Victoria, Australia, for kindly sharing the AB1 and AB1-HA tumor cells. This work was supported by the Ministère de la Recherche, the Université Pierre et Marie Curie (Paris VI), The Association pour la Recherche sur le Cancer (ARC) and the CNRS. We thank Charlotte Dalba for critical reading of this paper, Bruno Gouritin and Sylvie Grégoire for assistance with flow cytometry sorting, and Pierric Parent for animal care.
Glossary
- Ab
antibody
- dLN
draining lymph node
- Foxp3
forkhead/winged-helix protein 3
- HA
hemagglutinin
- Ins
insuline
- TAA
tumor-associated antigen
- Teff
effector T cell
- Treg
regulatory T cell
The authors declare no conflict of interest.
References
- Marzo AL, Lake RA, Lo D, Sherman L, McWilliam A, Nelson D, et al. Tumor antigens are constitutively presented in the draining lymph nodes. J Immunol. 1999;162:5838–5845. [PubMed] [Google Scholar]
- Cuenca A, Cheng F, Wang H, Brayer J, Horna P, Gu L, et al. Extra-lymphatic solid tumor growth is not immunologically ignored and results in early induction of antigen-specific T-cell anergy: dominant role of cross-tolerance to tumor antigens. Cancer Res. 2003;63:9007–9015. [PubMed] [Google Scholar]
- Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- Chaput N, Darrasse-Jeze G, Bergot AS, Cordier C, Ngo-Abdalla S, Klatzmann D, et al. Regulatory T cells prevent CD8 T cell maturation by inhibiting CD4 Th cells at tumor sites. J Immunol. 2007;179:4969–4978. doi: 10.4049/jimmunol.179.8.4969. [DOI] [PubMed] [Google Scholar]
- Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Tanaka J, Kjaergaard J, Shu S. Depletion of CD4+ CD25+ regulatory cells augments the generation of specific immune T cells in tumor-draining lymph nodes. J Immunother. 2002;25:207–217. doi: 10.1097/00002371-200205000-00003. [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Clements VK, Terabe M, Park JM, Berzofsky JA, Dissanayake SK. Resistance to metastatic disease in STAT6-deficient mice requires hemopoietic and nonhemopoietic cells and is IFN-gamma dependent. J Immunol. 2002;169:5796–5804. doi: 10.4049/jimmunol.169.10.5796. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Watanabe S, Neumann G, Kida H, Kawaoka Y. Immunogenicity and protective efficacy of replication-incompetent influenza virus-like particles. J Virol. 2002;76:767–773. doi: 10.1128/JVI.76.2.767-773.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolou I, Von Boehmer H. The TCR-HA, INS-HA transgenic model of autoimmune diabetes: limitations and expectations. J Autoimmun. 2004;22:111–114. doi: 10.1016/j.jaut.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur C, Hanahan D, Smith KM. T-cell tolerance toward a transgenic beta-cell antigen and transcription of endogenous pancreatic genes in thymus. Proc Natl Acad Sci USA. 1994;91:6707–6711. doi: 10.1073/pnas.91.14.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D, Benoist C. A decade of AIRE. Nat Rev Immunol. 2007;7:645–650. doi: 10.1038/nri2136. [DOI] [PubMed] [Google Scholar]
- Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Hengartner H, Odermatt B, Schneider R, Schreyer M, Walle G, MacDonald HR, et al. Deletion of self-reactive T cells before entry into the thymus medulla. Nature. 1988;336:388–390. doi: 10.1038/336388a0. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- Davis MR, Manning LS, Whitaker D, Garlepp MJ, Robinson BW. Establishment of a murine model of malignant mesothelioma. Int J Cancer. 1992;52:881–886. doi: 10.1002/ijc.2910520609. [DOI] [PubMed] [Google Scholar]
- Darrasse-Jeze G, Bergot AS, Durgeau A, Billiard F, Salomon BL, Cohen JL, et al. Tumor emergence is sensed by self-specific CD44hi memory Tregs that create a dominant tolerogenic environment for tumors in mice. J Clin Invest. 2009;119:2648–2662. doi: 10.1172/JCI36628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiard F, Litvinova E, Saadoun D, Djelti F, Klatzmann D, Cohen JL, et al. Regulatory and effector T cell activation levels are prime determinants of in vivo immune regulation. J Immunol. 2006;177:2167–2174. doi: 10.4049/jimmunol.177.4.2167. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage PA, Vosseller K, Kang C, Larimore K, Riedel E, Wojnoonski K, et al. Recognition of a ubiquitous self antigen by prostate cancer-infiltrating CD8+ T lymphocytes. Science. 2008;319:215–220. doi: 10.1126/science.1148886. [DOI] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- Sotomayor EM, Borrello I, Tubb E, Allison JP, Levitsky HI. In vivo blockade of CTLA-4 enhances the priming of responsive T cells but fails to prevent the induction of tumor antigen-specific tolerance. Proc Natl Acad Sci USA. 1999;96:11476–11481. doi: 10.1073/pnas.96.20.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon ED, Hurwitz AA, Foster BA, Madias C, Feldhaus AL, Greenberg NM, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110:2614–2627. doi: 10.1002/cncr.23086. [DOI] [PubMed] [Google Scholar]
- Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Montagut T, Chow A, Hirschhorn-Cymerman D, Terwey TH, Kochman AA, Lu S, et al. Glucocorticoid-induced TNF receptor family related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity. J Immunol. 2006;176:6434–6442. doi: 10.4049/jimmunol.176.11.6434. [DOI] [PubMed] [Google Scholar]
- Casrouge A, Beaudoing E, Dalle S, Pannetier C, Kanellopoulos J, Kourilsky P. Size estimate of the alpha beta TCR repertoire of naive mouse splenocytes. J Immunol. 2000;164:5782–5787. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, et al. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Brook MO, Carvalho-Gaspar M, Zhang J, Ramon HE, Sayegh MH, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci USA. 2007;104:19954–19959. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmowski MJ, Choi EM, Hermans IF, Gilbert SC, Chen JL, Gileadi U, et al. Competition between CTL narrows the immune response induced by prime-boost vaccination protocols. J Immunol. 2002;168:4391–4398. doi: 10.4049/jimmunol.168.9.4391. [DOI] [PubMed] [Google Scholar]
- Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- Fedoseyeva EV, Boisgerault F, Anosova NG, Wollish WS, Arlotta P, Jensen PE, et al. CD4+ T cell responses to self- and mutated p53 determinants during tumorigenesis in mice. J Immunol. 2000;164:5641–5651. doi: 10.4049/jimmunol.164.11.5641. [DOI] [PubMed] [Google Scholar]
- Bueter M, Gasser M, Lebedeva T, Benichou G, Waaga-Gasser AM. Influence of p53 on anti-tumor immunity (review) Int J Oncol. 2006;28:519–525. [PubMed] [Google Scholar]
- el-Shami K, Tirosh B, Bar-Haim E, Carmon L, Vadai E, Fridkin M, et al. MHC class I-restricted epitope spreading in the context of tumor rejection following vaccination with a single immunodominant CTL epitope. Eur J Immunol. 1999;29:3295–3301. doi: 10.1002/(SICI)1521-4141(199910)29:10<3295::AID-IMMU3295>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Markiewicz MA, Fallarino F, Ashikari A, Gajewski TF. Epitope spreading upon P815 tumor rejection triggered by vaccination with the single class I MHC-restricted peptide P1A. Int Immunol. 2001;13:625–632. doi: 10.1093/intimm/13.5.625. [DOI] [PubMed] [Google Scholar]
- McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechel MA, Krawetz MD, Singh B. Epitope dominance: evidence for reciprocal determinant spreading to glutamic acid decarboxylase in non-obese diabetic mice. Immunol Rev. 1998;164:111–118. doi: 10.1111/j.1600-065x.1998.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Lozupone F, Rivoltini L, Luciani F, Venditti M, Lugini L, Cova A, et al. Adoptive transfer of an anti-MART-1(27-35)-specific CD8+ T cell clone leads to immunoselection of human melanoma antigen-loss variants in SCID mice. Eur J Immunol. 2003;33:556–566. doi: 10.1002/immu.200310032. [DOI] [PubMed] [Google Scholar]
- Khong HT, Restifo NP. Natural selection of tumor variants in the generation of ‘tumor escape' phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong HT, Wang QJ, Rosenberg SA. Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: tumor escape by antigen loss and loss of MHC expression. J Immunother. 2004;27:184–190. doi: 10.1097/00002371-200405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine FM, Cherai M, Giverne C, Dimitri D, Rosenzwajg M, Trebeden-Negre H, et al. Massive expansion of regulatory T-cells following interleukin 2 treatment during a phase I-II dendritic cell-based immunotherapy of metastatic renal cancer. Int J Oncol. 2009;35:569–581. doi: 10.3892/ijo_00000368. [DOI] [PubMed] [Google Scholar]
- Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T, Hsu D, Hammond S, Hobeika A, Devi G, Clay T, et al. Metastatic colorectal cancer cells from patients previously treated with chemotherapy are sensitive to T-cell killing mediated by CEA/CD3-bispecific T-cell-engaging BiTE antibody. Br J Cancer. 2010;102:124–133. doi: 10.1038/sj.bjc.6605364. [DOI] [PMC free article] [PubMed] [Google Scholar]