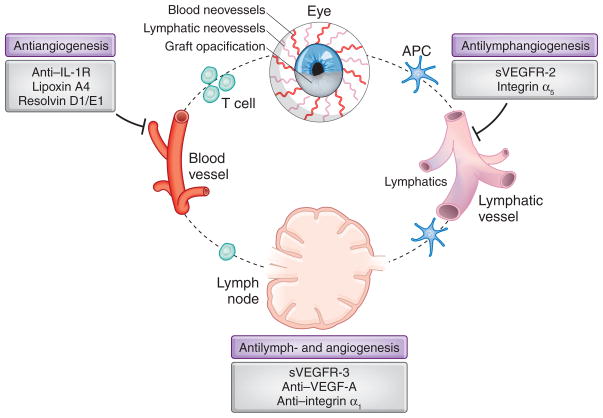
The cornea is one of the few avascular tissues. When blood or lymphatic vessels invade the cornea, usually after injury or infection, the tissue loses its transparency and vision weakens, accounting for the second most common cause of blindness worldwide. Corneal transplants can return sight, but they often fail when neovascularization takes place and causes them to succumb rapidly to immune rejection. The growth of new lymphatic vessels, or lymphangiogenesis, is crucial in the induction of immune response responsible for transplant rejection, because lymphatic vessels transport antigen-presenting cells from the eye to the regional lymphoid tissues where they initiate T cell response (Fig. 1). It is necessary, therefore, to maintain an avascular cornea, but little is known about how lymphangiogenesis is kept in check.
Figure 1.
Function of lymphatic and blood vessels in adaptive immunity and corneal transplant rejection. Lymphatics transport antigens and antigen-presenting cells (APCs) from the graft site to the draining lymph nodes, where the T cell response is primed and expanded. Effector (alloreactive) T helper 1 cells return to the cornea and mediate transplant rejection by primarily generating a delayed hypersensitivity response. Blocking lymphangiogenesis or angiogenesis can promote graft longevity by suppressing egress of APCs to the lymph nodes and by preventing homing of T cells to the transplant, respectively. Albuquerque et al.1 identify a factor that inhibits lymphangiogenesis that has potential as a therapeutic target.
In this issue of Nature Medicine, Albuquerque et al.1 identify an endogenous protein that specifically inhibits lymphangiogenesis, a secreted splice variant of VEGFR-2 (sVEGFR-2). The discovery provides insight into the mechanism by which avascular tissues such as cornea, brain and cartilage remain free of lymphatic vessels. It also furnishes a unique molecular tool for stopping aberrant lymphangiogenesis and for assessing the distinct contributions of the lymphatic vasculature to various pathologies.
Blood vessels and lymphatic vessels often run in parallel because they complement each other’s functions in regulating tissue fluid and protein balance and immune cell trafficking2. Members of the vascular endothelial growth factor (VEGF) family regulate growth of both lymphatic and blood vasculatures, making it difficult to decipher the mechanisms that selectively control lymphangiogenesis, particularly when both processes occur at the same time. Moreover, most of the molecules signaling for lymphangiogenesis have dual roles.
Much of the work has focused on lymphangiogenic factors VEGF-C and VEGF-D2, which also induce angiogenesis, and on recently developed inhibitors of lymphangiogenesis that are mostly antagonists of VEGF-C and its receptor. Several natural antiangiogenic factors also block lymphangiogenesis. In the cornea, a soluble splice variant of VEGF receptor-1 (VEGFR-1), which traps VEGF-A, inhibits corneal angiogenesis only3, whereas soluble VEGFR-3 receptor can suppress both lymphangiogenesis and angiogenesis4. But a selective endogenous inhibitor of lymphangiogenesis has not yet been described.
While studying the function of an antiangiogenic factor in the cornea3, Albuquerque et al.1 made a serendipitous discovery of a truncated splice variant of VEGFR-2 that localized in the corneal epithelium. The authors observed lymphatic vessels in the corneas of sVEGFR-2–deficient mice at birth1. In the adult mice, lack of sVEGFR-2 resulted in more lymphangiogenesis in corneas after injury. Surprisingly, these corneas were not invaded by blood vessels. One reason for this specificity is that only dimeric VEGFR-2 can bind VEGF-A and block angiogenesis5, whereas sVEGFR-2 is a monomer in the cornea. The authors proposed that sVEGFR-2 prevents lymphatic growth in the cornea because it is a selective VEGF-C antagonist1.
The absence of lymphatic vessels in the cornea grants the tissue a uniquely immune-privileged status; it prevents traffic of antigen-presenting cells into the lymph nodes and induction of immune response, and so it prevents graft rejection6. Inflammation, however, can induce lymphangiogenesis and lead to the loss of this immune privilege, rendering corneal allografts much more susceptible to rejection.
Because blocking lymphangiogenesis can prevent graft rejection, Albuquerque et al.1 tested the effects of sVEGFR-2 in corneal transplantation in mice. They found that administering sVEGFR-2 into the cornea inhibited lymphatic vessel formation and doubled transplant survival rate, and they ascribed this effect to the blockade of lymphangiogenesis1.
The authors acknowledge that sVEGFR-2 could promote allograft survival also by inhibiting dendritic cell trafficking to the lymph nodes7. Nevertheless, their study is in line with others that found lymphangiogenesis and VEGF-C to be important in transplant immunobiology7–9. Notably, chronic rejection of human kidney transplants is also associated with lymphangiogenesis9. The above studies provide a basis for evaluating the effects of sVEGFR-2 and other inhibitors of lymphangiogenesis on prolonging the life of various organ transplants.
Outside of the eye, the authors found sVEGFR-2 expression to be particularly abundant in the epidermis1. Removing sVEGFR-2 in the mouse epidermis caused lymphatic vessel hyperplasia, further arguing that sVEGFR-2 antagonizes VEGF-C. Albuquerque et al.1 also report that endothelial cells from blood vessels secrete sVEGFR-2. sVEGFR-2 might, speculatively, direct the proper separation of blood vessels from lymphatic vessels.
The identification of sVEGFR-2 as an endogenous, specific inhibitor of lymphangiogenesis is a major contribution to the field and complements research efforts by other labs to decouple blood and lymphatic vessel growth. This work, however, may also spark controversy, and additional studies will be required to confirm the selectivity of sVEGFR-2 for lymphangiogenesis. It is surprising that the authors did not report any effects of sVEGFR-2 on angiogenesis, because VEGF-C is a potent chemotactic factor for macrophages10, which are a rich source of angiogenic factors. Macrophages infiltrate the cornea during inflammation6,11, attracted by VEGF-C itself, and so blocking of VEGF-C with sVEGF-2 would be expected to suppress corneal angiogenesis at least to a certain degree.
The work by Albuquerque et al.1 could make a substantial contribution to the burgeoning field of lymphangiogenesis in various diseases. In cancer, the growth of lymphatic vessels can promote metastasis2,12, but whether endogenous inhibitors of lymphangiogenesis could affect tumor progression is unknown. It will be exciting to see whether sVEGFR-2 is expressed in tumors. The finding that sVEGFR-2 inhibits lymphangioma proliferation in vitro1 raises the possibility of its use in treatment of lymphangioma and other lymphatic vascular malformations. The discovery of sVEGFR-2 may even offer a fresh perspective for looking at the underlying cause of lymphedema, in which an endogenous inhibitor could hamper lymphatic regeneration. Finally, we are just beginning to learn about the interface of the lymphangiogenesis and adaptive immune responses. These research efforts have potential implications for modulation of antitumor immunity in addition to the allorejection immunity described above.
But what about the eye? This new tool could be used therapeutically to improve survival of corneal transplants, and it may prove useful for treatment of many immune-mediated destructive corneal diseases characterized by aberrant lymphangiogenesis. Let’s see!
Contributor Information
Mihaela Skobe, Email: mihaela.skobe@mssm.edu, Department of Oncological Sciences, Mount Sinai School of Medicine, New York, New York, USA.
Reza Dana, Schepens Eye Research Institute and Massachusetts Eye and Ear, Harvard Medical School Department of Ophthalmology, Boston Massachusetts, USA.
References
- 1.Albuquerque RJC, et al. Nat Med. 2009;15:1023–1030. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alitalo K, Tammela T, Petrova TV. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 3.Ambati BK, et al. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cursiefen C, et al. Proc Natl Acad Sci USA. 2006;103:11405–11410. doi: 10.1073/pnas.0506112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuh G, Li B, Crowley C, Cunningham B, Wells JA. J Biol Chem. 1998;273:11197–11204. doi: 10.1074/jbc.273.18.11197. [DOI] [PubMed] [Google Scholar]
- 6.Patel SP, Dana R. Semin Ophthalmol. 2009;24:135–138. doi: 10.1080/08820530902801320. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, et al. Nat Med. 2004;10:813–815. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 8.Cursiefen C, et al. Invest Ophthalmol Vis Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 9.Kerjaschki D, et al. Nat Med. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 10.Skobe M, et al. Am J Pathol. 2001;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maruyama K, et al. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skobe M, et al. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]