Abstract
Cellular metal ion fluxes are known in the case of alkali and alkaline earth metals but not well documented for transition metals. Here, we describe major changes in the zinc physiology of the mammalian oocyte as it matures and initiates embryonic development. Single-cell elemental analysis of mouse oocytes by synchrotron-based x-ray fluorescence microscopy (XFM) revealed a 50% increase in total zinc content within the 12-14 hour period of meiotic maturation. Perturbation of zinc homeostasis with a cell-permeable small molecule chelator blocked meiotic progression past telophase I. Zinc supplementation rescued this phenotype when administered prior to this meiotic block. However, following telophase arrest, zinc triggered parthenogenesis, suggesting that exit from this meiotic step is tightly regulated by the availability of a zinc-dependent signal. These results implicate the zinc bolus acquired during meiotic maturation as an important part of the maternal legacy to the embryo.
Introduction
Iron, copper, and zinc are three of the most abundant transition metals in biology and are essential components of most cells1-3. They serve many well-established roles as structural and catalytic components of a myriad of proteins and may also have intracellular signaling functions4,5 akin to the alkaline earth metal calcium. The intracellular availability of these transition metals is tightly regulated6-8, as both metal deficiency and excess are potentially toxic to a cell9. Accordingly, there is a growing body of evidence indicating that cells maintain total concentrations of these transiton metal ions within a narrow conserved range, which is defined as the resting or minimal “metal quota”6,10,11. Bulk analytical methods such as inductively coupled plasma mass spectrometry (ICP-MS) can be used to monitor concentration changes in samples available in large quantities, such as cells in culture10. However, cells such as mammalian oocytes are rare and there are few quantitative approaches appropriate for single cell analysis, making it difficult to establish the concentration of essential metals and their functions at key points in development.
Though unique in its singular ability to give rise to an entirely new organism, the oocyte shares many of the signaling mechanisms and nutritional needs driving somatic cells. To date, transition metal physiology within the oocyte has been studied exclusively using non-mammalian model systems where the cell is larger and easily isolated in significant quantities, such as Xenopus laevis and Caenorhabditis elegans12-14. Zinc is accumulated during oocyte growth in these systems and is thought to be stored in lipoproteins in preparation for later stages such as embryonic development15,16. Additionally, zinc-dependent kinases have been implicated in the control of cell cycle progression in maturing X. laevis oocytes14. While many similarities exist between non-mammalian and mammalian oocytes, a crucial biological difference lies in the fact that X. laevis and C. elegans oocytes must prepare for embryonic development completely outside of the maternal environment. This likely creates different demands in elemental composition than mammalian oocytes, which develop entirely in the female reproductive tract. For this reason, a murine model system is an important tool for understanding the roles of transition metals in the oocyte as it prepares for fertilization and transformation into a developmentally competent mammalian embryo.
The bulk of the embryo's cytoplasm originates from the oocyte. In fact, it is the oocyte that provides the necessary components to support development (such as mRNA and proteins) until the embryo's own genome is activated and it is able to sustain its own growth17. Therefore, the fate of the embryo relies heavily on the integrity of its oocyte predecessor18,19. An understanding of the biological processes that create a “good egg” in vitro is important to the fertility management options for young cancer patients where the culture of immature follicles and oocytes from frozen and fresh ovarian tissue is becoming a viable option20.
The oocyte undergoes a remarkable array of developmental changes immediately before and just after fertilization. Fully-grown oocytes display an intact nucleus (or germinal vesicle, GV) and are maintained in prophase I arrest until they are recruited for ovulation. This arrest can last weeks to months in rodents and months to decades in humans21, and is relieved in oocytes that are ‘selected’ for ovulation during each hormonal cycle upon a surge in luteinizing hormone (LH). The short time between the LH surge and ovulation (approximately 12-14 hours) is referred to as meiotic maturation, since the oocyte progresses through the bulk of meiosis. During meiotic maturation, the nuclear envelope breaks down (germinal vesicle breakdown, or GVBD) and the oocyte proceeds from meiosis I to meiosis II without an intervening interphase. The maturing oocyte also undergoes cytoplasmic modifications in preparation for early developmental events that occur upon fertilization, such as sperm nuclear decondensation22 and calcium oscillations23. The end of meiotic maturation is marked by the establishment of a second meiotic arrest at metaphase II (MII)24, at which point this single cell is now called an egg.
To determine the transition metal content of maturing mouse oocytes and early embryos, we have exploited synchrotron-based x-ray fluorescence microscopy (XFM) for single cell analysis. We show that the level of total zinc increases significantly during meiotic maturation, by an order of magnitude more than iron or copper. Furthermore, perturbation of intracellular transition metal homeostasis with a small molecule chelator (TPEN) during in vitro oocyte maturation led to a block in meiosis following telophase I. Zinc supplementation prior to the establishment of this meiotic block restored normal nuclear maturation to metaphase II. However, supplementation following the meiotic block triggered parthenogenetic activation of the oocyte. This phenomenon suggested that exit from meiosis is tightly regulated by a novel zinc-dependent signal in the oocyte. TPEN-treated oocytes (referred to here as “zinc-insufficient”) were able to undergo a true fertilization event, but the developmental potential of the resulting embryo was severely compromised. Thus, profound changes in the oocyte's zinc content over an extremely short period of time are required for normal progression of meiosis and proper cytoplasmic maturation in the development of a healthy mouse embryo.
Results
Single-cell elemental analysis of mouse oocytes and embryos
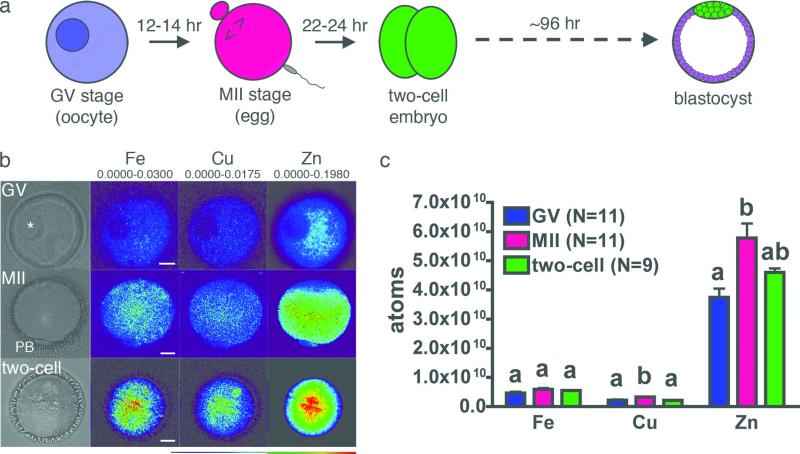
Fully-grown but immature mouse oocytes display an intact germinal vesicle (GV). They proceed through the maturation period described above and establish metaphase II (MII) arrest before undergoing fertilization and preimplantation embryo development (Fig. 1a). The subcellular distribution of the elements in individual mouse oocytes and embryos was determined using synchrotron-based x-ray fluorescence microscopy (XFM), performed at Beamline 2-ID-E of the Advanced Photon Source (Argonne National Laboratory, Argonne, IL). XFM provides the total elemental content of a sample through detection of x-ray emission spectra that are unique to each element. Emission data was integrated along the z-axis so that distributions are presented as two-dimensional images with total metal content presented in units of μg/cm2 after conversion using NIST calibration standards (representative data are shown in Fig. 1b).
Figure 1. Synchrotron-based x-ray fluorescence microscopy reveals intracellular distribution of the transition elements in oocytes and early embryo.
Fully-grown oocytes, eggs, and two-cell embryos (a) were prepared as whole mount samples for synchrotron-based xray fluorescence microscopy. Oocytes (N = 11) display an intact germinal vesicle (GV, asterisk) while mature (MII) eggs (N = 11) have a visible first polar body (PB). Two-cell embryos (N = 9) were obtained by in vitro fertilization. Representative brightfield images for each stage are shown, in addition to the elemental maps for iron, copper and zinc (b). The minimum and maximum elemental content (μg/cm2) are shown above each set of elements. Among the biologically relevant transition elements, zinc is an order of magnitude more abundant than iron and copper at all developmental stages (c). Scale bar = 20 μm. Data represent mean values ± s.e. Letters denote statistically significant differences between developmental stages for individual elements (p<0.05).
The total number of atoms per cell at each developmental stage was calculated from the integrated data (Fig. 1c). The number of iron atoms did not change significantly between the three stages examined. Copper and zinc both rose significantly during meiotic maturation but decreased between the egg and two-cell embryo stages. Zinc was the most abundant of these three transition metals by an order of magnitude (Supplementary Table 1) and experienced a dynamic change between each of the developmental stages studied. While approximately 2 × 1010 atoms of zinc were acquired per oocyte over the 12-14 hours required for progression from the GV to the MII stage, some of this bolus (approximately 1 × 1010 atoms) was lost between the MII egg and the two-cell embryo. The fact that the total zinc quota is nearly an order of magnitude higher than that of iron or copper and that the oocyte actively modifies the zinc quota at key stages in development implicated a potentially important role for this transition metal in the steps leading from fully-grown oocyte to nascent embryo.
Perturbation of intracellular zinc disrupts asymmetric division
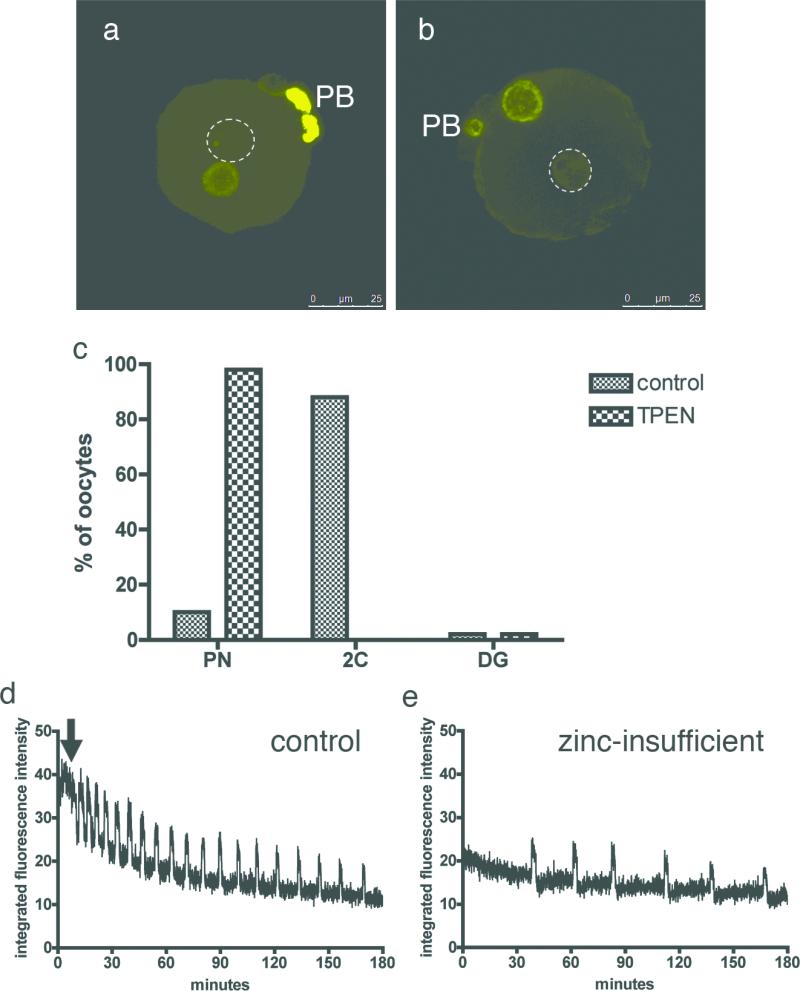
The significant increase in total zinc levels that occurs during meiotic maturation suggested that perturbation of its intracellular availability would affect the normal course of development. The heavy metal chelator N,N,N’,N’-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN) or other chelators shown in Supplementary Scheme 1 were added to the medium during in vitro oocyte maturation (IVM) to directly test this hypothesis. TPEN exhibits high affinity for transition metals such as iron, zinc and copper but low affinity for other metals such as magnesium and calcium25, and is capable of sequestering kinetically accessible pools of these metals within cells. Oocytes were treated with increasing doses of TPEN. Concentrations lower than 10 μM did not have any effect on meiotic maturation as evidenced by polar body extrusion, whereas concentrations of 20 μM or higher resulted in oocytes that failed to progress through meiosis (Supplementary Fig. 1). Oocytes treated with 10 μM TPEN were phenotypically distinct from the oocytes from either the low concentration or high concentration groups. Following IVM in the presence or absence of TPEN, more than 75% of oocytes in both groups emitted the first polar body (PB) (Supplementary Table 2). The remaining cells treated with vehicle or 10μM TPEN did not enter meiosis [as evidenced by lack of germinal vesicle breakdown (GVBD)] or were degenerate (DG). To test whether maturing oocytes could be deprived of zinc in an alternate manner, the GV oocytes were cultured in a zinc-reduced maturation medium produced via treatment with Chelex resin. The lowest achievable zinc level using the methods of Suhy et al.26 was 80 nM, and this concentration was still sufficient to support normal oocyte maturation (Supplemental Fig. 2). This protocol reduces total zinc in the medium from 4 μM to 80 nM; however, the latter concentration corresponds to an excess of 4 × 1013 zinc atoms in this culture experiment (800 μl medium containing 30 oocytes). This robust response underscores the efficiency of high affinity zinc uptake systems in the oocyte. Consequently, the stage-specific effects of 10 uM TPEN were investigated in greater detail.
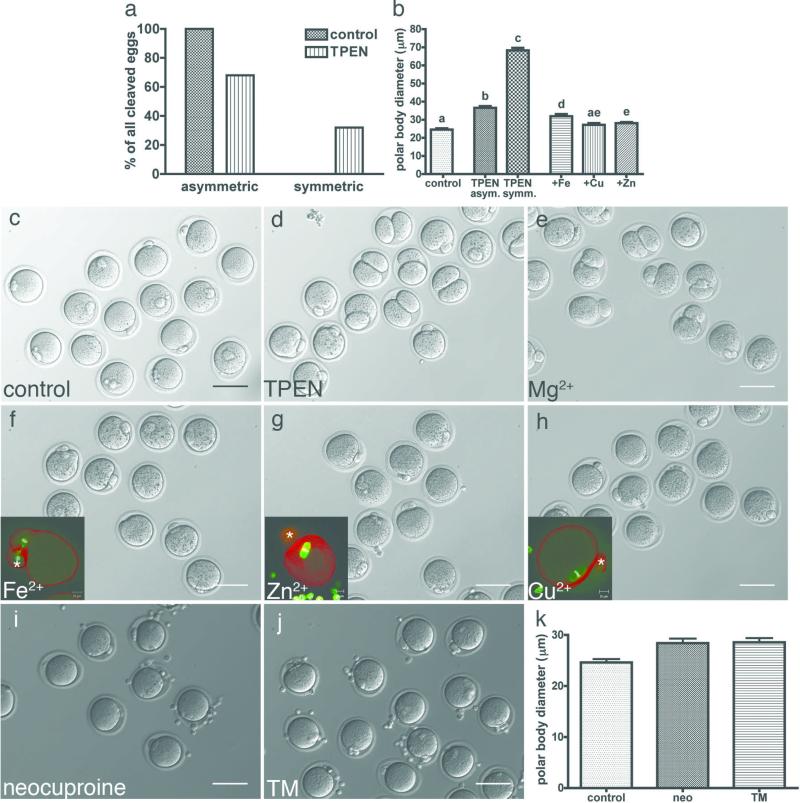
Although polar body emission occurred at a similar frequency with or without TPEN in the IVM medium, polar body size was strikingly different between the two groups. Of all eggs that extruded a polar body, 100% of the control group underwent an asymmetric division, producing a large egg and a small polar body (Fig. 2a). In contrast, only 68% of the TPEN-treated group divided asymmetrically while the remaining 32% divided symmetrically. Measurement of the polar body diameter at its widest point revealed that the polar bodies in the TPEN-treated group were significantly larger than those in the control group (Fig. 2b). Representative images of control and TPEN-treated oocytes are shown in Figs. 2c and 2d, respectively.
Figure 2. The heavy metal chelator TPEN disrupts asymmetric division of the oocyte, which can be rescued by exogenous zinc.
Among those oocytes that extruded a polar body, 100% of control oocytes (N = 62) displayed asymmetric division (a). Only 68% of TPEN-treated oocytes (N = 65) displayed asymmetric division, while the remaining 32% were symmetrically divided. Measurement of the polar body diameter at its widest point showed that TPEN-treated oocytes had significantly larger polar bodies than control oocytes (b). Representative images of control (c) and TPEN-treated (d) oocytes are shown. Exogenous sources of magnesium (e), iron (f), zinc (g) and copper (h) were added directly to the chelator-containing medium after an initial maturation period of 4 hrs. Magnesium was included as a negative control as TPEN has low affinity for this metal. Copper (N = 34) and zinc (N = 71) supplementation restored polar body diameter more effectively than iron (N = 38) (b). Copper chelators neocuproine (i) and tetrathiomolybdate (TM, j) did not disrupt asymmetric division and polar body size remained indistinguishable from the control group (k). Scale bar = 80 μm. Data represent mean values ± s.e. Letters denote statistically significant differences in polar body diameter in b (p<0.05).
The observation that zinc was the most abundant transition metal to undergo significant changes during meiotic maturation suggested that the phenotypes caused by TPEN might be specifically due to interference with zinc homeostasis. Therefore, a series of rescue experiments were performed to determine which metals could overcome the effect of TPEN and permit progression to normal meiotic arrest at MII. Oocytes were incubated in maturation medium containing 10 μM TPEN for four hours to ensure full penetration of the chelator into the intracellular space. The chelator-containing media was then supplemented with exogenous sources of magnesium (Fig. 2e), iron (Fig. 2f), zinc (Fig. 2g) or copper (Fig. 2h). Magnesium was included as a negative control because TPEN has low affinity for Mg(II)27 and therefore was not expected to rescue the oocyte phenotype. All three transition metals reduced polar body size to levels statistically different from TPEN-treated oocytes, copper and zinc more so than iron (Fig. 2b, p<0.01). However, we observed that the spindles in iron-rescued oocytes (inset in Fig. 2f) were strikingly different than those of copper- or zinc-rescued oocytes, which displayed a metaphase II spindle as would be expected of a normal, mature egg (insets in Fig. 2g and h, respectively). This confirmed that rescue with added copper or zinc, but not iron, could fully restore asymmetric division and normal spindle morphology.
Since TPEN preferentially binds Cu(II) (KD = 10-20) over Zn(II) (KD = 10-16)27, we addressed directly whether the effect of TPEN could actually be the result of disrupting intracellular copper homeostasis. Membrane-permeable, copper-selective chelators shown in Supplementary Scheme 1 were added to the IVM medium to ask directly whether they caused morphological phenotypes identical to TPEN. Neither neocuproine nor tetrathiomolybdate (Fig. 2i and j, respectively) had any effect on asymmetric division at the same concentration as TPEN (10 μM) or at a ten-fold higher concentration (data not shown). They also did not cause significant differences in polar body diameter when compared to the control group (Fig. 2k). Given that two independent copper-specific chelators could not cause the same phenotypes as TPEN, we concluded that copper was able to rescue by sequestering the chelator and preventing it from perturbing intracellular zinc levels.
Finally, oocytes matured in the absence (control) or presence of 10 μM TPEN were analyzed by XFM for their total zinc and copper content. Notably, while copper content is unchanged relative to control (Supplementary Fig. 3a), the oocyte's total zinc content is significantly lower in the TPEN-treated group than in the control group (Supplementary Fig. 3b, Supplementary Table 3). Intriguingly, TPEN-treated oocytes have the same zinc content as the GV stage oocytes, providing strong evidence that this treatment leads to a specific perturbation of zinc in the maturing oocyte. In light of these results, we modified our terminology to refer to TPEN-treated oocytes as “zinc-insufficient” oocytes.
Perturbation of intracellular zinc blocks exit from meiosis I
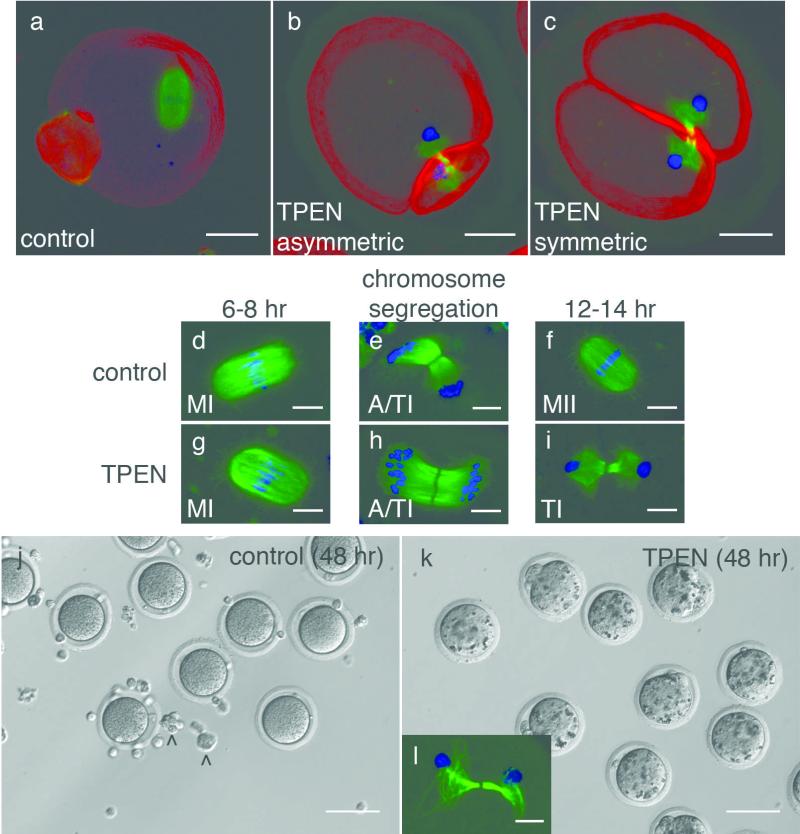
The large polar bodies observed in zinc-insufficient oocytes suggested that they might exhibit defects at the level of the meiotic spindle, which directs the plane of cleavage in the oocyte28,29. Therefore, spindle morphology was interrogated by probing the state of the chromatin and α-tubulin by immunofluorescence. Following IVM, control oocytes displayed a metaphase II spindle at the oocyte cortex (Fig. 3a); however, 100% of zinc-insufficient oocytes displayed a telophase spindle regardless of gross morphology. Immunohistochemical staining for F-actin revealed that cytokinesis was complete for both asymmetrically and symmetrically cleaved oocytes (Fig. 3b and 3c, respectively). DNA was separated to the two poles but we did not observe individual chromosomes, indicating that they had decondensed.
Figure 3. Zinc insufficient oocytes experience a meiotic block following telophase I.
Control oocytes display a metaphase II spindle (a), but zinc-insufficient oocytes have a telophase I-like spindle with decondensed chromatin whether they cleave asymmetrically (b) or symmetrically (c). Control oocytes proceed through metaphase I (MI, d), chromosome segregation (anaphase/telophase I, or A/TI as shown in e), and establish a meiotic arrest at metaphase II (f). Zinc-insufficient oocytes also proceed through MI (g) and individual chromosomes are segregated to two poles (A/TI shown in h). However, by the time control oocytes reach MII, zinc-insufficient oocytes retain a telophase I spindle with decondensed chromatin at each pole (i). Control oocytes remain morphologically unchanged after a total of 48 hrs in culture (j). Zinc-insufficient oocytes become increasingly granular with extended culture (k) and they retain a telophase-like spindle with decondensed chromatin, although the tubulin array becomes less compact over time (l). Carats (^) denote cumulus cells. Scale bar = 20 μm (a-c), 10 μm (d-i, l), or 80 μm (j, k).
The spindle morphologies of control and zinc-insufficient oocytes were further compared at key steps in meiotic maturation to determine the precise time when these defects occurred. Germinal vesicle breakdown (GVBD), as defined by the absence of a defined nucleus, proceeded normally whether TPEN was absent or present in the medium (data not shown). Control oocytes proceeded through metaphase I (MI) (Fig. 3d), segregated their chromosomes (Fig. 3e) and established meiotic arrest at metaphase II (MII) (Fig. 3f). Zinc-insufficient groups also proceeded through MI (Fig. 3g) and chromosome segregation (Fig. 3h), but did not proceed to MII. Instead, they retained a telophase spindle with decondensed chromosomes at each pole (Fig. 3i). The fact that we observed individual chromosomes being segregated in zinc-insufficient oocytes suggested that decondensation did not occur until the telophase spindle was established. To address whether TPEN simply caused a developmental delay, zinc-insufficient oocytes were cultured up to 48 hours in chelator-containing medium. These oocytes maintained their gross morphology, although their cytoplasm became increasingly granular compared to control oocytes cultured for the same duration (Fig. 3j, k). A telophase spindle with an intact midbody was maintained, although the tubulin array became more diffuse by this time (Fig. 3l).
Delayed rescue of zinc insufficiency triggers activation
Since zinc-insufficient oocytes proceeded through metaphase I in the expected timeframe (6-8 hrs) despite the presence of TPEN, we delayed zinc supplementation of the medium until 7, 8, 9 and 12 hrs post-maturation to determine when a full rescue of an MII spindle was no longer possible. Restoration of asymmetric division and an MII spindle was achieved by the addition of exogenous zinc as late as 9 hrs post-maturation (Supplementary Fig. 4a-d). The length and width of the spindle, however, increased significantly with delayed zinc supplementation (Supplementary Fig. 4e, f). This was also the trend observed with the effects of delayed rescue on polar body diameter (Supplementary Fig. 4g).
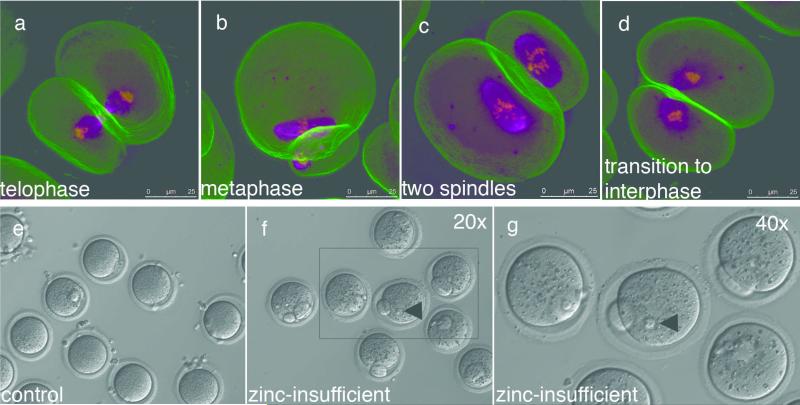
Zinc supplementation later than 10 hrs post-maturation was unable to restore polar body diameter, as a polar body had already been extruded by this time. Unlike earlier rescue time points, where a single metaphase II spindle was restored by the end of the 16 hr duration of IVM, we observed both telophase I and metaphase II spindles with late rescue (Fig. 4a, b). Strikingly, two metaphase spindles were observed in oocytes that had divided more symmetrically when compared to control oocytes (Fig. 4c); others displayed a diffuse spindle configuration resembling entry into interphase (Fig. 4d). The latter observation suggested a possible role for zinc in meiotic exit, so we investigated whether zinc-insufficient oocytes could be relieved of telophase-like arrest by transfer into a zinc-replete environment. Oocytes were matured for 16 hrs in the absence (control) or presence of TPEN, then washed and cultured further in chelator-free medium. Control oocytes did not change over this extended culture period (Fig. 4e). In contrast, 100% of zinc-insufficient oocytes (N = 62) formed pronuclear structures after 8 hrs (Fig. 4f, g), which persisted even after a total of 24 hrs in culture. 75% of the oocytes cleaved after a 48 hr culture period, as indicated by the presence of structures resembling two-cell embryos, but we did not observe more than two cleavage events even after 96 hrs in culture. The release from telophase I arrest was stimulated by the restoration of zinc and thus implicates a role for zinc in meiotic exit.
Figure 4. Zinc supplementation following telophase-like arrest induces spontaneous activation of the zinc-insufficient oocyte.
Zinc supplementation at 12 hrs post-maturation is too late to restore the metaphase II spindle and a mixture of spindle configurations are seen (ad). After a full 16 hr maturation period, control (e) and zinc-insufficient (f) oocytes were transferred into KSOM for further culture. Control oocytes do not change over the extended culture period in the absence of an activating stimulus. In contrast, all zinc-insufficient oocytes (N = 62) exhibited pronuclear structures after 8 hrs (arrowheads, f and g). Magenta = alpha-tubulin, yellow = DNA, green = F-actin. Scale bar = 25 μm (a-d) or 80 μm (e, f).
Fertilized zinc insufficient oocytes fail to form blastocysts
The presence of TPEN in the maturation medium causes the meiotic spindle to arrest in a telophase I by the time normal oocytes establish arrest at metaphase II. We investigated whether zinc-insufficient oocytes could be fertilized normally in spite of this phenotype and if so, how far they could develop as embryos. Control and zinc-insufficient oocytes were fertilized in vitro under identical conditions and embryonic development was monitored at pronuclear formation [8 hours post-fertilization (hpf)] and every 24 hrs thereafter up to blastocyst formation. Both control and zinc-insufficient oocytes form pronuclei by 8 hpf with comparable rates of fertilization (85% and 77%, respectively). Measurement of pronuclear diameters at their widest points confirmed that the small (maternal) and large (paternal) pronuclei had significantly different diameters in both control and zinc-insufficient groups (data not shown). Furthermore, it is known that the male pronucleus selectively undergoes demethylation during chromatin remodeling30,31. In all cases, we found that one of the two pronuclei had decreased 5-methylcytidine staining in both control (Fig. 5a, arrow) and zinc-insufficient oocytes (Fig. 5b, arrow), suggesting that a male genetic component was indeed integrated during IVF. However, while control embryos progressed to the two-cell stage by 24 hpf, embryos derived from zinc-insufficient oocytes remained at the pronucleus stage (Fig. 5c). By 48 hpf, a majority of the control group began to form four-cell embryos, but the zinc-insufficient group had only gone through the first round division. The control group reached the blastocyst stage by 120 hpf, but we never observed blastocyst formation among those embryos originating from zinc-insufficient oocytes.
Figure 5. Zinc-insufficient eggs can undergo a true fertilization event but display a delayed pronuclear stage and abnormal calcium oscillations upon activation.
Fertilized control (a) and zinc-insufficient (b) eggs were fixed 8 hpf for 5-methylcytidine staining. In both groups, one of the two pronuclei displayed an absence of signal (dotted circles), indicating demethylation and a male origin of the genetic material. However, embryos derived from zinc-insufficient oocytes experience a delayed pronuclear stage (c). At 24 hpf, when embryos from control eggs have proceeded to the two-cell stage, embryos from zinc-insufficient eggs still display two pronuclei. Upon activation, control eggs initiate a prolonged first calcium transient (d, arrow) followed by a regular series of shorter transients. Zinc-insufficient oocytes lack a large first transient and undergo a fewer total number of transients within the same imaging period. PB = polar body, scale bar = 25 μm.
The compromised quality of embryos derived from zinc-insufficient oocytes implied an important contribution of zinc homeostasis to oocyte quality. The earliest known event following sperm-egg fusion at fertilization are intracellular oscillations in calcium, which have been observed in some form in a wide variety of animals32. It is also believed that the total number and frequency of the calcium oscillations triggers different physiological responses in the oocyte including cortical granule exocytosis and pronuclear formation33,34. Therefore, we monitored the pattern of oscillations for three hours in the activated oocyte with the fluorophore Calcium Green-1 AM. Control oocytes displayed a prolonged first calcium transient (Fig. 5d, arrow) followed by shorter transients in a regular pattern. In contrast, all zinc-insufficient oocytes (Fig. 5e) lacked an extended initial transient and always displayed a fewer total number of transients than the controls within the same duration of imaging. This lower frequency of transients may account for the delay at the pronuclear stage seen in zinc-insufficient oocytes. Together, zinc-insufficient oocytes were capable of undergoing a true fertilization event despite their ability to parthenogenetically activate, but displayed calcium oscillations that were variant from normal oocytes. Altogether, our results (summarized in Figure 6) highlight the lasting effect of zinc insufficiency originating from oocyte development on embryo quality.
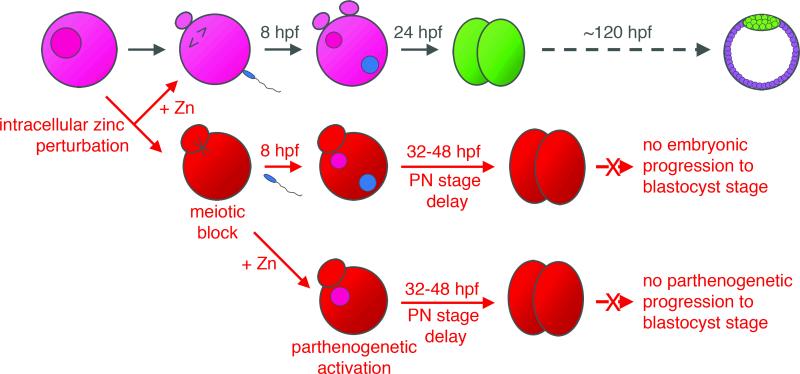
Figure 6. Summary of results.
Normal maturation of fully-grown oocytes begins with the progression of an immature, germinal vesicle (GV) stage oocyte to a mature, metaphase II (MII)-arrested oocyte. Fertilization triggers the completion of meiosis and the first mitotic division results in the two-cell embryo, which will in turn develop into a blastocyst. Induction of zinc insufficiency during meiotic maturation results in oocytes that are prematurely arrested in meiotic telophase. Fertilization of these oocytes results in embryos that experience an extended pronuclear stage and impaired viability. This can be ameliorated with zinc supplementation prior to the establishment of telophase arrest. Simply returning zinc-insufficient oocytes to a zinc-replete environment triggers parthenogenetic activation in the absence of any other stimulus, leading to approximately two rounds of division before the parthenotes are no longer viable.
Discussion
The brief period of development just prior to ovulation is a critical time for the oocyte, as it must proceed through a number of nuclear and cytoplasmic modifications in preparation for fertilization. The contribution of essential transition metals to oocyte maturation has yet to be determined, partially due to the lack of appropriate analytical methods for determining total elemental content and subcellular distributions for small numbers of cells. The oocyte is a unique cell, one of the largest in the body, which also houses most of the components to support the early stages of development of an entirely new organism. It is also a rare cell in mammalian systems; for example, in mice, only 8-12 oocytes are released during a natural ovulation35. Therefore, methods of elemental analysis that rely on large sample volumes are inappropriate and impractical for the determination of elemental content in this system. Here, using XFM for the determination of transition metal concentrations across oocyte development and early embryonic development, we discovered a unique feature of the oocyte's inorganic physiology: the oocyte's total zinc content is an order of magnitude larger than that of iron or copper. This represents a departure from the inorganic signatures of many cell types such as Escherichia coli, where zinc and iron content were similar and both are higher than copper10. Intriquingly, the zinc quota abruptly rises to still higher levels during the brief course of meiotic maturation and decreases again after fertilization. Several lines of evidence show that these extraordinary changes in the zinc quota are important for subsequent developmental steps and are consistent with a previously unrecognized role for zinc-dependent processes that regulate the exit of the oocyte from meiosis I.
The importance of this developmental flux in the intracellular abundance of zinc was established by lowering metal availability through controlled administration of TPEN, a high-affinity metal chelating agent. TPEN can sequester transition metals (but not alkaline earth metals such as calcium) both in the growth medium and, given the well established membrane permeability of the metal free form, it can also sequester intracellular pools of labile or kinetically accessible zinc36. Several phenotypes of TPEN treatment were observed, including enlarged polar bodies, an increased rate of symmetric division and telophase I arrest. Although supplementation with either copper or zinc reversed these phenotypes, XFM analysis showed that only the concentration of zinc was lowered by the presence of TPEN. This result suggests that the copper rescue phenotype was not the result of a TPEN alteration of copper-dependent processes in the oocyte. We propose instead that supplementation of exogeneous copper in the rescue experiments sequesters TPEN outside the cell and prevents it from perturbing zinc activity inside or outside the cell. The XFM analysis strongly corroborates this interpretation: the increase in total copper observed in the GV to MII transition also occurred in oocytes grown in the TPEN treated medium. Turning then to zinc, the XFM data also show that in TPEN-treated medium, the oocytes failed to accrue the significant zinc bolus observed for control oocytes and indicate that TPEN induces a zinc-insufficient state.
We consider three explanations for how 10 μM TPEN may disturb zinc physiology in these conditions: a) it may work after entering the cell by binding intracellular zinc and escorting it out of the cell; b) it may act inside the cell and directly effect zinc-dependent signaling homeostasis processes; or c) it may reduce zinc availability in the medium by complex ion formation and thus preventing zinc uptake. Given that the zinc-TPEN complex has a 2+ charge, it is unlikely that TPEN is passively removing zinc from the cell. The simplest explanation is that TPEN binds available zinc in the culture medium and that the complexed form of zinc is not a substrate for the oocyte's high-affinity zinc uptake systems. This would prevent zinc acquisition at a critical stage when a large bolus of this metal ion must be taken up over the 12-14 hour period of maturation; however, we underscore the fact that these results do not rule out intracellular sites of TPEN action.
The disruption of asymmetric division observed in zinc-insufficient oocytes suggested that the meiotic spindle might be influenced by intracellular zinc availability, since the meiotic spindle directs oocyte division28,29. Indeed, 100% of zinc-insufficient oocytes experienced an unusual meiotic block in which the spindle was in a telophase configuration. This was unique given that failed meiotic maturation in mouse oocytes is typically manifested as either retention of prophase I or arrest at metaphase I37-39. A full rescue of the meiotic spindle could be achieved by exogenous zinc supplementation as late as nine hours into maturation, corroborating our observation that nuclear maturation proceeds normally through metaphase I in spite of zinc insufficiency. However, zinc supplementation at 12 hours post-maturation was too late to restore metaphase II arrest and resulted in a variety of spindle phenotypes. Since meiotic block was already established by 12 hours post-maturation, supplementation at this time was too late to undo the developmental consequences of zinc insufficiency. Instead, the presence of interphase-like spindles suggested that delayed rescue had the same effect as transferring zinc-insufficient oocytes to a zinc-replete medium, where artificial activation was induced as evidenced by pronuclear formation. This phenomenon has been seen in Mos-deficient oocytes, in which nearly half of the knockout oocytes progressed directly from meiosis I into interphase40. However, Mos-deficient oocytes spontaneously progress from meiosis I to interphase, while zinc-insufficient oocytes maintain the meiotic block at telophase until exogenous zinc is administered or they are transferred to a zinc-replete environment. Based on these observations, we propose that newly acquired zinc bolus participates in a mechanism regulating the exit of oocytes from meiosis.
Despite their ability to spontaneously activate in a zinc-replete environment, zinc-insufficient oocytes were fertilized at rates comparable to control oocytes, and interrogation of the pronuclei revealed that one pronucleus is selectively demethylated as occurs during normal fertilization30,31. This concurs with the observation that nuclear status is not necessarily a reliable indicator of an oocyte's developmental potential. For example, oocytes arrested at metaphase I can be fertilized and become blastocysts41. In the case of zinc-insufficient oocytes, however, blastocyst formation was never achieved suggesting that they had not attained full cytoplasmic maturity. Furthermore, disruption of zinc physiology appears to alter essential calcium physiology in the oocyte. Calcium oscillations in activated zinc-insufficient oocytes were missing the initial prolonged calcium transient relative to control oocytes and also exhibited a lower frequency of subsequent calcium transients within the same imaging period. The calcium oscillations that occur at oocyte activation are critical for driving the events leading to formation of the zygote. The frequency and the amplitude of the oscillations, as well as the total number of oscillations dictates which events are triggered33,34,42. Therefore, the deviation in the oscillatory pattern found in zinc-insufficient oocytes is likely partially responsible for the compromised developmental competence of the resulting embryo.
In summary, the application of XFM to the elemental analysis of single mammalian oocytes and embryos spurred the exploration of a new elemental player, zinc, in the terminal steps of maturation to the earliest stages of embryonic development. The acute accumulation of zinc during meiotic maturation implicates this element as a potential player in the cytoplasmic maturation of the oocyte. In particular, the meiotic block caused by attenuation of intracellular zinc availability highlights mechanisms within the oocyte that rely on biochemistry at the most basal, elemental level. Furthermore, this fluctuation in zinc balance established during oocyte maturation had lasting effects on early embryonic development, implicating the metal ion as a critical factor in the maternal legacy from egg to embryo. These results underscore the idea that inorganic physiology is critical in the making of a competent and mature oocyte that is able to sustain the early development of a new organism.
Materials and Methods
Oocyte Collection
Cumulus-oocyte complexes (COCs) were isolated from the ovaries of sexually mature (6-8 weeks old) female CD-1 mice primed with 5 IU pregnant mare's serum gonadotropin (PMSG, Sigma-Aldrich, St. Louis, MO) 48 hours prior to sample collection. Large follicles were grazed using insulin-gauge needles to liberate COCs into Leibovitz's L-15 medium (Invitrogen, Carlsbad, CA) supplemented with 1% fetal bovine serum (FBS, Invitrogen) and 0.2 mM 3-isobutyl-1-methylxanthine (IBMX, Sigma-Aldrich). Prior to culture, COCs were washed through at least three drops of IBMX-free media. To collect eggs at the metaphase II (MII) stage, females were primed with 5 IU PMSG followed by 5 IU hCG 46 hours later. Eggs were isolated from the oviducts 13-14 hours post-hCG. Cumulus cells were denuded using 0.3% (w/v) hyaluronidase and gentle aspiration through a narrow-bore pipette. Animals were treated in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Food and water were given ad libitum. The Northwestern University Institutional Animal Care and Use Committee (IACUC) approved all protocols.
Synchrotron-based x-ray fluorescence microscopy
Oocytes and embryos were prepared whole-mount for synchrotron-based x-ray fluorescence microscopy (XFM). Cells were transferred with a minimal amount of media to an intact 5 mm x 5 mm silicon nitride window (Silson, Blisworth, U.K.) on a heated stage warmed to 37°C. When most of the media had evaporated without drying out the sample, 1 μl of ammonium acetate solution (100 mM, 4°C) was administered to each sample under a dissection microscope. This facilitated a quick wash and dehydration process, leaving the morphology of the sample intact without causing membrane rupture.
XFM was performed at Beamline 2-ID-E at the Advanced Photon Source (Argonne National Laboratory, Argonne, IL). 10 keV x-rays were monochromatized with a single bounce Si(111) monochromator, and focused to a spot size of 0.5 × 0.6 μM using Fresnel zone plate optics (X-radia, Concord, CA). Raster scans were done in steps of 1 μM. Fluorescence spectra were collected with a 1 sec dwell time using a silicon drift detector (Vortex-EM, SII NanoTechnology, CA). Quantification and image processing was performed with MAPS software43. The fluorescence signal was converted to a two-dimensional concentration in μg/cm2 by fitting the spectra against the thin-film standards NBS-1832 and NBS-1833 (National Bureau of Standards). It was assumed that no elemental content was lost during sample preparation.
In Vitro Maturation
Oocytes fully enclosed by cumulus cells were selected and transferred into in vitro maturation (IVM) medium consisting of minimum essential medium, alpha (α-MEM, Irvine Scientific, Santa Ana, CA) supplemented with 200 mM L-glutamine, 10% FBS, 1.5 IU/ml human chorionic gonadotropin (hCG, Sigma-Aldrich), and 5 ng/ml epidermal growth factor (EGF, Sigma-Aldrich). In some cases, the medium was supplemented with the chelators TPEN (Sigma-Aldrich), ammonium tetrathiomolybdate (TM, Sigma-Aldrich) or neocuproine hydrochloride (Sigma-Aldrich). TPEN and TM were prepared in Milli-Q water at stock concentrations of 1 mM and 10 mM, respectively. Neocuproine was prepared in DMSO at a stock concentration of 5 mM. All chelators were added at a final concentration of 10 μM remained in the culture media for the duration of each experiment unless noted otherwise. This concentration of TPEN is below toxic thresholds and is sufficient to induce a physiological effect (Supplementary Fig. 1).
30-40 COCs were cultured together in 800 μl of maturation medium in center-well organ culture dishes, at 37°C in an atmosphere of 5% CO2. The moment COCs were transferred into IVM medium was designated t = 0 hr. COCs were collected after 4, 8, 12, 16, or 48 hr duration. At the end of each culture period, cumulus cells were removed using 0.3% (w/v) hyaluronidase and gentle aspiration through a narrow-bore pipette. Brightfield images were captured on a DM IRB inverted microscope (Leica Microsystems, Bannockburn, IL) using 20x or 40x objectives. Polar body diameter was determined using ImageJ (NIH) by taking a line measurement at the widest point of the polar body. For TPEN-treated oocytes, asymmetrically and symmetrically divided oocytes were pooled separately for measurement. TPEN affected oocyte morphology in the same manner whether or not cumulus cells were present (data not shown). Therefore, all experiments were performed on fully enclosed COCs. For rescue experiments with exogenous metal supplementation, sulfate derivatives of Mg2+, Fe2+, Cu2+ and Zn2+ (all acquired from Sigma-Aldrich) were prepared as a 10 mM stock solution in Milli-Q water. Metal solutions were added directly to the medium to a final concentration of 10 μM at 4 hrs post-maturation. For delayed rescue experiments, ZnSO4 was added to a final concentration of 10 μM at 7, 8, 9, and 12 hrs post-maturation.
Immunofluorescence and Confocal Microscopy
Oocytes were fixed and extracted in a microtubule stabilizing buffer44 containing 2% formaldehyde and 1% Triton X-100 for 30 min at 37°C. After fixation, samples were washed in a blocking/washing buffer containing 0.2% sodium azide, 0.2% milk, 2% normal goat serum, 1% BSA, 100 mM glycine, and 0.1% Triton X-100. Following a 60 min blocking step, samples were maintained at 4°C for short-term storage. In vitro matured oocytes were incubated in monoclonal alpha-tubulin antibody (1:100, Sigma-Aldrich) for 60 min at 37°C. After three washes, samples were incubated in a cocktail of Alexa Fluor 488-conjugated goat anti-mouse IgG (1:500, Invitrogen) and rhodamine-phalloidin (1:100, Invitrogen) for 60 min at 37°C. Samples were washed then mounted in VectaShield solution containing DAPI (Vector, Burlingame, CA). Images were acquired on a LSM 510 confocal microscope (Zeiss) equipped with a 63x oil-immersion objective and UV (405 nm), Ar (488 nm), and HeNe (543 nm) laser lines. Images were processed using the LSM Image Browser software (Carl Zeiss Microimaging, Thornwood, NY).
Statistical Analysis
Elemental concentrations, polar body diameters and spindle parameters were analyzed for statistical significance using one-way ANOVA analysis. All statistical tests were performed using the software Prism 4.0 (GraphPad Software, San Diego, CA). P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Jennifer Jozefik, Sarah Kiesewetter, and Dragan Mackovic for animal care and concerns. We would also like to thank the P01 Histology Core (Tyler Wellington, Director), the Analytical Services Laboratory, and the Quantitative Bioelement Imaging Center in the Chemistry of Life Processes Institute at Northwestern University for reagents and discussions regarding sample processing. This work is supported by NIH grants P01 HD021921 and GM38784, the W. M. Keck Foundation Medical Research Award, and the Chicago Biomedical Consortium Spark Award. A.M.K. was a fellow of the Reproductive Biology Training Grant HD007068. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy, the Office of Science, the Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Footnotes
Competing Financial Interests Statement
The authors declare no competing financial interests.
References
- 1.Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14:331–41. doi: 10.1023/a:1012905406548. [DOI] [PubMed] [Google Scholar]
- 2.Turski ML, Thiele DJ. New roles for copper metabolism in cell proliferation, signaling, and disease. J Biol Chem. 2009;284:717–21. doi: 10.1074/jbc.R800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang AS, Enns CA. Iron homeostasis: recently identified proteins provide insight into novel control mechanisms. J Biol Chem. 2009;284:711–5. doi: 10.1074/jbc.R800017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamasaki S, et al. Zinc is a novel intracellular second messenger. J Cell Biol. 2007;177:637–45. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galaris D, Skiada V, Barbouti A. Redox signaling and cancer: the role of “labile” iron. Cancer Lett. 2008;266:21–9. doi: 10.1016/j.canlet.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763:711–22. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Kambe T, Weaver BP, Andrews GK. The genetics of essential metal homeostasis during development. Genesis. 2008;46 doi: 10.1002/dvg.20382. spcone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Halloran TV. Transition metals in control of gene expression. Science. 1993;261:715–25. doi: 10.1126/science.8342038. [DOI] [PubMed] [Google Scholar]
- 9.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 10.Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–92. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 11.Finney LA, O'Halloran TV. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science. 2003;300:931–6. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 12.Bruinsma JJ, Jirakulaporn T, Muslin AJ, Kornfeld K. Zinc ions and cation diffusion facilitator proteins regulate Ras-mediated signaling. Dev Cell. 2002;2:567–78. doi: 10.1016/s1534-5807(02)00151-x. [DOI] [PubMed] [Google Scholar]
- 13.Nomizu T, Falchuk KH, Vallee BL. Zinc, iron, and copper contents of Xenopus laevis oocytes and embryos. Mol Reprod Dev. 1993;36:419–23. doi: 10.1002/mrd.1080360403. [DOI] [PubMed] [Google Scholar]
- 14.Sun L, Chai Y, Hannigan R, Bhogaraju VK, Machaca K. Zinc regulates the ability of Cdc25C to activate MPF/cdk1. J Cell Physiol. 2007;213:98–104. doi: 10.1002/jcp.21090. [DOI] [PubMed] [Google Scholar]
- 15.Falchuk KH, Montorzi M. Zinc physiology and biochemistry in oocytes and embryos. Biometals. 2001;14:385–95. doi: 10.1023/a:1012994427351. [DOI] [PubMed] [Google Scholar]
- 16.Falchuk KH, Montorzi M, Vallee BL. Zinc uptake and distribution in Xenopus laevis oocytes and embryos. Biochemistry. 1995;34:16524–31. doi: 10.1021/bi00050a037. [DOI] [PubMed] [Google Scholar]
- 17.Stitzel ML, Seydoux G. Regulation of the oocyte-to-zygote transition. Science. 2007;316:407–8. doi: 10.1126/science.1138236. [DOI] [PubMed] [Google Scholar]
- 18.Gosden RG. Oogenesis as a foundation for embryogenesis. Mol Cell Endocrinol. 2002;186:149–53. doi: 10.1016/s0303-7207(01)00683-9. [DOI] [PubMed] [Google Scholar]
- 19.Gandolfi TA, Gandolfi F. The maternal legacy to the embryo: cytoplasmic components and their effects on early development. Theriogenology. 2001;55:1255–76. doi: 10.1016/s0093-691x(01)00481-2. [DOI] [PubMed] [Google Scholar]
- 20.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–11. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picton H, Briggs D, Gosden R. The molecular basis of oocyte growth and development. Mol Cell Endocrinol. 1998;145:27–37. doi: 10.1016/s0303-7207(98)00166-x. [DOI] [PubMed] [Google Scholar]
- 22.Perreault SD, Barbee RR, Slott VL. Importance of glutathione in the acquisition and maintenance of sperm nuclear decondensing activity in maturing hamster oocytes. Dev Biol. 1988;125:181–6. doi: 10.1016/0012-1606(88)90070-x. [DOI] [PubMed] [Google Scholar]
- 23.Ajduk A, Malagocki A, Maleszewski M. Cytoplasmic maturation of mammalian oocytes: development of a mechanism responsible for sperm-induced Ca2+ oscillations. Reprod Biol. 2008;8:3–22. doi: 10.1016/s1642-431x(12)60001-1. [DOI] [PubMed] [Google Scholar]
- 24.Cooley L. Oogenesis: variations on a theme. Dev Genet. 1995;16:1–5. doi: 10.1002/dvg.1020160103. [DOI] [PubMed] [Google Scholar]
- 25.Martell AE, Smith RM. NIST Standard Reference Database 46, v5.0. Plenum; NY: 1998. NIST Critical Stability Constants of Metal Complexes. [Google Scholar]
- 26.Suhy DA, Simon KD, Linzer DI, O'Halloran TV. Metallothionein is part of a zinc-scavenging mechanism for cell survival under conditions of extreme zinc deprivation. J Biol Chem. 1999;274:9183–92. doi: 10.1074/jbc.274.14.9183. [DOI] [PubMed] [Google Scholar]
- 27.Arslan P, Di Virgilio F, Beltrame M, Tsien RY, Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem. 1985;260:2719–27. [PubMed] [Google Scholar]
- 28.Barrett SL, Albertini DF. Allocation of gamma-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte. Biol Reprod. 2007;76:949–57. doi: 10.1095/biolreprod.106.057141. [DOI] [PubMed] [Google Scholar]
- 29.Brunet S, Maro B. Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: integrating time and space. Reproduction. 2005;130:801–11. doi: 10.1530/rep.1.00364. [DOI] [PubMed] [Google Scholar]
- 30.Santos F, Dean W. Using immunofluorescence to observe methylation changes in mammalian preimplantation embryos. Methods Mol Biol. 2006;325:129–37. doi: 10.1385/1-59745-005-7:129. [DOI] [PubMed] [Google Scholar]
- 31.Santos F, Hendrich B, Reik W, Dean W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol. 2002;241:172–82. doi: 10.1006/dbio.2001.0501. [DOI] [PubMed] [Google Scholar]
- 32.Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–76. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 33.Ducibella T, et al. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev Biol. 2002;250:280–91. [PubMed] [Google Scholar]
- 34.Toth S, Huneau D, Banrezes B, Ozil JP. Egg activation is the result of calcium signal summation in the mouse. Reproduction. 2006;131:27–34. doi: 10.1530/rep.1.00764. [DOI] [PubMed] [Google Scholar]
- 35.Nagy A, Gertsenstein M, Vintersten K, Behringer R, editors. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2003. [Google Scholar]
- 36.Taki M, Wolford JL, O'Halloran TV. Emission ratiometric imaging of intracellular zinc: design of a benzoxazole fluorescent sensor and its application in two-photon microscopy. J Am Chem Soc. 2004;126:712–3. doi: 10.1021/ja039073j. [DOI] [PubMed] [Google Scholar]
- 37.Wickramasinghe D, Ebert KM, Albertini DF. Meiotic competence acquisition is associated with the appearance of M-phase characteristics in growing mouse oocytes. Dev Biol. 1991;143:162–72. doi: 10.1016/0012-1606(91)90063-9. [DOI] [PubMed] [Google Scholar]
- 38.Sorensen RA, Wassarman PM. Relationship between growth and meiotic maturation of the mouse oocyte. Dev Biol. 1976;50:531–6. doi: 10.1016/0012-1606(76)90172-x. [DOI] [PubMed] [Google Scholar]
- 39.Erickson GF, Sorensen RA. In vitro maturation of mouse oocytes isolated from late, middle, and pre-antral graafian follicles. J Exp Zool. 1974;190:123–7. doi: 10.1002/jez.1401900112. [DOI] [PubMed] [Google Scholar]
- 40.Araki K, et al. Meiotic abnormalities of c-mos knockout mouse oocytes: activation after first meiosis or entrance into third meiotic metaphase. Biol Reprod. 1996;55:1315–24. doi: 10.1095/biolreprod55.6.1315. [DOI] [PubMed] [Google Scholar]
- 41.Eppig JJ, Schultz RM, O'Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol. 1994;164:1–9. doi: 10.1006/dbio.1994.1175. [DOI] [PubMed] [Google Scholar]
- 42.Ozil JP, Banrezes B, Toth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol. 2006;300:534–44. doi: 10.1016/j.ydbio.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 43.Vogt S. MAPS: A set of software tools for analysis and visualization of 3D X-ray fluorescence data sets. Journal De Physique Iv. 2003;104:635–638. [Google Scholar]
- 44.Ibanez E, Sanfins A, Combelles CM, Overstrom EW, Albertini DF. Genetic strain variations in the metaphase-II phenotype of mouse oocytes matured in vivo or in vitro. Reproduction. 2005;130:845–55. doi: 10.1530/rep.1.00558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.