Abstract
Background
Traditionally in pediatric HIV, the CD4+ T-lymphocyte percent is used in monitoring disease progression due to the variability in absolute CD4+ T-lymphocyte numbers. Because of the high cost of equipment, sophisticated and delicate technology, most laboratories in resource-limited settings use simple protocols that enumerate only the absolute CD4+ T-lymphocyte counts. We assessed the use of absolute CD4+ T-lymphocyte count as a surrogate marker of pediatric HIV disease progression.
Methods
We analyzed the CD4+ T-lymphocytes and HIV viral load over a 10-year period (1996 to 2006) of 97 HIV-infected children enrolled in the Yale Prospective Longitudinal Pediatric HIV Cohort using generalized linear mixed models. Both CD4+ T-lymphocytes and HIV viral load were assessed at baseline and every 2-3 months. The modeling approach utilized in this study allows the intercept and the rate at which outcome variables change over time to vary across participants.
Results
We determined that absolute CD4+ T-lymphocytes count was just as reliable at monitoring pediatric HIV as CD4+ T-lymphocyte percentage. Antiretroviral treatment, regardless of the regimen used, was associated with higher CD+ T-lymphocytes count (p < 0.01). Race was significantly associated with CD4+ T-lymphocytes counts (with lower values for blacks compared to non-blacks; p < 0.01). The presence of other infections was associated with lower CD4+ T-lymphocyte count (p=0.01) and higher viral load (p<0.01, respectively).
Conclusions
In situations where determination of CD4+ T-lymphocyte percentages is not readily available, the absolute count may provide an affordable and accessible laboratory surrogate marker of HIV disease progression in children.
Keywords: HIV disease progression, CD4+ T-lymphocytes, HIV viral load, pediatrics, resource-limited settings
INTRODUCTION
Two significant milestones have characterized the pediatric HIV epidemic. First, the landmark Pediatric AIDS Clinical Trials Group study (PACTG 076) in 1994 demonstrated that a 67% reduction in perinatal HIV transmission could be achieved with the administration of a combination of prenatal, intrapartum, and neonatal zidovudine (ZDV) 1. Second, the introduction of the highly active antiretroviral therapy (HAART) a decade ago has resulted in markedly reduced HIV-related morbidity and mortality in resource-rich countries 2. The next major hurdle is the containment of the epidemic in resource-limited settings, particularly in HIV-infected children. In resource-rich countries, initial measurement of the CD4+ T-lymphocyte count and the plasma HIV-1 RNA level (viral load) is used to determine whether antiretroviral treatment is indicated, and, later in the course of treatment, whether antiretroviral therapy (ART) is successful3. The quantification of HIV-1 RNA levels in plasma and the measurement of CD4+ T-lymphocyte counts have also been used as prognostic markers in HIV-1-infected individuals 4. In HIV-infected adults, absolute CD4+ T-lymphocyte count in peripheral blood predicts development of opportunistic illnesses and death due to AIDS 5. HIV-infected children differ from adults with regards to the natural history of HIV-1 infection, and the response to antiretroviral therapy. The use of CD4+ T-lymphocyte counts and HIV-1 RNA levels as monitoring and prognostic tools in children is a more recent practice and is based on studies with inherent limitations; usually cross-sectional study design, small sample sizes, or limited duration of follow-up 6-9.
Children are less likely than adults to achieve full viral suppression on ART; however, they often recover or gain CD4+ T-lymphocyte counts during therapy 10. The HIV-1 RNA levels remain relatively high and fluctuate over the years in symptomatic as well as in asymptomatic children, making it a less reliable tool for prediction of disease progression and for initiation of therapy 11. Moreover, changes in absolute CD4+ T-lymphocyte counts are difficult to interpret due to wide individual variations in the immunologic response to antiretroviral treatment. The percentage of CD4+ T-lymphocytes rather than their absolute number, has been used as a marker of HIV disease progression in children 12. Because of well-known large natural decline and variation in absolute CD4+ T-lymphocyte numbers in early childhood 13, 14, there is limited experience in the use of absolute CD4+ T-lymphocyte numbers in pediatrics 7. There is limited data on the long-term utility of the absolute CD4+ T-lymphocyte count to guide treatment changes in pediatric HIV.
The standard method for CD4+ T-lymphocyte enumeration is flow cytometry based on either dual or single platform technologies 15. The dual-platform technology determines absolute T-lymphocyte numbers using two different instruments (a hematology analyzer and a flow cytometer). The absolute CD4+ T-lymphocyte number is the product of three laboratory measurements: the white blood cell count, the percentage of white blood cells that are lymphocytes (determined by hematology analyzer), and the percentage of lymphocytes that are CD4+ T-lymphocytes (determined by flow cytometry). The single-platform technology is designed to determine both absolute and percentage lymphocyte subset values using a single tube. Because of the high cost of equipment and reagents, sophisticated and delicate technology, and the need for complex maintenance for the single and dual platform technologies, their applicability is very limited in resource-limited settings 16. A number of cheaper, more robust and simpler to use protocols for CD4+ T-lymphocytes analysis have been developed for resource-limited settings 17, 18, however, these equipments usually estimate the absolute CD4+ T-lymphocyte count, but not the CD4+ T-lymphocyte percentage 19.
In this study, we hypothesized that absolute CD4+ T-lymphocyte count will be equivalent to CD4+ T-lymphocyte percentage in the monitoring and evaluation of the natural history of HIV disease, and response to antiretroviral treatment in children. To test our hypothesis, we analyzed longitudinal data from HIV-infected children enrolled in the Yale Prospective Longitudinal Pediatric HIV Cohort study comprising of children born to HIV-infected mothers in the greater New Haven, Connecticut area since 1985. The current study is unique in assessing the performance of CD4+ T-lymphocyte count in both time-fixed and time-varying models with a longer follow-up duration spanning different treatment strategy eras (i.e., pre-HAART, and HAART), and also provide evidence to support the use of CD4+ T-lymphocyte absolute count as a surrogate marker of pediatric HIV disease progression in resource-limited settings.
METHODS
Study Participants
Longitudinal data were extracted from the Yale Prospective Longitudinal Pediatric Cohort study at Yale University and Yale-New Haven Hospital. The rationale, organization, and recruitment of the subjects for the cohort study have been described in detail elsewhere 20. The enrollment of children born to HIV-1-infected mothers in the greater New Haven area in Connecticut began in 1985. All the children either had mothers already known to be HIV-1 seropositive during pregnancy or at the time of delivery or were discovered to be infected with HIV-1 after presenting with an AIDS defining illness. In the analyses contained herein, we used data collected from HIV-infected children since 1996, when plasma HIV-1 RNA quantification became available. The study was reviewed and approved by the Yale-New Haven Hospital Human Investigation Committee.
Measurements
The outcome variables were absolute CD4+ T-lymphocyte count, CD4+ T-lymphocyte percentage, and viral load. The variables were extracted from participants’ medical and laboratory records with the first recorded set of values (CD4+ T-lymphocyte and HIV viral load) regarded as the baseline values. For participants born before or in 1996, the baseline values were taken in 1996 when viral load measurements were begun in our clinic; subsequently, these measures were obtained every 2-3 months. The data used for analysis were measures obtained between 1996 and December 2006. The predictor variables at baseline included; demographic characteristics of participants (e.g., gender, race, or caregiver type), treatment regimen (e.g., no therapy, monotherapy or combination therapy), history of AIDS defining illness, age at HIV diagnosis, and mode of transmission.
Determination of HIV infection status
Children were defined as infected with HIV-1 if they had a combination of physical, serologic, and HIV-1 antigen and culture-positive findings. Children were determined to be infected with HIV-1 if they had one or more positive cultures for HIV-1 or two positive antigen tests for HIV-120, 21. In addition seropositive children <24 months of age were defined as infected with HIV if the criteria for AIDS (based on the 1987 AIDS surveillance definition) were met 22. More recently, two PCR tests positive for HIV DNA have also been considered proof of HIV infection 23.
Clinic and follow-up visits
The study participants were seen and examined at the pediatric specialty clinic every 2-3 months and more frequently as necessary. Since 1996, HIV-1 RNA quantification and CD4+ T- lymphocyte counts and percentages as well as routine blood tests (e.g., complete blood count, chemistry, and liver function tests, etc.) were done every 2-3 months to follow HIV disease progression. The Amplicor Monitor™ test (Roche Diagnostic Systems, Inc., Branchburg, New Jersey, USA) was used for the quantification of the HIV-1 RNA, with a detection limit of 400 copies/ml, in accordance with the manufacturer’s instructions and the AIDS Clinical Trials Group (ACTG) quality assurance recommendations were followed, as described elsewhere 9, CD4+ T-lymphocytes levels were quantified by standard dual-platform flow cytometry technology by a certified clinical laboratory at Yale-New Haven Hospital.
Antiretroviral Treatment
In the early years of the epidemic, neither treatment to prevent vertical transmission of HIV nor regimens for either prophylaxis nor treatment of AIDS indicator diseases was available. Beginning in April 1989, antiretroviral treatment (initially zidovudine monotherapy) was prescribed for pediatric patients in New Haven who had rapid progressive HIV disease; subsequently, other antiretroviral drugs, alone or in combination, were prescribed. For the current analysis the regimens have been categorized as; mono therapy (group I), dual therapy (group II), and combination therapy (HAART, group III), and triple nucleoside analog therapy (group IV).
Statistical Analysis
Longitudinal data analyses using mixed models were conducted to examine differences in CD4+ T-lymphocyte counts and viral loads by treatment category, disease status, gender, ethnicity and other demographic factors. The modeling approach utilized in this study allows the intercept and the rate at which outcome variables change over time to vary across participants. This analysis was conducted using PROC MIXED in SAS and we assumed that all random effects are independent and have distinct variance components. This procedure provides efficient estimates and valid standard errors of the estimates 24. In addition, PROC MIXED does not require participants to have the same number of visits or measurements, and uses all available data instead of eliminating subjects with missing data, resulting in unbiased estimates of the model parameters when data are missing randomly. Therefore, this analysis makes use of all available data collected for each participant at each time point. The study participants were categorized into four treatment groups (mono, dual, HAART and triple nucleoside analogs).
RESULTS
The average age of the participants at the baseline assessment was 8.5 years (S.D of 4.6 years). The mean of the assessment times was 20.0 visits (S.D of 13.5 visits). The modes of transmission in the cohort were mother-to-child (93%), sexual (6%) and blood transfusion (1%). Table 1 shows the characteristics of the study population by gender, ethnicity, and other infections, for each of the outcome measures (CD4+ T-lymphocyte and viral load) at baseline. At baseline, one and five participants had missing viral load and CD4+ T-lymphocyte counts, respectively. The CDC Pediatric HIV classification 22 of the participants at baseline was 13%, 24%, 28%, and 35% for clinical class N, A, B, and C, respectively. The proportion of participants with immunologic class 1, 2 and 3 was 14%, 33%, and 53%, respectively. 63% and 86% of the children had moderate to severe clinical and immunologic disease, respectively.
Table 1.
Baseline Characteristics and Study Variables
| Viral Load* | CD4+ T-cell Counts† | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | P-Value | N | Mean | SD | P-Value | |
| Gender | ||||||||
| Male | 53 | 127.84 | 183.54 | 0.11 | 49 | 475.2 | 501.39 | 0.94 |
| Female | 44 | 202.33 | 267.18 | 43 | 482.21 | 403.79 | ||
| Race | ||||||||
| Black | 55 | 180.95 | 249.64 | 0.32 | 53 | 476.7 | 519.8 | 0.86 |
| Non-Black | 41 | 133.41 | 195.41 | 38 | 493.5 | 353.9 | ||
| Other Infections¶ | ||||||||
| No | 33 | 88.94 | 123.45 | 0.078 | 32 | 599.44 | 406.9 | 0.024 |
| Yes | 33 | 176.21 | 249.92 | 31 | 377.48 | 350.2 | ||
Number of HIV-1 RNA copies X 103 per ml of plasma
Number of cells per μl of whole blood
The other infections were recurrent invasive bacterial infections (mainly pneumococcal infections).
We first used a two-sample T-test analysis to determine whether there was evidence of significant differences in the mean score of each response variable between males and females; blacks and non-blacks; and baseline co-infection and/or an opportunistic infection status (Table 1). Females had higher CD4+ T-lymphocyte counts and viral loads in comparison with males, however, the difference did not reach statistical significance. Furthermore, there was no statistically significant difference between the CD4+ T-lymphocyte counts and viral load of blacks and non-blacks although blacks had lower CD4+ T-lymphocyte count and higher viral load as compared with non-blacks. The CD4+ T-lymphocyte counts for children who had other infections and/or an opportunistic infection at baseline were significantly lower than the CD4+ T-lymphocyte counts of HIV-infected children without other infections and/or an opportunistic infection (p = 0.024). Children with other infections and/or an opportunistic infection had higher viral loads compared with those without any other infections, however, this was not statistically significant (p = 0.078). During the study period, other infections were predominantly invasive bacterial infections with Streptococcus pneumoniae as the leading etiological agent. Herpes zoster, disseminated Mycobacterium avium complex (MAC), Pneumocystis jiroveci pneumonia (PCP), and candidiasis were more predominant before 1996.
Several longitudinal models were fitted in our analyses using viral load, CD4+ T-lymphocyte counts and CD4+ T-lymphocyte percent as outcome variables (Table 2). Logarithmic transformation of the CD4+ T-lymphocyte counts was performed to facilitate normalization of the data distribution. The parameter estimates for Model I showed no significant decrease in CD4+ T-lymphocyte counts over time (p = 0.195). However, there was a significant effect of treatment regardless of the regimen on CD4+ T-lymphocyte counts (p <0.001). The adjusted average log CD4+ T-lymphocytes for mono, dual, HAART and triple nucleoside analogs were 0.28, 0.11, 0.25 and 0.14 units, respectively, higher compared with no therapy. There was a significant negative effect of other infections (β = -0.25; p = 0.011), indicating that participants who had other infections or opportunistic infections had lower levels of CD4+ T-lymphocyte counts. In addition, there was a significant effect of race on CD4+ T-lymphocyte counts (p = 0.018). On average, the log CD4+ lymphocyte counts for blacks decreased by 0.24 units compared with that of non-blacks. Neither mode of transmission (p = 0.257) nor gender (p = 0.383) had a significant effect on CD4+ T-lymphocyte counts. Interestingly, when CD4+ T-lymphocyte percentage was substituted for absolute count in the longitudinal model (Table 2; model II- CD4+ %) the results were similar to that obtained with absolute count.
Table 2.
Parameter Estimates of Longitudinal Modeling of Outcome Variables
| Parameter | Estimate | S.E.* | p-value |
|---|---|---|---|
| Model-I: CD4 T-Cell Counts | |||
| Time | -0.003 | 0.003 | 0.195 |
| Gender | -0.088 | 0.101 | 0.383 |
| Race | -0.238 | 0.101 | 0.018 |
| Treatment | |||
| Mono Therapy | 0.279 | 0.055 | <0.001 |
| Dual Therapy | 0.105 | 0.029 | <0.001 |
| HAART | 0.246 | 0.026 | <0.001 |
| Triple Nucleoside | 0.137 | 0.031 | <0.001 |
| Other Infections | -0.253 | 0.099 | 0.011 |
| Mode of Transmission | -0.165 | 0.146 | 0.257 |
| Model-II: CD4-Percent | |||
| Time | -0.019 | 0.049 | 0.698 |
| Gender | -2.513 | 2.402 | 0.296 |
| Race | -5.560 | 2.399 | 0.021 |
| Treatment | |||
| Mono Therapy | 5.871 | 1.282 | <0.001 |
| Dual Therapy | 2.889 | 0.677 | <0.001 |
| HAART | 5.174 | 0.609 | <0.001 |
| Triple Nucleoside | 3.142 | 0.728 | <0.001 |
| Other Infections | -6.975 | 2.365 | 0.003 |
| Mode of Transmission | -3.358 | 3.471 | 0.333 |
| Model-III: Viral Load | |||
| Time | -0.539 | 0.349 | 0.129 |
| Gender | -8.874 | 18.052 | 0.623 |
| Race | 4.825 | 18.013 | 0.789 |
| Treatment | |||
| Mono Therapy | -16.692 | 21.599 | 0.439 |
| Dual Therapy | -57.206 | 11.192 | <0.001 |
| HAART | -86.023 | 10.150 | <0.001 |
| Triple Nucleoside | -22.382 | 12.172 | 0.066 |
| Other Infections | 52.850 | 17.878 | 0.003 |
| Mode of Transmission | 18.669 | 26.681 | 0.484 |
Standard error
Considering the greater variability of absolute CD4+ T-lymphocytes among children under 5 years of age 25, we investigated the reliability of the CD4+ T-lymphocyte absolute count as surrogate marker among children in the cohort followed from birth to 5 years of age. Only sixteen participants met the criteria for the sub analysis (data not shown). There was no statistically significant effect of time, treatment, and other infections on either absolute CD4+ T-lymphocyte count or CD4+ T-lymphocyte percentage. The data may imply that absolute CD4+ T-lymphocyte is as useful as CD4+ T-lymphocyte percentage in monitoring HIV disease progression in the 0 to 5 years age group. However, this should be interpreted with caution as the small sample size for the sub analysis does not have the power to detect differences if any.
The parameter estimates in Model III indicates that viral load significantly decreased with effective combination therapy (p < 0.01). The adjusted average viral load for dual and HAART were respectively 57 and 86 units lower compared with no therapy. However, there was no significant difference between the viral loads of mono therapy and triple nucleoside analogs as compared with no therapy. Moreover, the viral load of children with other infections was significantly higher compared with those without other infections (p<0.01). There was no significant change in viral load over time (p = 0.129), and neither gender (p = 0.62) nor mode of transmission (p = 0.48) had any significant effect on viral load. Additional models were considered for each outcome variable. For example, participants were nested according to caregiver to evaluate the influence of type of caregiver on outcome variables. The caregivers were predominantly biological relatives of the HIV-infected children. There was no statistically significant effect of caregiver type on the outcome variables (data not shown). Age at diagnosis of HIV infection was a significant predictor of CD4+ T-lymphocyte counts, with those diagnosed at earlier age having higher values.
The trajectories of the study outcomes (absolute CD4+ T-lymphocytes and viral loads) for the first fourteen visits are illustrated in Figure 1. There were missing CD4+ T-lymphocytes counts and viral loads for some participants during the first 14 visits. The missing values were due to; small volume of blood to run both CD4+ T-lymphocyte count and viral load, missing laboratory data from the patient’s chart, and lost of participant to follow up (relocation and six deaths). At baseline, 51 out of the 97 participants had severe immunologic suppression. There was steady improvement in the CD4+ T-lymphocyte count with the availability of effective antiretroviral combination regimens (Fig. 1A).
Figure 1.
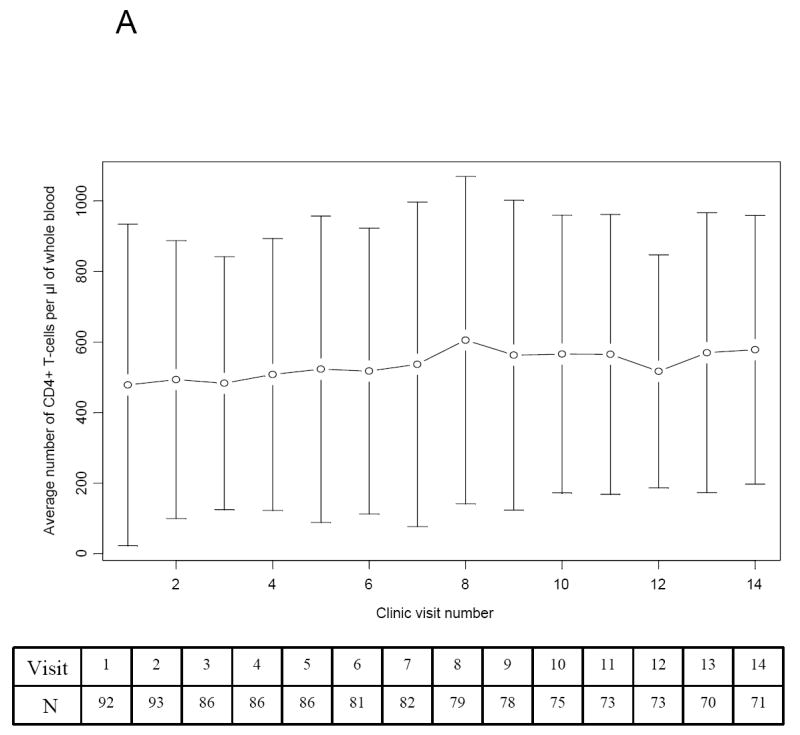
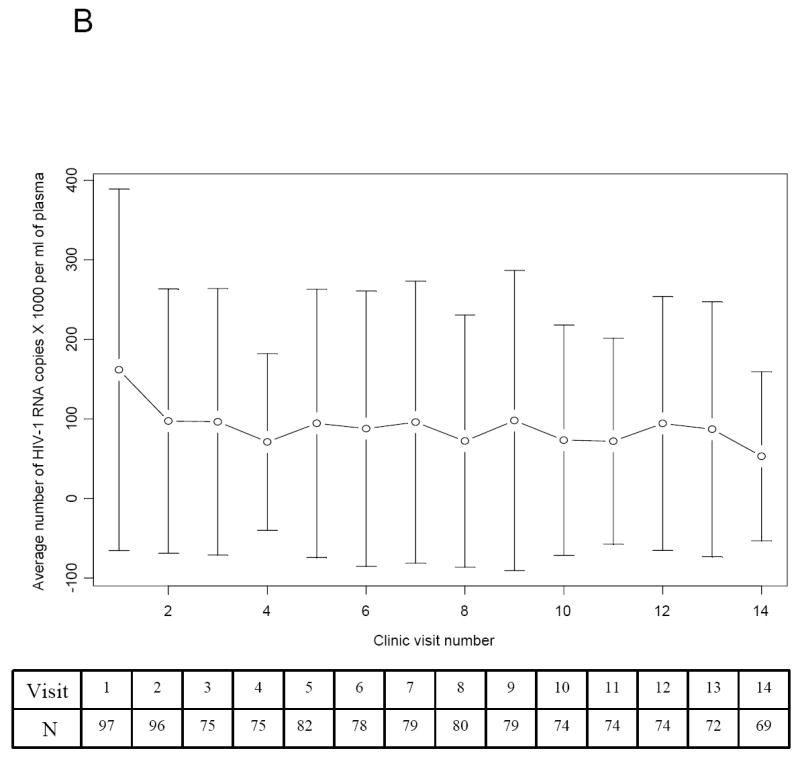
Trends in the Absolute CD4+ T-lymphocyte Count and Viral Load of Study Participants over the first 14 visits. The table inserted below the graph contains the number of participants (N) included in the analysis at each clinic visit. (A) The average (mean) CD4+ T-lymphocyte count (cell/μl of whole blood) and standard deviation plotted over the first 14 clinic visits. (B) The average (mean) viral load (HIV RNA copies X 1000/mL of plasma) and standard deviation plotted over the first 14 clinic visits.
The mean viral load for visits 1, 2 to 13, and 14 was 170,000, 70,000 - 100,000, and 50,000 copies/ml, respectively (Fig. 1B). The high mean viral load was due partly to extreme high viral loads (250,000 - 750,000 copies/ml) of participants who were not adherent to their treatment regimens. The proportion of participants with viral loads ≥ 250,000 copies/ml decreased over time. At visit 1, 20 of the participants had viral loads ≥ 250,000 copies/ml as compared with 8 and 3 participants at visits 7 and 14, respectively. Two of the 3 participants with high viral loads at visit 14 subsequently succumbed to opportunistic infections. About a third of the 77 participants who were diagnosed with HIV infection prior to 1996 continued on dual antiretroviral therapy regimens into the fourth visit. Two participants continued on mono therapy during the first 14 visits. One had viral load between 1,000 and 8,000 copies/ml, and the other had undetectable viral load at each visit. The rest of the participants were on either non nucleoside or protease inhibitor based-HAART. Though, adherence to therapy was not routinely recorded at each visit, 23 participants were noted as being intermittently or completely non adherent to prescribed regimens.
DISCUSSION
In this study, we sought to assess the performance of CD4+ T-lymphocyte absolute count as a surrogate marker of HIV-1 disease progression in children. The analysis included data extracted from the medical records of 97 HIV-infected children (from 1996 to 2006) of the Yale Prospective Longitudinal Pediatric HIV Cohort. There was no significant difference between males and females in CD4+ T-lymphocyte count at baseline. Most of the study participants had their baseline measurements in 1996 prior to the availability of HAART regimens.
An unresolved issue in the care of HIV-infected children is the role of absolute CD4+ T-lymphocyte count in monitoring HIV disease progression. Using a generalized linear mixed model, we found that absolute CD4+ T-lymphocyte counts were just as reliable at predicting disease progression as CD4+ T-lymphocyte percentages or viral loads. Due to the variations in CD4+ T-lymphocyte numbers in children, the CD4+ T-lymphocyte percentage is traditionally used to monitor the natural history, and the effects of antiretroviral treatment in HIV-infected children 13. However, in resource-limited settings low-cost assays available for enumeration of CD4+ T-lymphocyte mainly provide the absolute counts but not the percentages 19. We therefore compared the performance of absolute CD4+ T-lymphocyte count to CD4+ T-lymphocyte percentage in our pediatric cohort over a 10-year period. Race, treatment with antiretroviral agents, and other infections had similar effects on absolute CD4+ T-lymphocyte count and percentage CD4+ T-lymphocyte. Our findings suggest that the absolute CD4+ T-lymphocyte count has similar utility as CD4+ T-lymphocyte percentage in monitoring HIV infection in children. Our findings corroborate those of the HIV Pediatric Prognostic Markers Collaborative Study 25, 26, and provide a proof of the concept of using absolute CD4+ T-lymphocytes to monitor pediatric HIV disease progression in resources-limited settings. CD4+ T-lymphocyte count has been found to be less prognostic in younger children than CD4+ T-lymphocyte percentage 25. The sub analysis did not have the power to detect any differences in the use of either CD4+ T-lymphocyte count or percentage in children from 0 to 5 years of age. Further investigation are needed in resource-limited settings to compare the utility of CD4+ T-lymphocyte count with CD4+ T-lymphocyte percentage as a surrogate for monitoring HIV-disease progression in children aged 0 to 5 years.
CD4+ T-lymphocyte depletion is a hallmark of HIV disease, and a significant predictor of the short-term risk of progression to AIDS 27. The CD4+ T-lymphocyte decline in blacks was rapid than in non-blacks in our longitudinal model even though there was no significant correlation between viral load and race (Table 2). This finding becomes more relevant in resource-limited settings, where the traditional monitoring of patients on ART with regular viral load measures and CD4+ T-lymphocyte percentages is not readily available. In resource-limited settings, monitoring the efficacy of treatment is hampered by the lack of laboratory facilities, and even where such tests can be done most patients can not have access to them 28. The current criteria for immunological treatment failure in resource-limited settings by the World Health Organization (WHO) are CD4+ T-lymphocyte counts that fall below the baseline level or one that falls by more than 50% after an initial increase 29. Several investigators have looked at different clinical models and cost-effective assays for monitoring virological efficacy of ART in resource-limited settings 19, 30-32. Our finding of rapid decline in CD4+ T-lymphocytes of HIV-infected blacks suggests that when the decline in CD4+ T-lymphocyte count is used alone or as part of a clinical model to monitor the effect of HIV treatment, providers may have to consider race and/or population-based reference values in making treatment decisions 33. The changes in CD4+ T-lymphocyte count are often independent of the effects of treatment on viral load 34. In a multicenter cohort study of treatment naïve HIV-infected adults, Rodriguez et al. found an absolute CD4+ T-lymphocyte cell loss of 50.5 (CI of 46.2 -54.8) cells/μL per year irrespective of the HIV RNA level 35. They concluded that the magnitude of the viral replication, reflected by the plasma HIV RNA level, is not the main determinant of the rate of CD4+ T-lymphocyte loss. Taken together, we postulate that individual differences or race may play a role in the rate of CD4+ T-lymphocyte decline during HIV disease progression in infected children. In the European Collaborative Study (ESC), differences in numbers of total lymphocytes and subsets by race were reported to occur in uninfected children born to HIV-infected mothers; there were lower total lymphocyte, CD4+ and CD8+ cell counts in blacks than in whites 36, 37. In the ESC cohort, the majority of the children were born to HIV-infected mothers of African descent 38. The difference in CD4+ T-lymphocyte numbers among black and white children undermines how one uses standard CD4+ T-lymphocyte cutoff values for monitoring the clinical course of HIV-infected children. The association between race and CD4+ T-lymphocyte numbers and their decline in HIV-infected children needs further research to inform decisions on initiation and monitoring of treatment, particularly in resource-limited settings.
Another significant finding, consistent with other reports, is that the immunological response to antiretroviral in our cohort was independent of the virological response 10. There was recovery of CD4+ T-lymphocyte count irrespective of the treatment regimen in comparison with no therapy. However, there was no statistically significant reduction in viral load with mono therapy and triple nucleoside regimens (Table 2). This may imply that viral load reduction is a function of the potency of a regimen. Other infections at baseline or in the longitudinal models had a negative effect on both viral load and CD4+ T-lymphocyte count. The likely explanation is that these infections may produce cytokines that can directly enhance HIV replication or create an inflammatory microenvironment leading to increased numbers of HIV target cells, thereby resulting in increased HIV RNA production and ultimately CD4+ T-lymphocyte depletion. The most common infections observed among the study participants were recurrent invasive bacterial infections (with preponderance of pneumococcal infections). The type of caregiver had no significant effect on either viral load or CD4+ T-lymphocyte count. Two-thirds of the study participants were cared for by either a biological parent, or a family member; this may account for a failure to observe an effect. Considering the overall well-being of our study participants one can speculate that being cared for in a functional-family system may protect against HIV disease progression. This finding may be applicable to resource-limited settings.
Our study, like other population-based studies, is particularly relevant for evaluating the full spectrum of surrogate markers and the long-term effect on chronic diseases like HIV, however, it has some limitations and care should be taken in the interpretation of our findings. First, it spans a period of ten years during which time HIV medicine underwent rapid and complex changes in treatment regimens. Since these rapid changes were not envisaged at the start of the study crucial treatment data are missing making it difficult to assess the direct impact of the components of various regimens on viral load and CD4+ T-lymphocyte counts. Medical records at clinic visits were not standardized and in most instances records on treatment adherence were missing. Moreover, care should be exercised when extrapolating our findings to resource-limited settings where the full complement of care (i.e., medical, nutritional, housing, and psychosocial) may not be available. However, with these caveats, our findings are still significant and warrant further investigations in pediatric HIV care in resource-limited settings.
In conclusion, our findings have several implications for the management of HIV-infected children. First, absolute CD4+ T-lymphocyte counts are just as reliable at predicting disease progression as CD4+ T-lymphocyte percentages or viral loads. Second, race may play a critical role in the rate of CD4+ T-lymphocyte decline during the progression of HIV infection, and therefore it may be expedient to establish race-based or at the least a population-based CD4+ T-lymphocyte cell cutoffs for monitoring pediatric HIV infections 37. The CD4+ T-lymphocyte count had greater stability than viral load over the study duration. Therefore, in situations where determinations of both viral load and CD4+ T-lymphocyte percentages are not readily available, the CD4+ T-lymphocyte absolute count may provide an affordable, acceptable and accessible laboratory surrogate marker of HIV disease progression in children that might assist in therapeutic decision-making.
Acknowledgments
We are grateful to the clinical team of the Pediatric AIDS Care Program for providing specialized medical care and social work services for the children enrolled. We thank the children and families who participated in the HIV prospective longitudinal pediatric cohort study.
Support for this study came from the National Institute of Child Health and Human Development (5 N01 HD 3-3345), National Institute of Allergy and Infectious Disease (AI32397 and AI39015); and EP was supported by Child Health Research Center Award K12HD001401-08.
References
- 1.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 2.Yeni PG, Hammer SM, Hirsch MS, et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA. 2004;292:251–265. doi: 10.1001/jama.292.2.251. [DOI] [PubMed] [Google Scholar]
- 3.Hammer SM, Saag MS, Schechter M, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien WA, Hartigan PM, Daar ES, Simberkoff MS, Hamilton JD. Changes in plasma HIV RNA levels and CD4+ lymphocyte counts predict both response to antiretroviral therapy and therapeutic failure. VA Cooperative Study Group on AIDS. Ann Intern Med. 1997;126:939–945. doi: 10.7326/0003-4819-126-12-199706150-00002. [DOI] [PubMed] [Google Scholar]
- 5.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 6.Mofenson LM, Korelitz J, Meyer WA, 3rd, et al. The relationship between serum human immunodeficiency virus type 1 (HIV-1) RNA level, CD4 lymphocyte percent, and long-term mortality risk in HIV-1-infected children. National Institute of Child Health and Human Development Intravenous Immunoglobulin Clinical Trial Study Group. J Infect Dis. 1997;175:1029–1038. doi: 10.1086/516441. [DOI] [PubMed] [Google Scholar]
- 7.Palumbo PE, Raskino C, Fiscus S, et al. Predictive value of quantitative plasma HIV RNA and CD4+ lymphocyte count in HIV-infected infants and children. JAMA. 1998;279:756–761. doi: 10.1001/jama.279.10.756. [DOI] [PubMed] [Google Scholar]
- 8.Valentine ME, Jackson CR, Vavro C, et al. Evaluation of surrogate markers and clinical outcomes in two-year follow-up of eighty-six human immunodeficiency virus-infected pediatric patients. Pediatr Infect Dis J. 1998;17:18–23. doi: 10.1097/00006454-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Shearer WT, Quinn TC, LaRussa P, et al. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. Women and Infants Transmission Study Group. N Engl J Med. 1997;336:1337–1342. doi: 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- 10.De Rossi A. Virological and immunological response to antiretroviral therapy in HIV-1 infected children: genotypic and phenotypic assays in monitoring virological failure. New Microbiol. 2004;27:45–50. [PubMed] [Google Scholar]
- 11.Naver L, Ehrnst A, Belfrage E, et al. Long-term pattern of HIV-1 RNA load in perinatally infected children. Scand J Infect Dis. 1999;31:337–343. doi: 10.1080/00365549950163743. [DOI] [PubMed] [Google Scholar]
- 12.Hughes MD, Stein DS, Gundacker HM, Valentine FT, Phair JP, Volberding PA. Within-subject variation in CD4 lymphocyte count in asymptomatic human immunodeficiency virus infection: implications for patient monitoring. J Infect Dis. 1994;169:28–36. doi: 10.1093/infdis/169.1.28. [DOI] [PubMed] [Google Scholar]
- 13.Wade AM, Ades AE. Incorporating correlations between measurements into the estimation of age-related reference ranges. Stat Med. 1998;17:1989–2002. doi: 10.1002/(sici)1097-0258(19980915)17:17<1989::aid-sim891>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 14.Stein DS, Korvick JA, Vermund SH. CD4+ lymphocyte cell enumeration for prediction of clinical course of human immunodeficiency virus disease: a review. J Infect Dis. 1992;165:352–363. doi: 10.1093/infdis/165.2.352. [DOI] [PubMed] [Google Scholar]
- 15.Mandy FF, Nicholson JK, McDougal JS. Guidelines for performing single-platform absolute CD4+ T-cell determinations with CD45 gating for persons infected with human immunodeficiency virus. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2003;52:1–13. [PubMed] [Google Scholar]
- 16.Lyamuya EF, Kagoma C, Mbena EC, et al. Evaluation of the FACScount, TRAx CD4 and Dynabeads methods for CD4 lymphocyte determination. J Immunol Methods. 1996;195:103–112. doi: 10.1016/0022-1759(96)00094-4. [DOI] [PubMed] [Google Scholar]
- 17.Sherman GG, Galpin JS, Patel JM, Mendelow BV, Glencross DK. CD4+ T cell enumeration in HIV infection with limited resources. J Immunol Methods. 1999;222:209–217. doi: 10.1016/s0022-1759(98)00172-0. [DOI] [PubMed] [Google Scholar]
- 18.Cassens U, Gohde W, Kuling G, et al. Simplified volumetric flow cytometry allows feasible and accurate determination of CD4 T lymphocytes in immunodeficient patients worldwide. Antivir Ther. 2004;9:395–405. [PubMed] [Google Scholar]
- 19.Diagbouga S, Chazallon C, Kazatchkine MD, et al. Successful implementation of a low-cost method for enumerating CD4+ T lymphocytes in resource-limited settings: the ANRS 12-26 study. AIDS. 2003;17:2201–2208. doi: 10.1097/00002030-200310170-00008. [DOI] [PubMed] [Google Scholar]
- 20.Andiman WA, Simpson BJ, Olson B, Dember L, Silva TJ, Miller G. Rate of transmission of human immunodeficiency virus type 1 infection from mother to child and short-term outcome of neonatal infection. Results of a prospective cohort study. Am J Dis Child. 1990;144:758–766. doi: 10.1001/archpedi.1990.02150310026020. [DOI] [PubMed] [Google Scholar]
- 21.Simpson BJ, Andiman WA. Difficulties in assigning human immunodeficiency virus-1 infection and seroreversion status in a cohort of HIV-exposed in children using serologic criteria established by the Centers for Disease Control and Prevention. Pediatrics. 1994;93:840–842. [PubMed] [Google Scholar]
- 22.Classification system for human immunodeficiency virus (HIV) infection in children under 13 years of age. MMWR Morb Mortal Wkly Rep. 1987;36:225–230. 235–226. [PubMed] [Google Scholar]
- 23.Dunn DT, Brandt CD, Krivine A, et al. The sensitivity of HIV-1 DNA polymerase chain reaction in the neonatal period and the relative contributions of intra-uterine and intra-partum transmission. AIDS. 1995;9:F7–11. doi: 10.1097/00002030-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci. 1998;76:1216–1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- 25.Use of total lymphocyte count for informing when to start antiretroviral therapy in HIV-infected children: a meta-analysis of longitudinal data. Lancet. 2005;366:1868–1874. doi: 10.1016/S0140-6736(05)67757-4. [DOI] [PubMed] [Google Scholar]
- 26.Predictive value of absolute CD4 cell count for disease progression in untreated HIV-1-infected children. AIDS. 2006;20:1289–1294. doi: 10.1097/01.aids.0000232237.20792.68. [DOI] [PubMed] [Google Scholar]
- 27.Pantaleo G, Graziosi C, Fauci AS. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993;328:327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- 28.Nkengasong JN, Adje-Toure C, Weidle PJ. HIV antiretroviral drug resistance in Africa. AIDS Rev. 2004;6:4–12. [PubMed] [Google Scholar]
- 29.Scaling up antiretroviral therapy in resource-limited settings: guidelines for a public health approach. Executive summary. IAPAC. 2002;8:168–175. [PubMed] [Google Scholar]
- 30.Colebunders R, Moses KR, Laurence J, et al. A new model to monitor the virological efficacy of antiretroviral treatment in resource-poor countries. Lancet Infect Dis. 2006;6:53–59. doi: 10.1016/S1473-3099(05)70327-3. [DOI] [PubMed] [Google Scholar]
- 31.Kent DM, McGrath D, Ioannidis JP, Bennish ML. Suitable monitoring approaches to antiretroviral therapy in resource-poor settings: setting the research agenda. Clin Infect Dis. 2003;37:S13–24. doi: 10.1086/375368. [DOI] [PubMed] [Google Scholar]
- 32.Florence E, Dreezen C, Schrooten W, et al. The role of non-viral load surrogate markers in HIV-positive patient monitoring during antiviral treatment. Int J STD AIDS. 2004;15:538–542. doi: 10.1258/0956462041558159. [DOI] [PubMed] [Google Scholar]
- 33.Lugada ES, Mermin J, Kaharuza F, et al. Population-based hematologic and immunologic reference values for a healthy Ugandan population. Clin Diagn Lab Immunol. 2004;11:29–34. doi: 10.1128/CDLI.11.1.29-34.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Florence E, Lundgren J, Dreezen C, et al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. HIV Med. 2003;4:255–262. doi: 10.1046/j.1468-1293.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez B, Sethi AK, Cheruvu VK, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296:1498–1506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 36.Are there gender and race differences in cellular immunity patterns over age in infected and uninfected children born to HIV-infected women? J Acquir Immune Defic Syndr. 2003;33:635–641. doi: 10.1097/00126334-200308150-00013. [DOI] [PubMed] [Google Scholar]
- 37.Bunders M, Cortina-Borja M, Newell ML. Age-related standards for total lymphocyte, CD4+ and CD8+ T cell counts in children born in Europe. Pediatr Infect Dis J. 2005;24:595–600. doi: 10.1097/01.inf.0000168835.01233.64. [DOI] [PubMed] [Google Scholar]
- 38.HIV-infected pregnant women and vertical transmission in Europe since 1986. European collaborative study. AIDS. 2001;15:761–770. [PubMed] [Google Scholar]


