Abstract
BACKGROUND
Marshall bundles (MBs) are the muscle bundles within the ligament of Marshall.
OBJECTIVE
This trial sought to the electrophysiological characteristics of the MB and the anatomical connections between MB and left atrium (LA) in patients with persistent atrial fibrillation (AF).
METHODS
We enrolled 72 patients (male:female 59:13, age 59.9 ± 9.4 years) who underwent MB mapping and ablation for AF. MB mapping was done via an endocardial or epicardial approach during sinus rhythm and AF.
RESULTS
Recordings were successful in 64 of 72 patients (89%). A single connection was noted in 11 of 64 patients between the MB and the coronary sinus (CS) muscle sleeves. The MB recordings showed distinct MB potentials with a proximal-to-distal activation pattern during sinus rhythm. During AF, organized passive activations and dissociated slow MB ectopic activities were commonly observed in this type of connection. Double connections to both CS and LA around left pulmonary veins were noted in 23 of 64 patients (36%). After the ablation of the distal connection, MB recording showed typical double potentials as in single connection. Multiple connections were noted in 30 of 64 patients (47%). During sinus rhythm, the earliest activation was in the middle of the MB. The activation patterns were irregular and variable in each patient. During AF, rapid and fractionated complex activations were noted in all patients of this group.
CONCLUSION
We documented 3 different types of MB–LA connections. Rapid and fractionated activations were most commonly observed in the MB that had multiple LA connections.
Keywords: Atrial fibrillation, Electrophysiology, Ligament of Marshall, Radiofrequency catheter ablation
The ligament of Marshall (LOM) is an epicardial vestigial fold that contains the oblique vein of Marshall (VOM), myocardial sleeve (the Marshall bundle [MB]), and autonomic nerves.1 The left lateral ridge (LLR), a structure located in between the left atrial (LA) appendage and left pulmonary veins (PVs), is known to be important to atrial fibrillation (AF) ablation.2 The LOM is located in the epicardial aspect of the LLR. At the coronary sinus (CS) junction, the MB completely encircles the VOM and inserts directly into the CS musculature or more distally into the posterior wall of the LA.3 At the middle or distal portions of the LOM, the MB gradually changes into multiple muscle fibers and then disappears or inserts into the epicardium of the anterior wall of the LA and left PVs, dominantly in the left inferior PV.4 Scherlag et al5 first characterized the electrical activities of the LOM. The investigators reported that the MB was part of the inferior interatrial pathway that connects the left and right atria. The terminal end of the MB showed no insertion into atrial musculature in that study. However, subsequent postmortem human pathologic studies demonstrated multiple histological connections of the MB with the LA myocardium.3 The major connections are located in the CS juncture, LA–left PVs junction, and LA in between CS and PVs with wide multiple connections. Studies in canine models showed that the MB provided an electrical conduit between LA–LLR and CS.6 Rapid activations from the MB indeed were sources of atrial tachycardia and AF in canine models and in humans,7–9 and rapid activation at the MB was also noted during persistent AF.10,11 We hypothesize that the presence of multiple connections between MB and LA allow re-entrant excitations to occur between them, leading to more complex and rapid activations that help maintenance of the AF. However, little information is available about the relationship between electrophysiological characteristics of the MB and its connections to the surrounding atrial structures in human patients with AF. The purpose of this study was to: 1) define the electrophysiological characteristics of the MB, and 2) evaluate the relationship between the electrophysiological characteristics of the MB and its connections to the surrounding atrial structures. The data will be used to test the hypothesis that the complexities of MB–LA connections correlate with the complexities of electrical activities during AF.
Methods
Patients undergoing the electrophysiological studies
With the approval of the Institutional Review Board of the Utah Valley Medical Center, we retrospectively reviewed the data from all (72) patients (58 men and 14 women; ages 59.9 ± 9.4 years) with symptomatic persistent AF who underwent attempted MB mapping and radiofrequency catheter ablation from March 2006 to February 2007. In each patient, AF was not controlled by at least 2 antiarrhythmic drugs. Written, informed consent was obtained in all patients prior to the electrophysiological study and ablation procedure. All antiarrhythmic drugs were discontinued for ≥5 half-lives. No patients were treated with amiodarone. Fentanyl and midazolam were used for sedation. The patients who underwent open heart surgery, pregnant women, and those who were younger than 18 or older than 80 years old were excluded.
MB recordings
The endocardial approach included a CS angiogram followed by the cannulation of the VOM using a 1.5-F quadripolar catheter (Cardima, Cardima Inc, Fremont, California).9 However, the VOM cannulation was not always successful due to various anatomical and technical reasons. In our experience, endocardial mapping was possible in approximately 45% of patients who underwent attempted VOM cannulation. In patients whose VOM was not visible on CS angiogram or in whom it could not be cannulated, MB mapping was performed by the epicardial approach, which had the advantage of free catheter movement and was not limited by the size of the VOM.12,13 The epicardial approach was performed via a subxiphoid pericardial puncture14 followed by mapping with a deflectable duodecapolar catheter and 8-F Swartz SL 1 transseptal sheath (Fast-Cath guiding introducer, St. Jude Medical, Inc., St. Paul, Minnesota) to stabilize the MB recordings. In the early period of epicardial MB mapping, the epicardial mapping catheter was placed over the visible VOM by CS angiogram, and both endocardial and epicardial mapping were done simultaneously to validate the epicardial MB potentials (Figure 1). Thereafter, in cases in which the VOM was not visualized or cannulated, we placed the catheter between left PVs and LA appendage to record the MB potentials. If VOM was not visualized, the ostium of VOM was identified by a superior notch at the valve of Vieussens15 in CS angiograms. This notch was used as a surrogate marker of the starting point of VOM. If there was a premature ectopic beat from the MB, small-amplitude potentials followed by large-amplitude (atrial) potentials could be verified as MB potentials (Figures 2 and 3). Otherwise, MB potentials were verified by differential pacing from by CS, left PV, or appendage (Figure 4). The pacing from each site could separate the second small-amplitude potentials from the first large-amplitude (atrial) potentials, which allowed us to verify that the second potentials were not from other parts of the LA. After validating MB potentials, we performed MB pacing by varying the pacing output to selectively pace the MB (Figures 5A and 5B). The filter and gain settings for the MB recordings were the same as those for the CS and PV recordings. The electrograms were acquired during sinus rhythm and during spontaneous or induced AF. The length of the MB was estimated by the length and distances of each electrode of the mapping catheter.
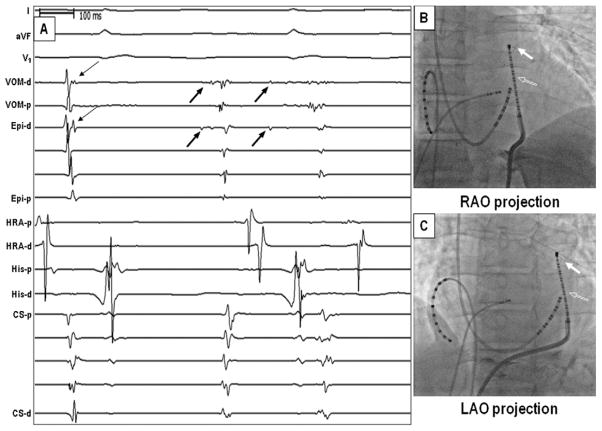
Figure 1.
Simultaneous endocardial and epicardial mapping of the MB. A: Sinus rhythm and MB ectopic activity. The thin arrows point to VOM, and Epi channels were MB potentials during sinus rhythm. Ectopic beats from the MB (thick arrows) were recorded on both VOM-d and on Epi-d at the same time, indicating that the deflection on Epi-d is the VOM activation. B, C: Fluoroscopic images show that the Cardima catheter (filled arrow) within the VOM and the duodecapolar catheter (unfilled arrow) used for epicardial mapping. These 2 were close to each other. CS = coronary sinus; d = distal; Epi = epicardial; His = His bundle; HRA = high right atrium; MB = Marshall bundle; p = proximal; VOM = vein of Marshall.
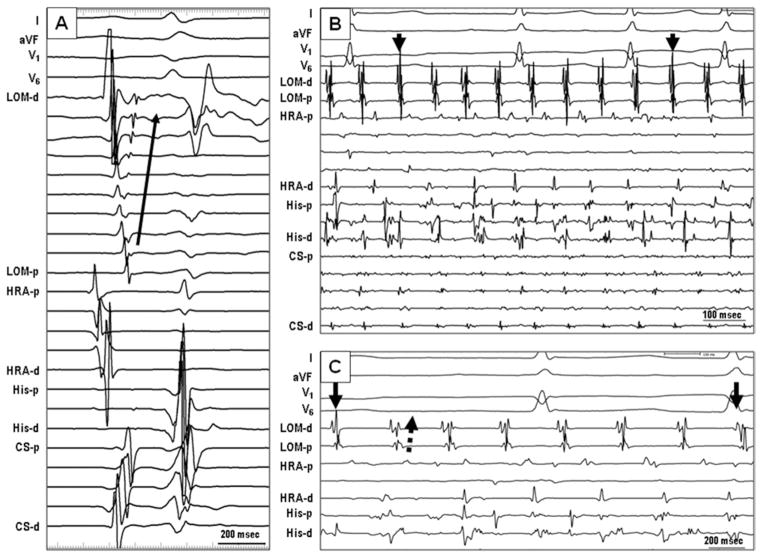
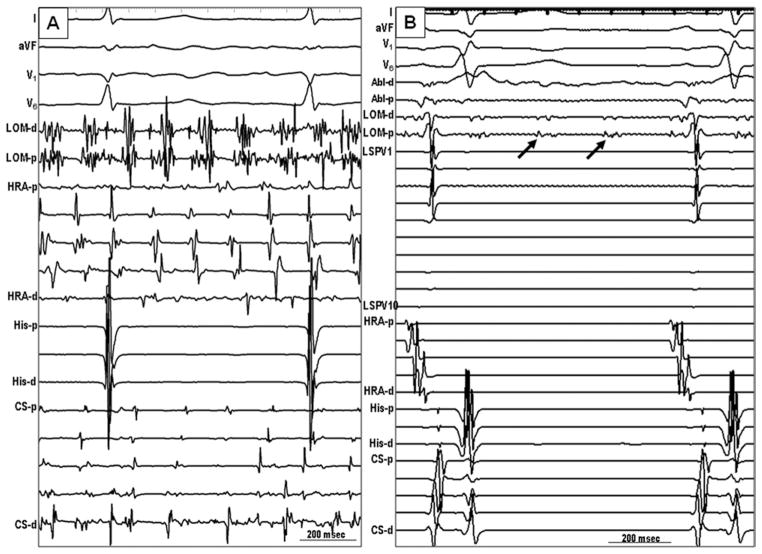
Figure 2.
Electrogram characteristics of single MB-CS connection. A: Sinus rhythm. The second potentials in LOM channel, which were recorded by a duode-capolar catheter placed epicardially on the LOM, were MB potentials. The earliest activation site was the proximal site near the coronary sinus. The activation propagated to the distal MB (arrow). B: MB recordings of a single connection type during AF. The electrical activities of the MB (marked as LOM) were regular and organized without chaotic fibrillatory activities. Independent of passive activities of MB, ectopic beats (arrow) from the distal MB were observed. C: The MB is activated from proximal to distal (dashed arrow) during persistent AF, but the MB ectopy activated in a distal to proximal direction (arrow). AF = atrial fibrillation; LOM = ligament of Marshall; other abbreviations as in Figure 1.
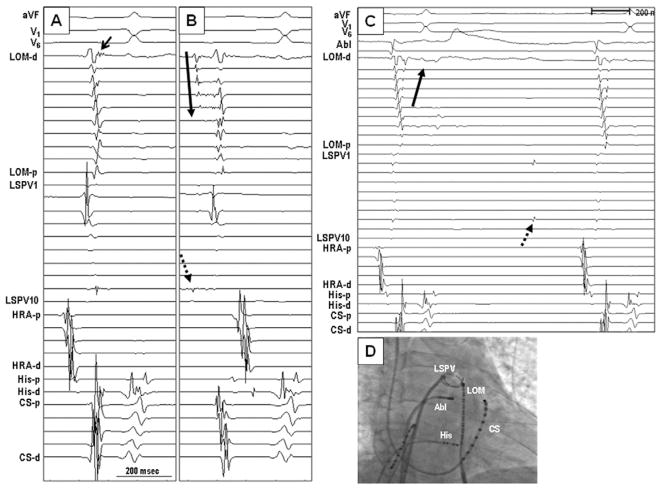
Figure 3.
Electrogram characteristics of double-MB connections (to CS and to LA–PVs). A: Sinus rhythm. The MB electrogram (arrow) and the local LA electrogram were close to each other. Therefore, distinct MB potentials were not seen in most recordings. B: A premature beat from the LSPV (dashed arrow) propagated first into the distal MB and then activated the MB in a distal-to-proximal direction (solid arrow). C: A recording from a double-connection type after the ablation of the distal connection between the MB and LSPV. During sinus rhythm, the MB activation was consistent with a single-connection type (proximal-to-distal activation pattern, solid arrow). Note that there was dissociated PV potential (dashed arrow). D: Fluoroscopic image in the left anterior oblique projection. A circular mapping catheter was located in the LSPV, and a duodecapolar catheter was placed epicardially on the LOM. LA = left atrium; LSPV = left superior pulmonary vein; PV = pulmonary vein; other abbreviations as in Figure 2.
Figure 4.
Example of differential pacing to map the MP potentials. A: Intracardiac recordings during sinus rhythm. B: Activation during CS pacing. The earliest activation was in CS, followed by proximal-to-distal propagation within the VOM. These VOM potentials (arrows) clearly preceded the onset of PV and HRA potentials. C: Pacing from distal pole of the ablation catheter located within the LAA. The potentials recorded by the VOM catheter (arrows) are completely separated from atrial and PV potentials. D: Pacing from left PV separated the VOM potentials (arrows) from the PV potentials. E: Pacing from the distal VOM showed that the proximal VOM potential (arrow) preceded the PV potential, indicating that these potentials are not from the PVs. F and G: Fluoroscopic images during differential pacing. Abl = ablation; HRA = high right atrium; LAA = left atrial appendage; LAO = left anterior oblique; LPV = left pulmonary vein; RAO = right anterior oblique; other abbreviations as in Figure 3.
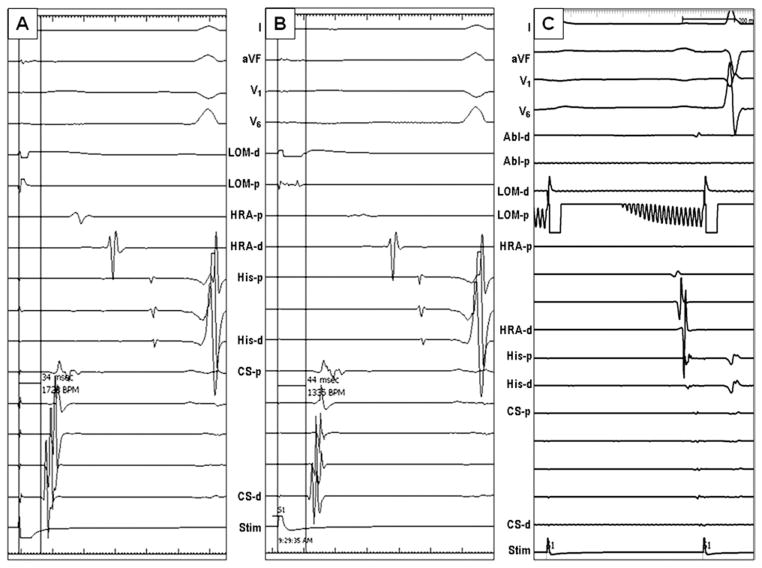
Figure 5.
Electrogram characteristics of MB pacing. A: Pacing at the LOM with 1.1 mA output resulted in simultaneous LA and MB capture. B: When the pacing output was decreased to 0.3 mA, the stimulus selectively captured MB without capturing the LA. Therefore, the interval between stimulus and the earliest CS activation was 44 ms, which is 10 ms longer than that in A. C: MB pacing after ablation of the MB–LA junction showed loss of MB capture with the same pacing output as pre-ablation. This finding is one of the end points of MB ablation. Abl = ablation catheter; Stim = stimulus, other abbreviations as in Figure 3.
Ablation strategy
Common ablation procedures in all patients were bi-antral electrical isolation using a large circular mapping catheter (30 to 35 mm in diameter, Biosense Webster Inc., Diamond Bar, California), accompanied by cavotricuspid isthmus ablation to prevent right atrial flutter.16 After finishing common procedures, we tried to isolate the MB activity from the LA. Because the LOM is located in the epicardial aspect of the LLR, radiofrequency energy application on the LLR may result in the ablation of the MB. However, a majority of patients needed additional ablation at the left lateral isthmus, CS, or epicardial site. Elimination of all MB potentials, loss of MB capture with the same pacing output of pre-ablation (Figure 5C), exit block by selective MB pacing, or dissociated MB activities were used to confirm the successful MB isolation (Figure 6B).
Figure 6.
MB potentials before and after radiofrequency catheter ablation in a patient with multiple MB connections. A: Recording during AF. The MB activity demonstrated sustained complex fractionated activations. B: Recording after isolation and during infusion of 1 μg/ml isoproterenol. The MB recording showed fast but organized tachycardia (arrows), which was dissociated with the rest of the LA. Abbreviations as in Figure 3.
Data analyses
The data were presented as the mean ± SD. The data were compared with an analysis of variance and Chi-square test using SPSS 16.0 software (SPSS Inc, Chicago, Illinois). A value of P ≤ .05 was considered significant.
Results
Successful MB recordings were achieved in 64 patients (89%), whose mean age was 59.1 ± 9.4 years. Among them, 52 were male. The LA volume and left ventricular ejection fraction (LVEF) measured by magnetic resonance image were 149.7 ± 19.7 ml and 46.4 ± 6.5%, respectively. The endocardial approach was used in 21 patients, the epicardial approach in 34. In the remaining 9 patients, both approaches were used to verify the epicardial mapping in the beginning period of the study. We classified the number of MB–LA connections based on electrogram characteristics.
Single connection
A single connection is the type of connection first reported by Scherlag et al.5 In patients with a single connection, the activation from the sinus node crosses to the LA via the inferior interatrial pathway and activates the MB from a proximal-to-distal direction. Because there is no other connection between the MB and LA, the MB was not pre-excited by the sinus wavefront from the Bachmann bundle.6 This type of connection has also been previously documented in humans.9 Eleven of 64 patients (17%) had distinct MB potentials with a typical proximal-to-distal activation sequence during sinus rhythm (Figure 2A). The same sequence of activation occurs during AF (Figures 2B and 2C). Note that these activations were relatively regular as compared with the activations within the LA. In 7 of 11 patients, there were additional ectopic activities from the distal LOM during AF. Those activities (downward arrows in Figures 2B and 2C) activated the LOM in a distal-to-proximal direction. The MB isolation was achieved in all patients by ablating the junction between the LOM and the CS.
Double connections
If the MB has connections with both the CS and the LA (or PVs), the wavefronts from the CS and LA (or PVs) compete for MB activation.6 As a result, the MB electrograms might not be clearly visible. Figure 3A shows the recordings during sinus rhythm at baseline. Note that the MB electrogram and the local LA electrogram were very close to each other at the distal MB. In most of the MB recordings, MB electrograms were indistinguishable from the local LA electrogram in sinus rhythm. However, during premature contraction from the left superior PV (dashed arrow, Figure 3B), multiple MB potentials were present. The activation in the MB was distal to proximal, consistent with the existence of a distal connection within the left superior PV. After successful radiofrequency catheter ablation of the distal MB–LA (PV) connection, only 1 connection (MB–CS) was left conducting. The MB activity was then visible in sinus rhythm. In this situation, the propagation was proximal to distal (solid arrows, Figure 3C), simulating the activation patterns of a single connection (Figure 2A). A total of 23 of 64 patients (36%) did not show distinctive MB potentials during sinus rhythm (Figure 3A), but apparent MB potentials were noted after catheter ablation of the MB–PV or LA junction (Figure 3C).
Antral ablation in most patients resulted in conduction block between distal LOM and the LA. However, in 12 of 23 patients (52%), the MB directly connected to the muscle sleeves of the left PVs. Direct ablation inside the PVs was needed to eliminate these distal connections and to achieve complete antral isolation. The sites of successful ablation within the PV were either at the origin of MB ectopy or at the site of earliest activation during MB pacing.
Multiple connections
In case of multiple MB–LA connections, the electrical activation patterns depend on the earliest MB–LA breakthrough sites and the number of MB–LA connections. In these patients, the electrical potentials from the MB were generally small and did not propagate in a uniform proximal-to-distal activation pattern as in a single connection (Figure 2A) or distal-to-proximal activation pattern during premature complex (Figure 3B). Rather, both central and peripheral sites could serve as an independent early site, and the earliest MB activation was not at either end of the MB (Figures 7A and 7B). Due to the presence of these additional connections, the interval between the local atrial electrograms and the MB may be either short or nonexistent. Therefore, distinct MB potentials were often difficult to identify during sinus rhythm. In patients with multiple connections, we might observe a premature MB activity breakthrough into the middle of the LA–LLR earlier than into either end of the LA. An example of the latter phenomenon is shown in Figure 7C, where the bold arrow points to the earliest LA–LLR activation. Due to conduction delay, there was a clear separation between MB and LA, shown in Figure 7C. A total of 30 of 64 patients (47%) had variable activation patterns in the MB during sinus rhythm. The electrical potentials from the MB were generally small due to the thin muscle bundles in this type, and the activation patterns were irregular and variable in each patient. During AF, rapid and complex fractionated activations of the MB were noted in all patients with multiple connections (Figure 6A). After isolation of the MB, 4 patients showed dissociated MB potentials, and 2 of 4 patients had isoproterenol (1 μg/min)-induced dissociated, organized MB tachycardia (Figure 6B). Due to complete MB–LA block, the MB tachycardia failed to trigger atrial arrhythmias.
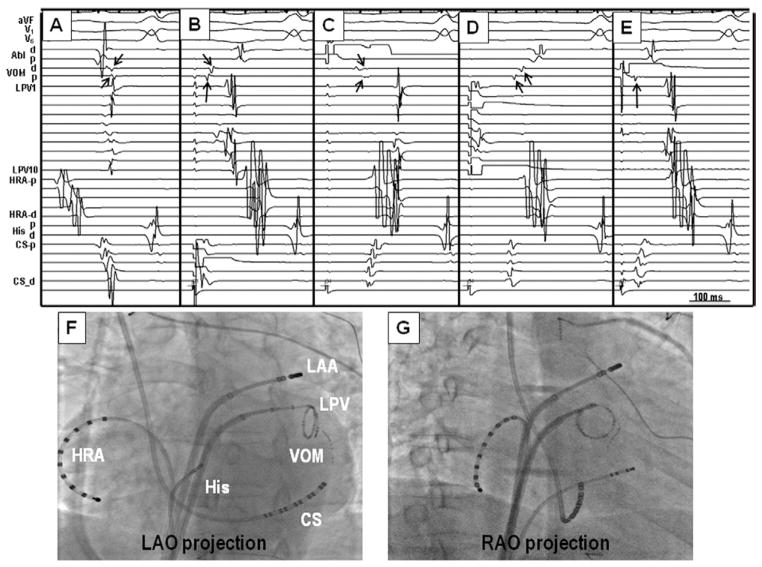
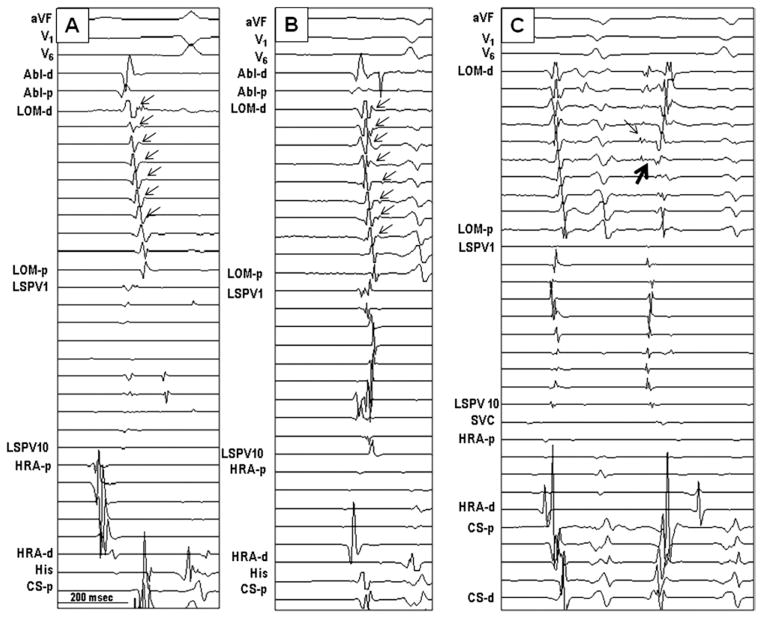
Figure 7.
Electrogram characteristics of multiple connections. A, B: MB recordings during sinus rhythm. The earliest breakthrough was in the middle of the MB. The MB potentials were typically small (arrows), and the activation sequence of the MB was irregular and did not show either a distal or a proximal activation pattern. C: During premature complex from the MB (thin arrow), the earliest atrial breakthrough site (thick arrow) was not at either end but was in the middle. SVC = superior vena cava; other abbreviations as in Figure 3.
Patient characteristics and types of MB connection
The patient characteristics and the results of the study are summarized in Table 1. The baseline LA volume and LVEF were not significantly different among connection types. The length of the MB in the single-connection type was the shortest (P = .000). There was no significant difference in MB length between double-connection and multiple-connection groups. The MB pacing threshold was significantly lower in the single-connection type than the other groups (P = .009). The procedure time and ablation time were the longest in multiple-connection type (P < .05), but total fluoroscopic times were similar in these 3 types of connections. Epicardial ablation12 to achieve complete MB isolation was done in 2, 2, and 4 patients, respectively, in single-, double-, and multiple-connection types.
Table 1.
Patient characteristics and types of MB connection
| Proximal (n = 11) | Double (n = 23) | Multiple (n = 30) | P value | |
|---|---|---|---|---|
| Age (yrs) | 61.1 ± 6.31 | 58.0± 9.0 | 59.3± 10.7 | .67 |
| Sex (male:female) | 11:0 | 16:7 | 25:5 | .10 |
| Left atrial volume at baseline (ml) | 143.6± 14.1 | 152.3± 24.8 | 149.9± 17.0 | .49 |
| Baseline left ventricular ejection fraction (%) | 47.0± 6.9 | 46.5± 6.2 | 46.0± 6.7 | .90 |
| Marshall bundle length (mm) | 22.0± 2.4 | 32.0± 4.0 | 30.8± 3.6 | .000 |
| Marshall bundle pacing threshold (mA) | 0.29± 0.4 | 0.67± 0.5 | 1.06± 0.8 | .009 |
| Procedure time (min) | 221.8± 28.2 | 240.0± 29.7 | 253.3± 21.9 | .004 |
| Fluoroscopic time (min) | 49.6± 9.7 | 53.2± 10.8 | 52.6± 11.7 | .66 |
| Ablation time (min) | 48.2± 6.2 | 46.3± 8.4 | 52.1± 8.8 | .04 |
| Epicardial ablation (n) | 2 | 2 | 4 | .72 |
Discussion
The major findings of this study are as follows: 1) The MB electrical activity can be divided into 3 basic patterns based on the number of connections between MB and other LA structures such as LA–LLR, left PV, or CS. 2) In addition to serving as a trigger to paroxysmal AF, MB may serve as a bypass tract between CS and left PVs when more than 1 connection is present. 3) MB with multiple LA connections show complex and fractionated electrograms, whereas MB with a single connection usually shows slower and more regular activation patterns during AF.
Role of the MB activity during sustained AF Single connection
The electrical activity of the MB during AF in the single-connection type was much slower and more organized than in other 2 types of connections. Because the MB only connects to CS muscle sleeves, the communication between the LA and MB was indirect. It is possible that delayed conduction with the CS muscle sleeves has prevented some of the AF wavefronts from reaching the MB. Therefore, the MB activation is slow. Although MB of this type may initiate AF through ectopic activity,9 the slow activation during persistent AF suggests that it may not be a major contributor to AF maintenance.
Double connections
In this type, the MB may serve as a bypass tract that connects the CS to the left PVs without any LA involvement. This connection might provide a substrate for macro–re-entry. The rapid electrical interaction between the left PVs and the MB has been shown to participate in re-entry during electrically induced AF in canine models.17 An important clinical implication of this finding is that the PV–MB connection provides an epicardial conduit between PV and LA through the CS muscle sleeves. If this epicardial conduit (accessory pathway) is not eliminated, electrical stimulation within the PV would be followed by LA activation. A clinical electrophysiologist performing AF ablations might interpret these findings as failed PV isolation. We have always pursued these connections to ensure that there is no PV–LA connection at the end of ablation. However, whether or not eliminating this connection helped to prevent AF recurrence remains unclear.
Multiple connections
Experimental studies showed that the dominant source of AF maintenance may be the rotors that generate high-frequency spiraling waves and create spatially distributed frequency gradients.18–20 Clinical studies demonstrated the existence of a hierarchical distribution of the rate of activation in the different regions during AF.21,22 Ablation of the highest-frequency site was associated with slowing and termination of AF.22 Thus, a very rapid focus producing fibrillatory conduction may be a driver of AF, and these drivers are often the targets of the catheter ablation.23,24 Animal and human studies have shown that the MB had the highest dominant frequency during sustained AF.10,11 The complex pattern of the electroanatomical connections between the MB and the LA can provide a substrate for re-entry, leading to complex and fragmented MB activities. After isolating the MB from LA, localized MB tachycardia could be induced by sympathetic stimulation while the atria remain in sinus rhythm. These findings document that MB is capable of independent rapid activation, and is not always activated passively by the neighboring AF wavefronts.
Technical considerations
In most human hearts, the MB is <3 mm from the endocardial surface of the LLR. The MB around the VOM increases in density at the junction between LOM and CS.2 Hwang et al12 showed that the shortest distance from the LA to the MB was located at the posterolateral region of the LA, just under the left inferior PV ostium. These findings suggest that endocardial catheter ablation at those locations is feasible in eliminating MB–LA connections. However, in some patients, the MB–LA and MB-PV connections may be located at sites that are out of reach of the endocardial radiofrequency energy application. In those patients, an epicardial approach is needed to complete the ablation.
Study limitations
We used 2 different catheters in each endocardial and epicardial mapping. We have moved the Cardima (quadripolar) catheter up and down inside the VOM, which allowed us to define the connection types. However, because 2 different catheters were used, and not all patients have data from both catheters, there might be a discrepancy in determining the connection types between these 2 mapping techniques.
The epicardial fat pad over the LLR accompanied by a high pacing threshold prevents consistent MB selective capture. Accordingly, we verify the MB signals using differential pacing from the adjacent sites. This might have some limitations to verify the epicardial MB potentials.
Conclusion
We documented 3 different types of MB–LA connections. During AF, rapid and fractionated activations were most commonly observed in the MB that had multiple MB–LA connections.
Acknowledgments
The authors thank Stephanie Plummer for her assistance.
This study was supported by the National Institutes of Health; National Heart, Lung, and Blood Institute grant number P01 HL78931, R01 HL78932, 71140; a Korean Ministry of Information and Communication Through Research and Development Support Project (Dr. Joung); and a Medtronic-Zipes Endowment (Dr. Chen). This manuscript was processed by a Guest Editor.
ABBREVIATIONS
- AF
atrial fibrillation
- CS
coronary sinus
- LA
left atrium/atrial
- LLR
left lateral ridge
- LOM
ligament of Marshall
- LVEF
left ventricular ejection fraction
- MB
Marshall bundle
- PV
pulmonary vein
- VOM
vein of Marshall
References
- 1.Marshall J. On the development of the great anterior veins in man and mammalia; including an account of certain remnants of foetal structure found in the adult, a comparative view of these great veins in the different mammalia, and an analysis of their occasional peculiarities in the human subject. Philos Trans R Soc Lond B Biol Sci. 1850;140:133–170. [Google Scholar]
- 2.Cabrera JA, Ho SY, Climent V, et al. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J. 2008;29:356–362. doi: 10.1093/eurheartj/ehm606. [DOI] [PubMed] [Google Scholar]
- 3.Kim DT, Lai AC, Hwang C, et al. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol. 2000;36:1324–1327. doi: 10.1016/s0735-1097(00)00819-6. [DOI] [PubMed] [Google Scholar]
- 4.Makino M, Inoue S, Matsuyama TA, et al. Diverse myocardial extension and autonomic innervation on ligament of Marshall in humans. J Cardiovasc Electrophysiol. 2006;17:594–599. doi: 10.1111/j.1540-8167.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 5.Scherlag BJ, Yeh BK, Robinson MJ. Inferior interatrial pathway in the dog. Circ Res. 1972;31:18–35. doi: 10.1161/01.res.31.1.18. [DOI] [PubMed] [Google Scholar]
- 6.Omichi CCC, Lee MH, Chang CM, Lai A, Chen PS. Demonstration of electrical and anatomic connections between Marshall bundles and left atrium in dogs: implications on the generation of P waves on surface electrocardiogram. J Cardiovasc Electrophysiol. 2002;13:1283–1291. doi: 10.1046/j.1540-8167.2002.01283.x. [DOI] [PubMed] [Google Scholar]
- 7.Doshi RN, Wu TJ, Yashima M, et al. Relation between ligament of Marshall and adrenergic atrial tachyarrhythmia. Circulation. 1999;100:876–883. doi: 10.1161/01.cir.100.8.876. [DOI] [PubMed] [Google Scholar]
- 8.Hwang C, Karagueuzian HS, Chen PS. Idiopathic paroxysmal atrial fibrillation induced by a focal discharge mechanism in the left superior pulmonary vein: possible roles of the ligament of Marshall. J Cardiovasc Electrophysiol. 1999;10:636–648. doi: 10.1111/j.1540-8167.1999.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 9.Hwang C, Wu T-J, Doshi RN, et al. Vein of Marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation. 2000;101:1503–1505. doi: 10.1161/01.cir.101.13.1503. [DOI] [PubMed] [Google Scholar]
- 10.Kamanu S, Tan AY, Peter CT, et al. Vein of Marshall activity during sustained atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:839–846. doi: 10.1111/j.1540-8167.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 11.Wu TJ, Ong JJ, Chang CM, et al. Pulmonary veins and ligament of Marshall as sources of rapid activations in a canine model of sustained atrial fibrillation. Circulation. 2001;103:1157–1163. doi: 10.1161/01.cir.103.8.1157. [DOI] [PubMed] [Google Scholar]
- 12.Hwang C, Fishbein MC, Chen PS. How and when to ablate the ligament of Marshall. Heart Rhythm. 2006;3:1505–1507. doi: 10.1016/j.hrthm.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Pak HN, Hwang C, Lim HE, et al. Hybrid epicardial and endocardial ablation of persistent or permanent atrial fibrillation: a new approach for difficult cases. J Cardiovasc Electrophysiol. 2007;18:917–923. doi: 10.1111/j.1540-8167.2007.00882.x. [DOI] [PubMed] [Google Scholar]
- 14.Sosa E, Scanavacca M, d’Avila A, et al. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 15.Corcoran SJ, Lawrence C, McGuire MA. The valve of Vieussens: an important cause of difficulty in advancing catheters into the cardiac veins. J Cardiovasc Electrophysiol. 1999;10:804–808. doi: 10.1111/j.1540-8167.1999.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 16.Feld GK, Fleck RP, Chen PS, et al. Radiofrequency catheter ablation for the treatment of human type 1 atrial flutter. Identification of a critical zone in the reentrant circuit by endocardial mapping techniques. Circulation. 1992;86:1233–1240. doi: 10.1161/01.cir.86.4.1233. [DOI] [PubMed] [Google Scholar]
- 17.Tan AY, Chou CC, Zhou S, et al. Electrical connections between left superior pulmonary vein, left atrium, and ligament of Marshall: implications for mechanisms of atrial fibrillation. Am J Physiol Heart Circ Physiol. 2006;290:H312–322. doi: 10.1152/ajpheart.00369.2005. [DOI] [PubMed] [Google Scholar]
- 18.Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res. 2002;54:204–216. doi: 10.1016/s0008-6363(02)00223-7. [DOI] [PubMed] [Google Scholar]
- 19.Mandapati R, Skanes A, Chen J, et al. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–199. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 20.Skanes AC, Mandapati R, Berenfeld O, et al. Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation. 1998;98:1236–1248. doi: 10.1161/01.cir.98.12.1236. [DOI] [PubMed] [Google Scholar]
- 21.Sahadevan J, Ryu K, Peltz L, et al. Epicardial mapping of chronic atrial fibrillation in patients: preliminary observations. Circulation. 2004;110:3293–3299. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 22.Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 23.Oral H, Chugh A, Good E, et al. A tailored approach to catheter ablation of paroxysmal atrial fibrillation. Circulation. 2006;113:1824–1831. doi: 10.1161/CIRCULATIONAHA.105.601898. [DOI] [PubMed] [Google Scholar]
- 24.Waldo AL. Mechanisms of atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14:S267–274. doi: 10.1046/j.1540-8167.2003.90401.x. [DOI] [PubMed] [Google Scholar]