Abstract
Snell dwarf mice have multiple hormonal deficits, but the way in which these deficits postpone aging are still uncertain. In this study, Snell dwarf mice received 11 weeks of growth hormone and thyroxine injections that increased their weight by approximately 45%, although they remained much smaller than controls. The hormone treatment also restored fertility to male dwarf mice. Despite these effects on growth and maturation, the hormone treatments did not diminish life span or lower the resistance of dwarf mice to cataracts and kidney disease. Administration of thyroxine in food throughout adult life did diminish longevity of Snell dwarf mice, although these mice remain longer lived than control animals. These results show that a 45% increase in body size does not impair longevity or disease resistance for dwarf mice of either sex, and that the exceptional longevity of Snell dwarf mice does not, at least for males, depend on prepubertal immaturity.
Mutations that extend mean and maximum life span have now been reported at eight mouse loci (1–7). In five of these loci, the mutation modulates either production of or response to insulin-like growth factor-I (IGF-I). The first such mutant to be reported, the Ames dwarf mouse (1), has a mutation at the Prop1 locus that interferes with development of the anterior pituitary gland. The Ames dwarf mutants (df/df) have abnormally low levels of growth hormone (GH), thyroid-stimulating hormone (TSH), and prolactin. The GH deficit results in drastic diminution of IGF-I, and the low TSH levels lead to low levels of the thyroid hormones thyroxine (T4) and triiodothyronine (T3). The Ames dwarf mutation has been shown in several studies (8) to be long-lived when compared to controls; homozygous wild-type (+/+) and heterozygotes (+/df) are indistinguishable in their longevity and hormonal profiles. The product of the Prop1 locus affects pituitary development by induction of the PIT1 protein (9), and mutations at the Pit1 gene lead to similar defects in GH, IGF-I, TSH, T4, and prolactin, as well as increased longevity (3). Pit1 mutants (the Snell dwarf mouse, dw/dw) have been shown not only to have longer life spans, but also to have delayed or decelerated aging in their connective tissue, immune system (3), and joint pathology (10).
It is not known how the hormonal defects in the Snell dwarf mice lead to extended longevity and delayed aging in multiple tissues. Life-span extension and disease resistance might reflect the inhibition of disease processes, in old age, by the altered hormonal milieu. Alternately, hormonal deficits during development or adolescence might induce long-lasting changes in cell or tissue function that result, later in life, in resistance to many forms of disease and tissue malfunction. The longevity effect might also represent a secondary consequence of small body size or of the reproductive immaturity noted in the dwarf model. To address these issues, we measured life span, cataract severity, and terminal pathology in a population of Snell dwarf mice that were exposed to GH and thyroid hormone injections for an 11-week period (i.e., from 4 to 15 weeks of age), sufficient to lead to substantial increase in body size and pubertal maturation of the male mice. In addition, half of the hormone-injected mice were placed on a regimen of oral thyroid hormone sufficient to establish euthyroid levels throughout adult life. The data show that neither reproductive maturation nor increased growth during adolescence diminishes the extended longevity of dwarf mice.
Method
Experimental Overview
The longevity colony under study contained three groups of dwarf mice and three of nonmutant littermate mice. From 4 to 15 weeks of age, some mice in each genotype received a series of 55 injections of GH plus T4, and subsequently received T4 in their food for the rest of their lives. Some mice received the 55 injections, but did not receive supplementary T4 in their food. Some mice received 55 saline injections, but were never exposed to hormone by injection or in their food. Each mouse was then examined for cataracts, life span, and pathology; in addition, males were tested to see if they could impregnate females.
Mouse Husbandry
DW/J-Pit1dw/+ female and C3H/HeJ-Pit1dwJ/+ male heterozygote breeders purchased from The Jackson Laboratory (Bar Harbor, ME) were crossed to produce the (DW × C3H)F1 mice used in this experiment. The (DW × C3H)F1 progeny included homozygote dw/dwJ, heterozygote dw/+ and +/dwJ, and wild-type +/+ genotypes. Mice with the dw/dwJ genotype were identified at approximately 3 weeks of age by their small body size (dwarfs). Mice of the other two genotypes were not distinguishable from one another, and were used as nonmutant littermate controls (genotype +/?). The mice were housed in microisolator cages with 1/8 inch Bed-O-Cob bedding (The Andersons, Maumee, OH), free access to tap water and Purina 5001 Rodent Chow (St. Louis, MO); in addition, moist or crushed pellets of chow were placed on the floor of cages housing dw/dw animals. Dwarf mice were caged with normal sized females (“warmer” mice) to prevent premature death of the dwarf mice from hypothermia; females were used instead of male “warmers” to avoid aggression of normal male mice against dwarf males. Male dwarf mice were housed in cages containing two dw/dw males and two normal sized female mice, which were not part of the longevity study. Male control mice used in the longevity study were housed two per cage. Female mice were housed in cages containing two dw/dw and two normal sized littermate controls per cage. When either a normal sized female warmer or female test mouse died, she was replaced with a fresh warmer female. In addition, warmer females housed with hormone-treated male dwarf mice frequently became pregnant, and were replaced with nonpregnant females. The mice were weighed weekly during the 11-week hormone injection phase and then monthly thereafter until death. Entry of mice into the experiment was staggered over a period of 26 calendar months.
Quarterly serologic tests using sentinel mice were conducted throughout the study, and were uniformly negative for the initial 38 months. Titers against mouse hepatitis virus were noted in 39 of the 95 cages for two consecutive quarters at this point; affected mice ranged in age from 10 to 36 months.
Parenteral Hormone Treatments
Each mouse in the population was given a series of injections beginning at 28 ± 6 days of age. Injections were given 5 days per week for 11 weeks. One group of dwarf mice received saline injections. A second group of dwarf mice received 50 μg of ovine or porcine GH (a gift from Dr. A. F. Parlow through the National Hormone and Pituitary Program of the National Institute of Diabetes & Digestive & Kidney Diseases) plus 2 μg of L-thyroxine (T0397; Sigma, St. Louis, MO). The first 9 of the 47 dwarf mice in this group received GH injections without T4; these mice did not differ appreciably in any of the outcome measures from those that received injections of both GH and T4, and the two groups were thus pooled for analyses. Groups of nonmutant littermate control mice were similarly given either saline injections or injections of GH plus T4.
Oral Supplementation With Thyroid Hormone
Dwarf mice and nonmutant control mice that had received injections of GH and T4 were split into two subgroups at the end of the series of injections. Mice in one of these subgroups were provided with a diet supplemented with T4. For the first set of 12 mice (8 normal and 4 dwarf), the diet consisted initially of Purina AIN-76A supplemented with 250 mg/kg thyroid powder. Blood levels of thyroid hormone were measured 45–60 days later, and were found to be higher than expected: 21 ± 1 μg/dl serum in the treated nonmutant mice, and 19 ± 1 in the treated dwarf mice, as compared to 7 ± 1 in nonmutant mice that had not received T4-laced food. Therefore, the dose of oral T4 was reduced to 63 mg/kg by mixture of 1 part of the Purina AIN-76A chow with 3 parts of crushed moistened Purina 5001 chow, and this mixture was used for the remainder of the study both for the original group of 12 mice and for all other mice completing their injection protocol. Serum T4 levels taken from a subset of these mice 19 days after the change to 63 mg/kg chow were within the normal range: 6 ± 1 for the hormone-treated nonmutant mice, 7 ± 2 for the hormone-treated dwarf mice, and 5 ± 0.3 for normal controls without supplementary T4. Serum T4 levels measured at two further intervals (6 and 17 months after initiation of the T4 food supplementation) were also within normal limits: 3 to 5 μg/dl serum in hormone-supplemented nonmutant and dwarf mice.
Thyroid Hormone Assays
Serum T4 levels were estimated in venous tail blood samples by using a kit purchased from ICN (Total T4 MAb Kit 06B56100-R6; ICN Biomedicals [now MP Biomedicals], Irvine, CA) according to the manufacturer’s instructions except that all volumes were reduced by a factor of four.
Cataract Assessment
Mice were tested at age 18 months and again at age 24 months for lens turbidity using a handheld slit lamp after pupillary dilation with a solution of equal parts 1% tropicamide and 0.5% phenylephrine. Turbidity was scored on a scale from 0 (no evidence of cataract) to 4 (severe) for each eye separately, and the mean score from the left and right eye was used as an index of cataract severity. One hundred sixteen mice were evaluated for cataract status at both ages, 28 mice were evaluated at 18 months only, and 51 mice were evaluated at 24 months only.
Necropsy Methods
Mice were examined for clinical signs at least daily. Mice suspected to be ill (because of weight loss, poor grooming, or visible tumor) were observed twice daily except on weekends during which they were inspected once daily. Mice judged by an experienced technician to be moribund were humanely euthanized and necropsied. This group made up approximately 65% of the total. Mice found dead were also submitted for necropsy. The necropsy protocol has been described in detail elsewhere (11), and it involved both gross inspection and histological examination of sections from 37 organs and any additional tissues which had gross lesions. Glomerular basement membrane thickening was graded from 1 to 3 upon evaluation of 5-micron kidney sections stained with hematoxylin and eosin as well as PAS (Periodic Acid Schiff) stains. Animals with Grade 1 thickening had minimal thickening of glomerular capillary loops noted only in PAS-stained sections. Grade 2 thickening was evident on examination of PAS-stained sections and was characterized by mild to moderate regular thickening of capillary walls in the outer portions of the glomerular tuft. Rare animals with Grade 3 lesions had marked irregular thickening of capillary walls evident in both hematoxylin and eosin-stained and PAS-stained sections usually accompanied by glomerular sclerosis (fibrous scarring).
Results
Snell dwarf mice were subjected to a course of injections with GH and T4, with five injections each week between 4 and 15 weeks of age to see if exposure to these hormones over the period of puberty and early adolescence would stimulate growth, reproductive maturation, and, potentially, loss of the anti-aging effects of this mutation. In addition, some of these hormone-treated mice were given T4 in food from 15 weeks until their death. Control mice were given injections of sterile saline in place of GH/T4 injections. A set of nonmutant littermates (composed of +/+ homozygotes and +/dw heterozygotes, which are phenotypically indistinguishable) was treated with saline or with GH/T4 injections in parallel.
Body weight gain was used as an indicator of the effectiveness of the hormone treatment protocols. Table 1 shows body weight prior to treatment, at 6 months, and at 18 months in each of the six groups of mice. GH/T4 injections led to an increase of 45% to 46% in body weight, for females and males respectively, when compared to saline-injected dwarf mice. Hormone injections followed by T4 ingestion led to increases of 76% to 86% over saline-treated dwarf mice. Thus the hormone treatments did, as expected from previous work, lead to a partial repair of the growth defect. Nonetheless, hormone-injected mice remained substantially smaller than saline-treated littermate controls: females were still only 45% as large as controls, and males only 48% as large as controls. The two hormone treatment protocols had a smaller effect on the non-dwarf littermates, with mean weights 3% to 7% higher than those of saline-injected control mice. Weight measured at 18 months showed a similar pattern: Hormone-injected dwarf mice were 24%–38% heavier than the saline-injected dwarf controls, but only 36%–44% of saline-treated nonmutant littermates. Provision of T4 in food produced 18-month weights that were 108% to 122% above those of saline-treated dwarfs, but were still substantially less than those of normal mice (i.e., 60%–71% of saline-treated nonmutant controls).
Table 1.
Weight at Various Ages in Each Treatment Group
| Genotype | Treatment* | Age: 4 Weeks |
Age: 6 Months |
Age: 18 Months |
|||
|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | ||
| Normal | Saline | 19.0 ± 2.6 (21) | 17.5 ± 2.2 (21) | 39 ± 5.5 (16)† | 38 ± 4.3 (21) | 41.6 ± 5.7 (15) | 42.4 ± 5.1 (19) |
| Normal | Rx | 19.8 ± 2.9 (21) | 16.9 ± 2.8 (20) | 42 ± 5.7 (17) | 39 ± 4.7 (20) | 45.0 ± 6.8 (16) | 44.7 ± 6.4 (18) |
| Normal | Rx + T4 | 20.0 ± 2.7 (20) | 17.2 ± 3.0 (14) | 45 ± 2.9 (14) | 40 ± 5.5 (11) | 45.5 ± 5.1 (18) | 45.5 ± 8.3 (12) |
| Dwarf | Saline | 6.4 ± 0.9 (17) | 6.5 ± 0.8 (20) | 13 ± 2.2 (13) | 12 ± 1.3 (20) | 13.7 ± 2.3 (13) | 12.7 ± 1.3 (20) |
| Dwarf | Rx | 6.0 ± 1.1 (18) | 6.4 ± 0.8 (20) | 19 ± 2.6 (22) | 17 ± 2.7 (23) | 18.7 ± 3.4 (18) | 16.3 ± 1.9 (18) |
| Dwarf | Rx + T4 | 6.6 ± 1.1 (16) | 6.4 ± 1.2 (12) | 24 ± 5.5 (12) | 21 ± 4.8 (11) | 30.5 ± 8.2 (15) | 24.4 ± 5.1 (11) |
Notes:
Rx: injected with growth hormone and thyroid hormone from 4 weeks to 15 weeks of age; Rx + T4: Rx followed by administration of T4 in food.
Mean weight ± SD (standard deviation).
Number of mice/group shown in parentheses.
Figure 1 shows survival curves for each of the six groups of mice, and Table 2 collects summary statistics. Of the 47 mice in the dwarf Rx group, 38 received injections containing both GH and T4, and the other 9 received injections containing GH only. There was not a significant difference in survival between these two groups (median survival was 1149 days for the GH/T4 group and 1220 days for the mice injected with GH only; p = .89 by log-rank test); for this reason, both sets of mice are considered together throughout this report. There was no significant difference in longevity (by log-rank test) between males and females in any of the six treatment groups; for that reason, male and female mice were pooled together for all subsequent analyses. At the time of analysis (July 2004) 30% of the dwarf mice (33 of 110) were still alive, as were 4% (4 of 109) of the littermate controls, making it impossible to provide a useful estimate of maximum life span. The oldest of these mice died at the age of 1472 days, which we believe may be longer than any other published longevity value for mice, excepting experiments involving caloric restriction protocols. The saline-treated dwarfs lived 38% longer than saline-treated littermate controls (mean values; median life span was 35% of controls). By log-rank test, the difference between saline-treated dwarfs and littermate controls was significant at p < .00001. Hormone-injected dwarfs had a mean life span that was 2% longer than saline-injected dwarfs. Hormone injections also had no effect on life span of nonmutant littermates (100% of mean life control life span). Hormone-injected dwarf mice given T4 in their food had a mean longevity that was only 83% of that seen in hormone-injected dwarf mice not given lifelong exposure to T4 (p = .03), and this group was also significantly short-lived (p = .05) when compared to all dwarf mice regardless of hormone injections. Thus we infer that lifelong T4 exposure diminishes life expectancy of dwarf mice, although it should be noted that this is an interim calculation with 30% of these mice still alive. Providing T4 in food had a small and statistically insignificant effect on life span in the nonmutant littermate mice, whether considered in comparison to mice given hormone injections (90% of control, p = .6) or to mice given saline only.
Figure 1.
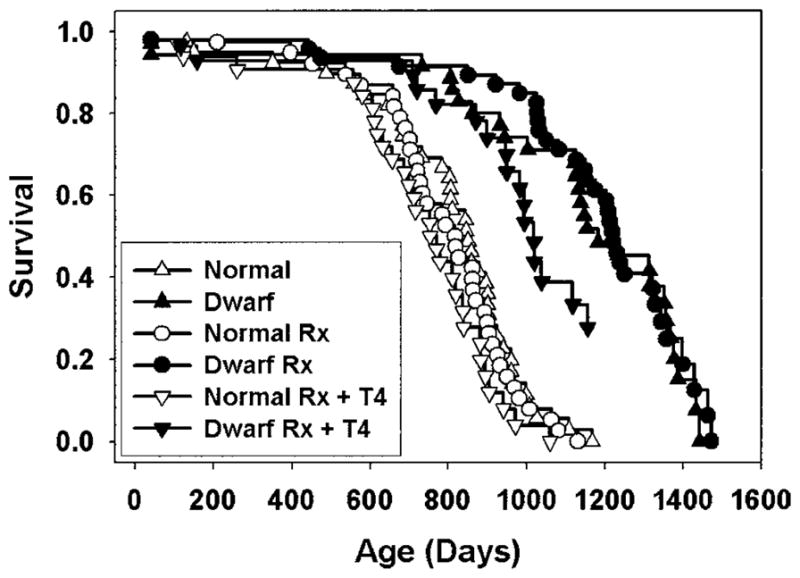
Survival curves for each of the six treatment groups. Each symbol represents one mouse. Rx: injected with growth hormone (GH) and thyroxine (T4) between 4 and 15 weeks of age. Rx + T4: received injections followed by treatment with T4 in food. Filled symbols represent dwarf mice; open symbols show nonmutant littermate control mice.
Table 2.
Life Table Statistics
| Genotype | Treatment* | Life Span† |
Deaths | Alive | Total N | |
|---|---|---|---|---|---|---|
| Median | Mean ± SD | |||||
| Normal | Saline | 850 | 794 ± 224 | 38 | 1 | 39 |
| Normal | Rx | 818 | 791 ± 190 | 38 | 0 | 38 |
| Normal | Rx + T4 | 761 | 714 ± 216 | 29 | 3 | 32 |
| Dwarf | Saline | 1146 | 1095 ± 332 | 27 | 8 | 35 |
| Dwarf | Rx | 1203 | 1121 ± 278 | 33 | 14 | 47 |
| Dwarf | Rx + T4 | 993 | 928 ± 273 | 17 | 11 | 28 |
Notes:
Rx: 11 weeks of injection with growth hormone and thyroid hormone; Rx + T4: Rx followed by administration of T4 in food.
Median life span, in days, is given for all mice in the population. Mean life span, ±SD (standard deviation), is given for those that had died at the time of analysis (April 2004). Mice still alive at this point are included as censored observations in the calculation of log-rank statistics testing differences between groups.
Many of the mice were examined at age 18 and/or 24 months by slit lamp to provide a score for severity of lens turbidity (cataract score). A two-way analysis of variance was used to test for the effects of genotype (normal vs dwarf), treatment (saline vs hormone injections vs hormone injections plus T4), and interactions between genotype and treatment. At 18 months, the data showed a strong effect of genotype (p < .00001), with dwarf mice showing less severe cataracts than normal controls. There was no effect of treatment group on cataract severity at 18 months, and no interaction of genotype with treatment. At 24 months, the difference between dwarf and normal mice was still apparent (p < .00001), and there was also at this age a significant effect of treatment (p < .001). A post hoc analysis using the Tukey–Kramer method showed a difference between the hormone-injected mice and the mice that received both hormone injections and T4 in food, with cataracts significantly more severe (p < .05) in the latter group; neither group was significantly different from saline-treated mice, which had an intermediate cataract score. Figure 2 depicts mean cataract scores in each of the six groups of mice at 24 months of age.
Figure 2.
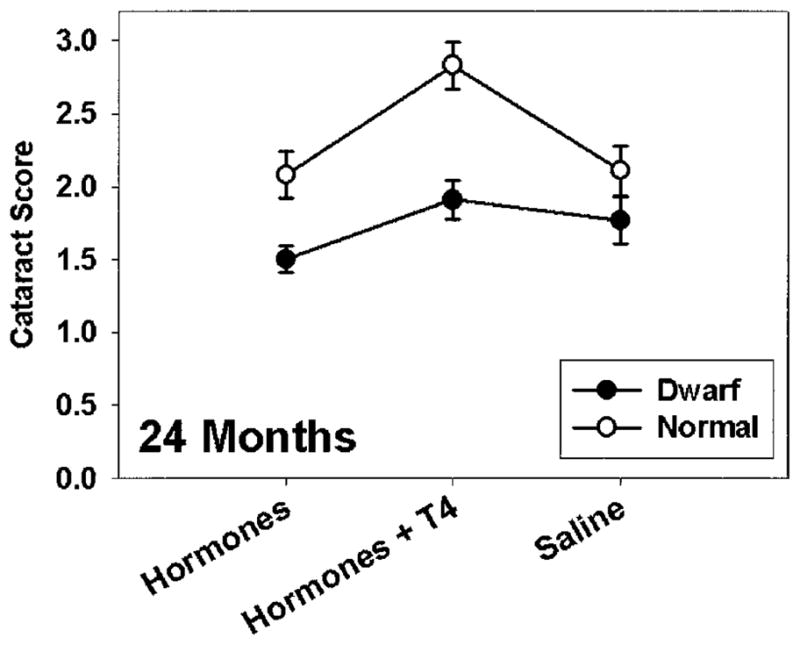
Mean cataract scores for mice in each of the six test groups evaluated at 24 months of age. Error bars indicate standard error of the mean. Number of dwarf mice tested (left to right): 39, 22, and 32. Number of normal mice tested, left to right: 32, 15, and 27.
Our husbandry system provided a convenient opportunity to assess the effects of these treatments on fertility of the dwarf male mice, in that each cage with dwarf mice also contained one or more female mice as “warmer” mice to protect against hypothermia (3,12). All 34 of the mice that had received injections of GH and T4, including some that had received T4 in food and others that had not, were able to impregnate female cohabitants at least once. The earliest litter was born when the sire was 98 days of age. Thirty of these hormone-treated male mice had reached the age of 24 months at the time of this report and, of these 30, 8 had been able to impregnate female mice after reaching the 2-year milepost. Five of these males sired pups at the ages of 28, 28, 30, 31, and 34 months (1012 days of age). This anecdotal evidence provides only a minimal estimate of late-life male fecundity in this system, because impregnated young females were in many cases replaced by elderly females past the normal breeding age. A more systematic program to test for fertility using young female tester mice would be expected to yield a higher success ratio for the elderly treated dwarf mice. Four of the saline-injected male dwarf mice were housed with female mice throughout their life span, and never induced pregnancies. In addition, no pregnancies have been noted among 38 cages of uninjected male dwarf mice, not part of this longevity study, that had been housed with normal female mice over intervals from 6 to 29 months.
Necropsies were performed on all mice found dead and on those sacrificed at the end of their life span when they became so ill that they seemed unlikely to survive for more than another week. Table 3 presents data on inferred cause of death for the 45 dwarf and 45 control mice for which microscopic evaluation was not precluded by advanced autolysis. Mice have been pooled with respect to genotype without regard for hormone regimen; there were no obvious differences in lesion frequency attributable to hormone exposure, although small group size precluded detailed analysis of this issue. No histologically obvious cause of death could be identified in 47% of the dwarf cases, a proportion significantly higher than in the control population (7% of cases; p < .001). This finding, consistent with those in a previous report (13), suggests that in dwarf mice death may often be caused either by the combined effects of multiple illnesses or, more likely, by illnesses which are not easily identified by histopathological examination alone. The data also suggested that death caused by lymphoma (20% of controls and 4% of dwarfs) or caused by mammary adenocarcinoma (8 of 21 female controls = 38%, compared to 1 of 14 dwarf controls = 7%) was more common among control mice, but these do not reach statistical significance (p = .06 in each case), and have not been adjusted for competing risks (i.e., the likelihood that dwarf mice would die of some other cause).
Table 3.
Cause of Death Analysis in Dwarf and Control Mice
| Cause of Death | Number of Cases |
Percent Cases |
Comment | ||
|---|---|---|---|---|---|
| Dwarf | Normal | Dwarfs | Normals | ||
| Fibrosarcoma | 2 | 1 | 4 | 2 | |
| Hemangiosarcoma | 6 | 9 | 13 | 20 | |
| Histiocytic sarcoma | 3 | 3 | 7 | 7 | |
| Lymphoma | 2 | 9 | 4 | 20 | p < .06 |
| Mammary adenocarcinoma | 1 | 8 | 2 | 18 | p < .06 for females |
| Other, neoplastic | 4 | 9 | 9 | 20 | |
| Other, nonmalignant | 6 | 3 | 13 | 7 | |
| Unknown cause of death | 21 | 3 | 47 | 7 | p < .001 |
| Grand Total | 45 | 45 | |||
Sections of kidney tissue were also evaluated in 40 dwarf and 46 control mice and were scored for severity of glomerular basement membrane lesions on a scale of 0 to 3. Table 4 shows the score distributions. The frequency of scores differs (p = .02) between normal and dwarf mice, with dwarf mice less likely to show advanced kidney pathology at the time of death, even though the average age at death is higher in the dwarf group. A similar statistical result (p = .03 by Fisher exact test) is obtained if the renal samples were instead scored as positive or negative for basement membrane pathology.
Table 4.
Severity of Glomerular Basement Membrane Damage in Dwarf and Control Mice
| Genotype | Grade of Severity (0–3) |
Total Cases | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| Dwarf | 22 | 16 | 2 | 0 | 40 |
| Control | 14 | 20 | 8 | 4 | 46 |
Discussion
The data shown in Figure 1 replicate our previous findings (3) of extended mean and maximal longevity in Snell dwarf mice and, when combined with parallel findings in the Ames dwarf mouse (1), they show that the ability of pituitary dwarf mutations to delay aging in mice is robust across several backgrounds in at least three animal colonies. Dwarf mice have been shown previously to exhibit delays or decelerations in multiple cells and tissues, including evidence for reduced arthritic change (10), slower immune aging and collagen cross-linking (3), preserved cognitive function (14), and neoplastic change (13). Our new data extend this listing, showing that dwarf mice are slower to develop cataracts (Figure 2) and have less severe glomerular basement membrane pathology (Table 4) compared to normal controls. Thus the case is now strong that the dwarf mutations, like calorie restriction (15), do not merely retard death and lethal illnesses, but seem to diminish the rate or extent of aging itself, with secondary effects on both lethal and nonlethal pathological lesions as well as retardation of changes in proliferating and nonproliferating tissues, both cellular and extracellular.
Small body size is associated with extended longevity in several varieties of mice (16–18) and dogs (19–21), and possibly in people (22). Thus one goal of our study was to determine if Snell dwarf mice whose body sizes were increased by hormone treatment would thereby lose their longevity advantage over normal sized controls. We found that an 11-week course of injected GH and T4 was sufficient to increase mean body size, assessed at 6 months of age, by 42% in females and 46% in males, but had no apparent effect on life span. The difference in cataract severity between normal and dwarf mice was also not diminished by the hormone injection protocol. These data thus argue against the hypothesis that the extended longevity of Snell dwarf mice is due to some undefined effect of small body size per se. We note, however, that hormone-injected dwarf mice did not reach the body size of saline-injected normal controls, and it remains possible that a more intensive or prolonged exposure to hormones might have both established normal full body size and diminished longevity.
Our data are consistent with the idea that the extended longevity of Snell and presumably Ames dwarf mice reflects diminished levels of both thyroid hormones and diminished influence of the GH/IGF-I axis. In particular, we find that hormone-injected mice that received lifelong supplementation with T4 have a survival curve that falls between that of dwarf mice (saline- or hormone-injected) and that of nonmutant controls (regardless of hormone exposure). The most straightforward interpretation would be that low levels of thyroid hormones throughout life contribute to the longevity of the dwarf mice; this is consistent with the observation that mice with isolated defects in GH/IGF-I signals, such as the Ghrhr-deficient lit/lit mutants (3) and IGF-I receptor hemizygous mice (7), do not show as large a life span effect as do pituitary dwarfs with mixed thyroid and IGF-I diminutions. In contrast, mice homozygous for a null allele of the growth hormone receptor show life span extensions that do, on some backgrounds, rival those of the Snell and Ames mutants (23). Our survival results for mice given lifelong oral T4 thus might be interpreted either as the restoration by T4 of some anti-aging effects seen in dwarf mice or, alternately, a toxic effect of pharmacologically administered thyroid hormone. We note that our limited data show thyroid hormone levels in the normal range for mice in the treated group (except for a small number of mice exposed to thyroid hormone prior to adjustment of dietary dose), and that nonmutant mice exposed to the same treatment regimen exhibit a much smaller (and statistically insignificant) decline in longevity compared to controls not given dietary T4. As with any treatment or mutation that diminishes life span, it is thus difficult to discriminate toxicity from promotion of authentic aging processes (24).
Several scientists have proposed that delayed aging accompanies, and in some cases may be in part a consequence of, declines in reproductive function. The suggestion of the popular “disposable soma” model of aging has postulated a balance between commitment of unspecified resources either to reproduction or to self-maintenance, with slow aging a result of preservation of repair and maintenance functions at the cost of full-bore reproduction (25–27). Studies of fly and worm populations have documented many instances in which commitment to reproduction increases mortality risks (28–30), although the complexities and idiosyncrasies of these invertebrate life histories make it uncertain how often similar connections will be found in mammals. Although it is clear that some mutations and some dietary interventions do both increase life span and decrease reproductive effort, our own data show that the excess longevity of the Snell dwarf mice does not require maintenance of a prepubertal, nonreproductive state, at least for males, in that mice injected with GH and T4 from 4 through 15 weeks of age attained long-lasting reproductive competence without a loss of life span. The long life span of miniature dogs, which are in at least two cases attributable to declines in IGF-I levels (31,32), is also attained without loss of reproductive abilities.
Our data also add to the slowly growing literature on late life pathology in long-lived mutant mice. In a study of terminal pathology in Ames dwarf mice, Ikeno and colleagues (13) concluded that the dwarf mutation increased the age of occurrence of fatal neoplastic diseases; this is also true on our own series of cases. We noted, however, that death in most of the nonmutant control mice (42 of 45 cases) could plausibly be attributed to one major form of illness, but that nearly half of the dwarf mice (21 of 45 cases) could not be diagnosed in this way. Associated with this significant (p < .001) increase in the proportion of deaths from unknown cause, there were trends toward a decrease in the incidence of lethal lymphoma (p < .06) and mammary adenocarcinoma (p < .06 among females) in the dwarf case series. It is likely that many of the deaths among dwarf mice may reflect severe biochemical shifts whose effects are not easy to discern at the histopathological level, perhaps representing in individual cases a combination of metabolic, neurological, and hormonal abnormalities rather than a single overwhelming neoplastic, degenerative, or infectious cause. However, it was not feasible to measure clinical chemistries and/or specific hormone levels in animals found dead or sacrificed when moribund.
It is not at all clear that the hormonal conditions required to create the long-lived body of the Snell dwarf mouse are also optimal for maintenance of excellent health in later life. Cell lines established from the skin of dwarf mice are relatively resistant to oxidative stress, ultraviolet light, toxic metals, and heat [even when the skin samples are taken in mice 3–4 months of age (33)], and, although it is not proven that this cellular stress resistance underlies late life disease resistance in these mutant mice, we think it likely that the foundation for diminished mortality risk at old age reflects epigenetic cellular changes established early in adult life. The evidence that middle-aged dwarf mice differ from controls in collagen and immune system aging (3) is also consistent with models in which at least some of the effects of the dwarf mutations are generated in the first half of the life span. We speculate that low levels of GH and/or TSH, while responsible for producing long-lasting cells and tissues in the juvenile and adolescent stages of life, may have detrimental effects in middle and old age. If this is true, it would suggest that the longevity of dwarf mice might be increased even further by judicious administration of GH and/or T4 at later ages. More generally, much remains to be learned about the stages in the developmental and aging process at which mice are susceptible to improvement of long-term health by manipulation of endocrine status. Mice engineered to permit transient reduction of GH and TSH levels at defined stages of life would be valuable tools for the exploration of this class of hypothesis.
Acknowledgments
We are grateful to Amanda Pilling, Jessica Sewald, and Gretchen Buehner for technical assistance. This work was supported by National Institute on Aging grants AG08808 and AG040426.
References
- 1.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 2.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 3.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miskin R, Masos T. Transgenic mice overexpressing urokinase-type plasminogen activator in the brain exhibit reduced food consumption, body weight and size, and increased longevity. J Gerontol Biol Sci. 1997;52:B118–B124. doi: 10.1093/gerona/52a.2.b118. [DOI] [PubMed] [Google Scholar]
- 5.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 6.Migliaccio E, Giorgio M, Mele S, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 7.Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 8.Bartke A, Coschigano K, Kopchick J, et al. Genes that prolong life: relationships of growth hormone and growth to aging and life span. J Gerontol Biol Sci. 2001;56:B340–B349. doi: 10.1093/gerona/56.8.b340. [DOI] [PubMed] [Google Scholar]
- 9.Sornson MW, Wu W, Dasen JS, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- 10.Silberberg R. Articular aging and osteoarthritis in dwarf mice. Pathol Microbiol (Basel) 1972;38:417–430. doi: 10.1159/000162458. [DOI] [PubMed] [Google Scholar]
- 11.Chrisp CE, Turke P, Luciano A, Swalwell S, Peterson J, Miller RA. Lifespan and lesions in genetically heterogeneous (four-way cross) mice: a new model for aging research. Vet Pathol. 1996;33:735–743. doi: 10.1177/030098589603300620. [DOI] [PubMed] [Google Scholar]
- 12.Flurkey K, Harrison DE. Use of genetic models to investigate the hypophyseal regulation of senescence. In: Harrison DE, editor. Genetic Effects on Aging II. Caldwell, NJ: The Telford Press; 1990. pp. 437–456. [Google Scholar]
- 13.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol Biol Sci Med Sci. 2003;58A:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 14.Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001;39:277–284. doi: 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- 15.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller RA, Chrisp C, Atchley W. Differential longevity in mouse stocks selected for early life growth trajectory. J Gerontol Biol Sci. 2000;55A:B455–B461. doi: 10.1093/gerona/55.9.b455. [DOI] [PubMed] [Google Scholar]
- 17.Miller RA, Harper JM, Dysko RC, Durkee SJ, Austad SN. Longer life spans and delayed maturation in wild-derived mice. Exp Biol Med. 2002;227:500–508. doi: 10.1177/153537020222700715. [DOI] [PubMed] [Google Scholar]
- 18.Miller RA, Harper JM, Galecki A, Burke DT. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell. 2002;1:22–29. doi: 10.1046/j.1474-9728.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Deeb B, Pendergrass W, Wolf N. Cellular proliferative capacity and life span in small and large dogs. J Gerontol Biol Sci. 1996;51A:B403–B408. doi: 10.1093/gerona/51a.6.b403. [DOI] [PubMed] [Google Scholar]
- 20.Speakman JR, van Acker A, Harper EJ. Age-related changes in the metabolism and body composition of three dog breeds and their relationship to life expectancy. Aging Cell. 2003;2:265–275. doi: 10.1046/j.1474-9728.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- 21.Miller RA. Kleemeier Award Lecture: are there genes for aging? J Gerontol Biol Sci. 1999;54A:B297–B307. doi: 10.1093/gerona/54.7.b297. [DOI] [PubMed] [Google Scholar]
- 22.Samaras TT, Elrick H. Height, body size and longevity. Acta Med Okayama. 1999;53:149–169. [PubMed] [Google Scholar]
- 23.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 24.Miller RA. ‘Accelerated aging’: a primrose path to insight? Aging Cell. 2004;3:47–51. doi: 10.1111/j.1474-9728.2004.00081.x. [DOI] [PubMed] [Google Scholar]
- 25.Kirkwood TBL. Comparative and evolutionary aspects of longevity. In: Finch CE, Schneider EL, editors. Handbook of the Biology of Aging. New York: Van Nostrand Reinhold Company; 1985. pp. 27–44. [Google Scholar]
- 26.Westendorp RG, Kirkwood TB. Human longevity at the cost of reproductive success. Nature. 1998;396:743–746. doi: 10.1038/25519. [DOI] [PubMed] [Google Scholar]
- 27.Muller HG, Chiou JM, Carey JR, Wang JL. Fertility and life span: late children enhance female longevity. J Gerontol Biol Sci. 2002;57A:B202–B206. doi: 10.1093/gerona/57.5.b202. [DOI] [PubMed] [Google Scholar]
- 28.Barnes AI, Partridge L. Costing reproduction. Animal Behav. 2002;66:199–204. [Google Scholar]
- 29.Sgro CM, Partridge L. A delayed wave of death from reproduction in Drosophila. Science. 1999;286:2521–2524. doi: 10.1126/science.286.5449.2521. [DOI] [PubMed] [Google Scholar]
- 30.Carey JR, Liedo P, Muller HG, Wang JL, Vaupel JW. Dual modes of aging in Mediterranean fruit fly females. Science. 1998;281:996–998. doi: 10.1126/science.281.5379.996. [DOI] [PubMed] [Google Scholar]
- 31.Eigenmann JE, Patterson DF, Froesch ER. Body size parallels insulin-like growth factor I levels but not growth hormone secretory capacity. Acta Endocrinol (Copenh) 1984;106:448–453. doi: 10.1530/acta.0.1060448. [DOI] [PubMed] [Google Scholar]
- 32.Eigenmann JE, Amador A, Patterson DF. Insulin-like growth factor I levels in proportionate dogs, chondrodystrophic dogs and in giant dogs. Acta Endocrinol (Copenh) 1988;118:105–108. doi: 10.1530/acta.0.1180105. [DOI] [PubMed] [Google Scholar]
- 33.Murakami S, Salmon A, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]


