Abstract
Rationale
Tumor necrosis factor-α (TNFα) acts within the brain to induce sickness behavior, but the molecular mechanisms are still unknown. TNFα binding induces receptor trimerization, activation of c-Jun N-terminal kinase (JNK), and induction of downstream transcription factors.
Objectives
We hypothesized that TNFα-induced sickness behavior can be blocked by a novel JNK inhibitor.
Methods
To test this idea, we used a bipartite protein consisting of a ten-amino-acid sequence of the trans-activating domain of the viral TAT protein (D-TAT) linked to a 19-amino-acid peptide that specifically inhibits JNK activation (D-JNKI-1). C57BL/6J mice were pre-treated intracerebroventricularly (i.c.v.) with D-JNKI-1 or the control peptide containing only the protein transduction domain, D-TAT. Mice were then injected centrally with an optimal amount of TNFα (50 ng/ mouse) to induce sickness behavior. Sickness was assessed as a decrease in social exploration of a novel juvenile, an increase in duration of immobility and loss of body weight.
Results
Pre-treatment with D-JNKI-1 (10 ng/mouse), but not D-TAT, significantly inhibited all three indices of sickness induced by central TNFα.
Conclusions
These findings demonstrate that D-JNKI-1 can abrogate TNFα-induced sickness behavior and suggest a potential therapeutic target for treating major depressive disorders that develop on a background of cytokine-induced sickness behavior.
Keywords: Mouse, D-JNKI-1, Social exploration, Cytokine, TNFα, Protein transduction domain
Introduction
Originally identified as a secretory product of macrophages, TNFα is now known to be synthesized by microglia in the brain where its expression is associated with a number of behavioral responses (Fiore et al. 1996, 1998). TNFα binds to two distinct receptors, the 55 kDa TNF-R1 and the 75 kDa TNF-R2 (Vandenabeele et al. 1995). TNFα binding trimerizes either receptor and recruits adaptor proteins such as TNF-R-associated factor-2. This action initiates a plethora of signaling cascades, including activation of nuclear factor (NF)-κB, p38 mitogen-activated protein kinase (MAPK), c-jun N-terminal kinase (JNK), caspases, and sphingomyelinases (McCusker et al. 2006; O'Connor et al. 2008b). Despite well-characterized receptors and identification of several intracellular signaling intermediates, the molecular details that lead to TNFα-induced sickness behavior remain unknown.
Intracerebroventricular (i.c.v.) administration of either human or murine TNFα into mice causes a reduction in social interaction of a novel juvenile, an increase in immobility, a decrease in food intake, and a loss of body weight (Bluthe et al. 1994, 2000). Murine TNFα acts on neurons through TNF-R1 (Yang et al. 2002), which indirectly links TNF-R1 to the behavioral responses. Although human TNFα is an agonist of only TNF-R1 in mice, it fully induces sickness behavior in this species (Bluthe et al. 1991). This finding directly implicates TNF-R1 in sickness behavior. Furthermore, mice lacking TNF-R2 are fully responsive to murine TNFα (Palin et al. 2007), which strongly supports signaling through TNF-R1 as a requirement for TNFα-induced sickness behavior. Recent findings support the hypothesis that TNFα-induced JNK activation via TNF-R1 likely plays an important role in neuroinflammation (Borsello et al. 2003; Bubici et al. 2004; Waetzig et al. 2005), the reduction in food intake (Moraes et al. 2006) and altered learning and memory (Medeiros et al. 2007). Collectively, these data suggest that TNFα-induced sickness behavior is mediated by TNF-R1 and might be blocked by inhibitors of JNK in the brain.
The recent discovery, development, and application of short protein transduction domains, formerly known as cell-penetrating peptides, to deliver much larger cargos into cells is now being exploited in a variety of diseases (Tilstra et al. 2007). These proteins can transport peptides, liposomes and oligonucleotides across cell membranes, thereby providing a new and powerful technique to regulate distinct intracellular events and link specific pathways to discrete physiological outcomes. One of these reagents, D-JNKI-1, is coupled to the arginine-rich HIV Tat protein. D-JNKI-1 readily crosses cellular membranes and then directly and specifically blocks JNK activation (Kuan and Burke 2005; Repici and Borsello 2006). We recently demonstrated that when used in vitro, activation of JNK by TNFα is completely blocked by D-JNKI-1, thereby preventing TNFα from inhibiting cellular differentiation (Strle et al. 2006). We hypothesized that this same JNK inhibitor, when given centrally, would also act in vivo to prevent the development of TNFα-induced sickness. Experiments reported here confirm this hypothesis and demonstrate for the first time that a specific JNK inhibitor coupled to a protein transduction domain can be used to block sickness behavior induced by central TNFα.
Materials and methods
Animal housing
C57BL/6J male mice, 6 weeks of age at arrival (Charles River Laboratories), were maintained in polycarbonate transparent cages with corn cob litter in a temperature (23± 1°C) and humidity (40%) controlled room. This room was maintained on a 12–12 h light/dark cycle (lights off at 09:00 h). The mice were group-housed in cages (40 × 25 × 15 cm) under these conditions for 1 week prior to surgery. Two weeks later, the treatments were initiated. Animals had free access to food and water. Juvenile male Crl:CD1(ICR) BR mice (3–4 weeks of age; Charles River Laboratories) were used to provide stimuli for the social investigation behavioral paradigm. Animal protocols were approved by the Institutional Animal Care and Use Committees.
Surgical procedures
Mice were anesthetized with a mixture of ketamine and xylazine (i.p., 13 mg/kg and 0.9 mg/kg body weight, respectively). Animals were placed in a Kopf stereotaxic instrument (Tujunga, CA) and surgically implanted unilaterally with a stainless-steel guide cannula (23-gauge, 7 mm length) 0.6 mm posterior and 1.5 mm lateral of the bregma and to protrude 2 mm below the skull surface at the point of entry, which was 1 mm above the lateral ventricle (Paxinos and Franklin 2001). Mice were allowed to recover for two weeks prior to initiation of behavioral tests, so mice were tested at approximately 9 weeks of age. Cannula placement was confirmed by injection of India ink at the conclusion of each experiment. The brain of every mouse was then removed, sliced, and the site of injection was verified.
Treatments
Treatments were administered to non-anesthetized animals over 90 s in a volume of one microliter via a 30-gauge needle placed through the guide cannulae into the lateral ventricle. Treatments were administered within 1 h of initiation of the dark phase, which permitted the behavioral tests to be performed during the dark. All reagents were reconstituted in artificial cerebrospinal fluid (aCSF; NaHCO3 26.2 mM; glucose 10 mM; NaCl 120 mM; Na2HPO4 1 mM; KCl 2.5 mM; MgCl2 1 mM; CaCl2 2.5 mM), and aCSF was used as the control. The protease-resistant all-D-retroinverso stereoisomer inhibitor of JNK, D-JNKI-1, is a peptide that contains a 19-amino-acid inhibitory peptide derived from the functional JNK-binding domain of JNK interacting protein (JIP, (D)-hJIP[175–157]). Cell permeability of the inhibitory peptide is achieved by the covalently linked ten-amino-acid protein transduction trans-activating domain of the viral TAT protein (HIV-TAT[57–48]). The bipartite protein contains two proline residues to link the two domains and to act as spacers to allow maximal flexibility (GRKKRRQRRR-PP-RPKRPTTLNLFPQVPRSQD-amide). D-JNKI-1 and DTAT were prepared in aCSF. Two behavioral experiments using D-JNKI-1 were conducted, the first being a dose–response experiment for D-JNKI-1. Specificity of D-JNKI-1 action was then confirmed in a second experiment using one dose of D-JNKI-1 and the same dose of control peptide. The inactive inhibitor control peptide, D-TAT, is composed of only the ten-amino-acid TAT protein transduction sequence. D-JNKI-1 and D-TAT (Axxora, San Diego, CO) were administered 1 h before TNFα administration. An optimal amount of recombinant murine TNFα (50 ng i.c.v.; R&D Systems Inc., Minneapolis, MN) that induces sickness behavior was previously established in dose–response experiments with both CD1 (Bluthe et al. 2006) and C57BL/6J mice (Palin et al. 2007). The mice used to collect body weight and behavioral measurements were the same. Mice were treated only once with TNFα.
Behavioral measurements
Prior to initiation of experiments, C57BL/6J mice were handled for 5 min each day for one week. Mice were individually housed in polycarbonate transparent cages (26 × 20 × 14 cm) for 24 h prior to administration of treatments. Three well-established criteria were used to quantify sickness: (a) Duration of social exploration directed towards a novel juvenile during the first 4 min following introduction of the juvenile into the home cage of the treated mouse, as previously described (Crestani et al. 1991). Treated mice were exposed to different juveniles across time; (b) length of time the test animal remained immobile during this 4 min observation period, and (c) change in body weight over a 6-h period following treatment. Behavioral observations were recorded using a video camera under red light illumination between 09:00 h and 17:00 h (dark phase) and scored by a trained observer blinded to the experimental treatments. Behavior was recorded 2, 6, and 24 h after treatment with TNFα.
Experimental design and statistical analysis
Mice were treated with D-JNKI-1 or D-TAT in a completely randomized design. Behavioral data were analyzed by a three-way ANOVA with pre-treatment (aCSF, D-TAT or DJNKI-1 at 5 ng or 10 ng) and treatment (aCSF or TNFα)as between-subject factors, and time as a within-subject factor. Change in body weight was analyzed at 6 h after TNFα injection by a two-way (pre-treatment×treatment) ANOVA. Post-hoc comparisons were conducted with the protected LSD. Results were presented as means±SEM; n=6 for all data points.
Results
The reduction in social exploration caused by central TNFα is blocked by D-JNKI-1
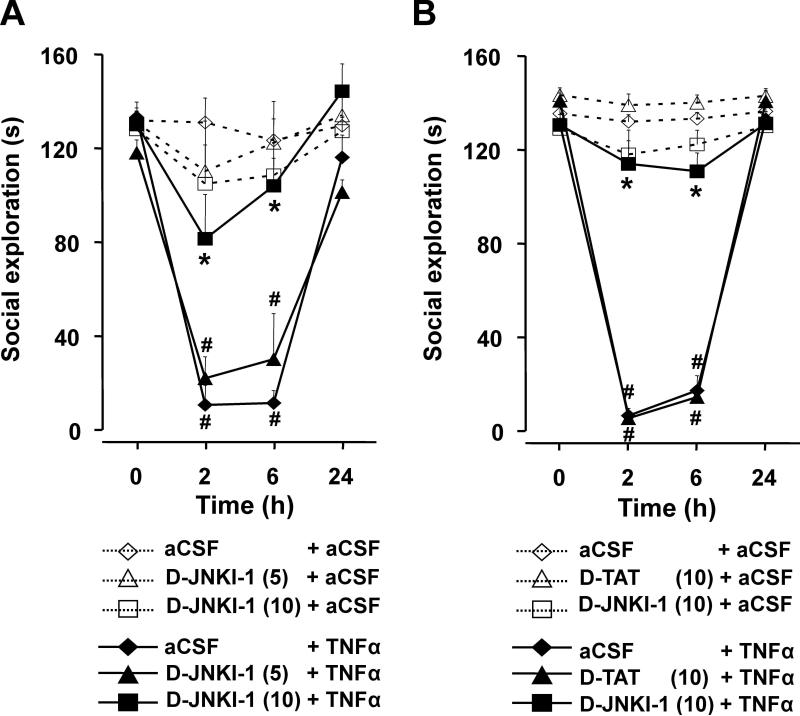
Mice were pre-treated i.c.v. with D-JNKI-1 (5 and 10 ng) 1 h prior to treatment with TNFα (50 ng). The mean duration of social exploration ranged from 118±5 s to 133± 6 s (time 0) for the six treatment combinations (Fig. 1a). As expected, TNFα given i.c.v. decreased duration of social exploration in a time-dependent manner (treatment×time: F (3,90)=22.0, p<0.001) and this reduction was abrogated by the JNK inhibitor at 10 ng by not at 5 ng (pre-treatment× treatment×time: F(6,90)=3.4, p<0.01). Post-hoc comparisons demonstrated that duration of social exploration did not change over time when mice were given aCSF i.c.v. (Fig. 1a). When TNFα was given centrally, duration of social exploration was significantly reduced at both 2 and 6 h, returning to baseline at 24 h. The ability of TNFα to decrease social exploration was blocked by D-JNKI-1 at 10 ng but not 5 ng.
Fig. 1.
Reduction in social exploration caused by central TNFα is abrogated by an inhibitor of JNK, D-JNKI-1. a Mice were treated i.c. v. with either aCSF, D-JNKI-1 (5 ng) or D-JNKI-1 (10 ng) 1 h before i.c.v. administration of either aCSF or TNFα (50 ng). b In a separate experiment, mice were treated with either aCSF, D-JNKI-1 (10 ng),or D-TAT (10 ng) followed 1 h later by aCSF or TNFα (50 ng). Data are expressed as means±SEM, n=6. **p<0.01 for the effect of TNFα within dose of D-JNKI-1; *p<0.01 comparing TNFα+D-JNKI-1 to TNFα within time
To confirm the specificity of D-JNKI-1 activity, mice were pre-treated with aCSF, the control peptide D-TAT (10 ng), or D-JNKI-1 (10 ng) prior to treatment with TNFα (50 ng). In Fig. 1b, the average baseline (time 0) for duration of social exploration ranged from 129±3 s to 143±3 s for the six treatment combinations. TNFα decreased social exploration in a time-dependent manner (treatment×time: F (3,90)=239.0, p<0.001) and this effect was abrogated by 10 ng of D-JNKI-1 but not by 10 ng of D-TAT (pre-treatment×treatment×time: F(6,90)=48.1, p<0.001).
The increase in immobility caused by TNFα is prevented by D-JNKI-1
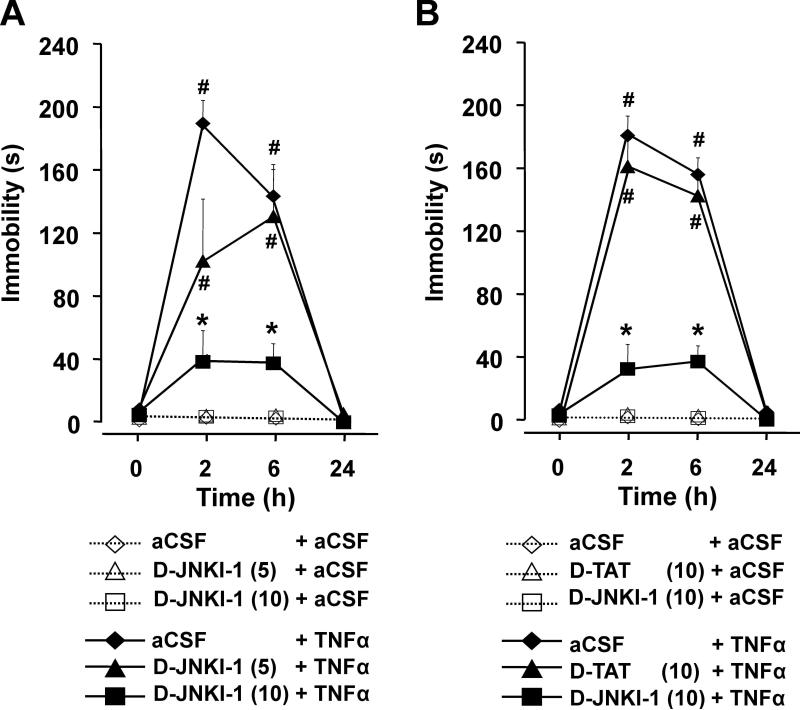
Mice were pre-treated i.c.v. with aCSF or D-JNKI-1 (5 or 10 ng) prior to treatment with TNFα. No immobility was observed at time zero (Fig. 2a). TNFα increased immobility in a time-dependent manner (treatment×time: F(3,90)= 43.6, p<0.001) and this effect was attenuated by D-JNKI-1 in a dose-dependent manner (pre-treatment×treatment× time: F(6,90)=6.61, p<0.001). In the absence of TNFα, mice remained fully active during each test period (no immobility). As expected, TNFα alone increased duration of immobility at 2 and 6 h. This increase was inhibited by 10 ng, but not 5 ng, of D-JNKI-1.
Fig. 2.
D-JNKI-1 inhibits immobility caused by central administration of TNFα. a Mice were treated i.c.v. with either aCSF, D-JNKI-1 (5 ng),or D-JNKI-1 (10 ng) 1 h before administration of aCSF or TNFα (50 ng). b In a separate experiment, mice were treated with aCSF, D-JNKI-1 (10 ng) or D-TAT (10 ng) 1 h before injection of aCSF or TNFα (50 ng). Data are expressed as of means±SEM, n=6. **p<0.01 the effect of TNFα within dose of D-JNKI-1; *p<0.01 comparing TNFα+D-JNKI-1 to TNFα within time
Specificity of the inhibition caused by D-JNKI-1 was confirmed in a second experiment in which D-JNKI-1 was compared with its control peptide, D-TAT (Fig. 2b). TNFα again induced immobility in a time-dependent manner (treatment×time: F(3,90)=179.0, p<0.001). The increase in immobility was inhibited by D-JNKI-1 (10 ng) but not by D-TAT (10 ng; pre-treatment×treatment×time: F(6,90)= 22.9, p<0.001). As in Fig. 2a, post-hoc comparisons revealed that TNFα increased duration of immobility at 2 and 6 h. These increases were inhibited by D-JNKI-1 at both time points but not by the control peptide, D-TAT.
D-JNKI-1 prevents loss of body weight induced by TNFα
Mice were pre-treated i.c.v. with aCSF, the JNK peptide inhibitor D-TAT or the D-JNKI-1 1 h prior to treatment i.c.v. with aCSF or TNFα. At 6 h post-treatment, TNFα reduced body weight (F(1,30)=46.2, p<0.001), and this reduction was significantly attenuated by pre-treatment with D-JNKI-1 but not D-TAT (pre-treatment×treatment: F(2,30)=4.8, p<0.05, Table 1).
Table 1.
Central pre-treatment with a JNK inhibitor, D-JNKI-1, but not its control peptide (D-TAT), inhibits the loss of body weight induced by TNFα given i.c.v.
| Pre-treatment |
|||
|---|---|---|---|
| TNFα (ng) | aCSF | D-TAT (10 ng) | D-JNK-1 (10 ng) |
| 0 | –0.06±0.23 | –0.03±0.26 | –0.15±0.23 |
| 50 | –1.88±0.18* | –1.55±0.15* | –0.32±0.29 |
Body weight changes (g) were calculated as the difference between weights obtained immediately after and 6 h after treatment with TNFα. Means±SEM.
p<0.01 for both aCSF and D-TAT pre-treatments when comparing 0 vs. 50 ng TNFα.
Discussion
These results are the first to establish that sickness behavior induced by central TNFα is antagonized by i.c.v, administration of a JNK peptide inhibitor, D-JNKI-1. We show that central injection of D-JNKI-1 (5 or 10 ng) or its control peptide, D-TAT, do not affect body weight or behavior in the absence of TNFα, as assessed by investigation of a juvenile mouse or duration of immobility of C57BL/6J mice. However, when administered i.c.v. 1 h before central injection of TNFα, D-JNKI-1 (10 ng) markedly impairs the TNFα-induced reduction in social exploration, induction of immobility, and loss of body weight. Although others have reported that D-JNKI-1 is neuroprotective in the central nervous system (Borsello et al. 2003; Minogue et al. 2003), this is the first report to show that D-JNKI-1 can prevent behavioral changes induced by central TNFα. These behavioral responses caused by central TNFα and their reversal by D-JNKI-1 occur in a paradigm independent of any cell death.
The ability of D-JNKI-1 to depress TNFα activity is exciting because of its novel nature. D-JNKI-1 may well represent a future generation of behaviorally-active therapeutic agents. The use of protein transduction domains to introduce regulatory peptides into cells remains a relatively new field of investigation (Becker-Hapak et al. 2001; Chauhan et al. 2007). Although current limitations do not permit targeting specific cell types, the ability to introduce sequence-specific peptide inhibitors or even other types of cargo into cells that act as agonists or antagonists of specific intracellular targets lends a high degree of targeting specificity rarely accomplished with conventional drugs. Additionally, the use of TAT or other, perhaps more efficient, protein transduction domains to transport cargo into cells avoids the laborious and inefficient use of transfection expression vectors or microinjection (Becker-Hapak et al. 2001). We initially used D-JNKI-1 to block TNFα inhibition of cell differentiation in vitro (Strle et al. 2006). Similar to the work presented in this manuscript, D-JNKI-1 was able to block TNFα activity in vitro and in vivo, demonstrating the efficacy of this agent and technique. Most importantly, in both our in vivo and in vitro systems, the control peptide was without effect, attesting to the specificity of the technique. The use of this approach in a number of biological models, including cancer, cardiac function, and inflammation (Chauhan et al. 2007;Gustafsson et al. 2005; Wadia and Dowdy 2005), attests to the vast potential of this method. One extension of the current work would be the use of this technique with other behavior models, including depressive-like behavior.
The behavioral effects caused by i.c.v. administration of TNFα in C57BL/6J mice are similar to those previously reported with outbred CD-1 and inbred DBA2 mice (Bluthe et al. 1991, 1994). However, the molecular mechanisms involved in TNFα-induced sickness behaviors have not yet been reported in any strain of mice. TNFα initiates a variety of signaling cascades downstream of both the TNF-R1 or TNF-R2 (MacEwan 2002). By comparing TNF-R2-deficient and wild-type mice, we recently established that in the absence of TNF-R2, mice are fully responsive to central TNFα induction of sickness behavior (Palin et al. 2007). These new findings strongly implicate the involvement of TNF-R1 in sickness behavior. The data in this manuscript significantly extend these recent findings and are the first to directly demonstrate that inhibition of JNK, a key signaling molecule downstream of TNF-R1 (McCusker et al. 2006), prevents sickness behavior induced by central TNFα. Although these data are the first to establish that D-JNKI-1 acts in vivo to block centrally-induced sickness behavior, it is not yet clear which cell types are involved in proinflammatory cytokine induction of sickness behavior. For example, IL-1β is known to strongly activate c-fos in non-neuronal cells, and this activity is correlated with the induction of sickness behavior (Nadjar et al. 2005). However, no direct evidence yet exists to prove that glial activation is the cause of IL-1β-induced sickness behavior. Conversely, neurons may play a more critical role than glia in induction of sickness behavior by TNFα since TNF-R1 is strongly expressed in neurons, whereas TNF-R2 is primarily localized to glia (Bette et al. 2003; Pradillo et al. 2005). This receptor distribution is maintained in vitro, wherein neurons, but not astrocytes, express abundant TNF-R1 (Figiel and Dzwonek 2007). Indeed, central TNFα causes sickness behavior in TNF-R2-deficient mice (Palin et al. 2007) and human TNFα, which does not activate murine TNF-R2, fully induces sickness in mice (Bluthe et al. 1991). Collectively, these findings suggest that TNFα may act directly on neurons via TNF-R1 to activate JNK followed by the induction of sickness behavior. Future experiments are needed to confirm the site of action responsible for TNFα-induced sickness behavior.
The rapid induction of sickness behavior by central TNFα suggests a direct mode of action. This is particularly true since TNFα is able to induce sickness behavior in IL-1R1 KO mice (Bluthe et al. 2000). Although these data indicate that IL-1β is not required to induce sickness behavior, this does not rule out the possibility that TNFα may induce the production of other cytokines within the brain that mediate behavioral changes (Dantzer et al. 2006). Additional models need to be tested to clarify the molecular mechanisms by which cytokines induce sickness. In addition to its involvement in sickness behavior, JNK is frequently implicated in central events associated with TNFα signaling. For example, TNFα acts within the hypothalamus to depress food intake, and this physiologic response requires nitric oxide synthesis which is associated with an elevation in JNK activity (Moraes et al. 2006). Furthermore, inhibition of JNK activity diminishes excitotoxic neuronal loss associated with ischemic brain insults (Borsello et al. 2003; Repici et al. 2007). In an Alzheimer's disease model, D-JNKI-1 prevents β-amyloid-induced apoptosis of cortical neurons and suppresses the inhibition of long-term potentiation (Minogue et al. 2003). In addition, regulation of JNK activation in hypothermia serves as a possible neuroprotective event following moderate fluid-percussion traumatic brain injury (Lotocki et al. 2006). These findings demonstrate that JNK is implicated in the pathophysiology of a plethora of neuro-inflammatory and neurodegenerative diseases and that regulation of JNK activity has promising therapeutic clinical implications (Manning and Davis 2003).
Major depressive disorders and depressive-like behaviors can develop on a background of cytokine-induced sickness behavior (Dantzer et al. 2006; Dantzer and Kelley 2007; Dantzer et al. 2008a, b; O'Connor et al. 2008a). Indeed, in patients with major depressive disorders, a reduction in serum TNFα is directly correlated with psychopathological improvement (Lanquillon et al. 2000). Whether sickness behavior and depression utilize the same TNFα-dependent intracellular signaling events is not yet known. A better understanding of the role of key signaling mediators like JNK in sickness behavior, depression, and a variety of neurological disorders could aid the development of novel pharmacological agents that might be used to treat these inflammation-associated disorders.
Acknowledgements
This research was supported by grants from the National Institutes of Health (NIH) to K.W.K. (MH51569 and AG029573) and R.D. (MH071349 and MH079829). The authors do not have a financial relationship with sponsoring organizations.
Contributor Information
K. Palin, Laboratory of Integrative Immunophysiology, Integrative Immunology and Behavior Program, Department of Animal Sciences, College of ACES, Urbana, IL 61801, USA
R. H. McCusker, Laboratory of Integrative Immunophysiology, Integrative Immunology and Behavior Program, Department of Animal Sciences, College of ACES, Urbana, IL 61801, USA
K. Strle, Laboratory of Integrative Immunophysiology, Integrative Immunology and Behavior Program, Department of Animal Sciences, College of ACES, Urbana, IL 61801, USA
F. Moos, Laboratoire Psynugen, CNRS, UMR5226, University Bordeaux, INRA, UMR1286 INRA, 146 rue Léo Saignat, Bordeaux F-33077, France
R. Dantzer, Laboratory of Integrative Immunophysiology, Integrative Immunology and Behavior Program, Department of Animal Sciences, College of ACES, Urbana, IL 61801, USA Department of Pathology, College of Medicine, University of Illinois, Urbana, IL 61801, USA.
K. W. Kelley, Laboratory of Integrative Immunophysiology, Integrative Immunology and Behavior Program, Department of Animal Sciences, College of ACES, Urbana, IL 61801, USA Department of Pathology, College of Medicine, University of Illinois, Urbana, IL 61801, USA; Laboratory of Integrative Immunophysiology, Integrative Immunology and Behavior Program, 227 Edward R. Madigan Laboratory, University of Illinois at Urbana-Champaign, 1201 West Gregory Drive, Urbana, IL 61801-3873, USA kwkelley@uiuc.edu.
References
- Becker-Hapak M, McAllister SS, Dowdy SF. TAT-mediated protein transduction into mammalian cells. Methods. 2001;24:247–256. doi: 10.1006/meth.2001.1186. [DOI] [PubMed] [Google Scholar]
- Bette M, Kaut O, Schafer MK, Weihe E. Constitutive expression of p55TNFR mRNA and mitogen-specific up-regulation of TNF alpha and p75TNFR mRNA in mouse brain. J Comp Neurol. 2003;465:417–430. doi: 10.1002/cne.10877. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW. Interleukin-1 mediates behavioural but not metabolic effects of tumor necrosis factor alpha in mice. Eur J Pharmacol. 1991;209:281–283. doi: 10.1016/0014-2999(91)90184-r. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Pawlowski M, Suarez S, Parnet P, Pittman Q, Kelley KW, Dantzer R. Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology. 1994;19:197–207. doi: 10.1016/0306-4530(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Laye S, Michaud B, Combe C, Dantzer R, Parnet P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447–4456. [PubMed] [Google Scholar]
- Bluthe RM, Kelley KW, Dantzer R. Effects of insulin-like growth factor-I on cytokine-induced sickness behavior in mice. Brain Behav Immun. 2006;20:57–63. doi: 10.1016/j.bbi.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- Bubici C, Papa S, Pham CG, Zazzeroni F, Franzoso G. NF-kappaB and JNK: an intricate affair. Cell Cycle. 2004;3:1524–1529. doi: 10.4161/cc.3.12.1321. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Tikoo A, Kapur AK, Singh M. The taming of the cell penetrating domain of the HIV Tat: myths and realities. J Control Release. 2007;117:148–162. doi: 10.1016/j.jconrel.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Seguy F, Dantzer R. Behavioural effects of peripherally injected interleukin-1: role of prostaglandins. Brain Res. 1991;542:330–335. doi: 10.1016/0006-8993(91)91587-q. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Castanon N, Kelley KW, Konsman JP, Laye S, Lestage J, Parnet P. Cytokines, sickness behavior and depression. In: Ader R, editor. Psychoneuroimmunology. Fourth Edition Academic Press; New York, NY: 2006. pp. 281–318. [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008a;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Capuron L, Irwin MR, Miller AH, Ollat H, Hugh Perry V, Rousey S, Yirmiya R. Identification and treatment of symptoms associated with inflammation in medically ill patients. Psychoneuroendocrinology. 2008b;33:18–29. doi: 10.1016/j.psyneuen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figiel I, Dzwonek K. TNFalpha and TNF receptor 1 expression in the mixed neuronal-glial cultures of hippocampal dentate gyrus exposed to glutamate or trimethyltin. Brain Res. 2007;1131:17–28. doi: 10.1016/j.brainres.2006.10.095. [DOI] [PubMed] [Google Scholar]
- Fiore M, Probert L, Kollias G, Akassoglou K, Alleva E, Aloe L. Neurobehavioral alterations in developing transgenic mice expressing TNF-alpha in the brain. Brain Behav Immun. 1996;10:126–138. doi: 10.1006/brbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Fiore M, Alleva E, Probert L, Kollias G, Angelucci F, Aloe L. Exploratory and displacement behavior in transgenic mice expressing high levels of brain TNF-alpha. Physiol Behav. 1998;63:571–576. doi: 10.1016/s0031-9384(97)00514-3. [DOI] [PubMed] [Google Scholar]
- Gustafsson AB, Gottlieb RA, Granville DJ. TAT-mediated protein transduction: delivering biologically active proteins to the heart. Methods Mol Med. 2005;112:81–90. [PubMed] [Google Scholar]
- Kuan CY, Burke RE. Targeting the JNK signaling pathway for stroke and Parkinson's diseases therapy. Curr Drug Targets CNS Neurol Disord. 2005;4:63–67. doi: 10.2174/1568007053005145. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Lotocki G, de Rivero Vaccari JP, Perez ER, Alonso OF, Curbelo K, Keane RW, Dietrich WD. Therapeutic hypothermia modulates TNFR1 signaling in the traumatized brain via early transient activation of the JNK pathway and suppression of XIAP cleavage. Eur J Neurosci. 2006;24:2283–2290. doi: 10.1111/j.1460-9568.2006.05123.x. [DOI] [PubMed] [Google Scholar]
- MacEwan DJ. TNF ligands and receptors—a matter of life and death. Br J Pharmacol. 2002;135:855–875. doi: 10.1038/sj.bjp.0704549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–565. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- McCusker RH, Strle K, Broussard SR, Dantzer R, Bluthé RM, Kelley KW. Crosstalk between insulin-like growth factors and pro-inflammatory cytokines. In: Adler R, Dantzer R, Glaser R, Heijnen C, Irwin M, Padgett D, Sheridan J, editors. Psychoneuroimmunology. Academic Press; New York: 2006. pp. 171–191. [Google Scholar]
- Medeiros R, Prediger RD, Passos GF, Pandolfo P, Duarte FS, Franco JL, Dafre AL, Di Giunta G, Figueiredo CP, Takahashi RN, Campos MM, Calixto JB. Connecting TNF-alpha signaling pathways to iNOS expression in a mouse model of Alzheimer's disease: relevance for the behavioral and synaptic deficits induced by amyloid beta protein. J Neurosci. 2007;27:5394–5404. doi: 10.1523/JNEUROSCI.5047-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minogue AM, Schmid AW, Fogarty MP, Moore AC, Campbell VA, Herron CE, Lynch MA. Activation of the c-Jun N-terminal kinase signaling cascade mediates the effect of amyloid-beta on long term potentiation and cell death in hippocampus: a role for interleukin-1beta? J Biol Chem. 2003;278:27971–27980. doi: 10.1074/jbc.M302530200. [DOI] [PubMed] [Google Scholar]
- Moraes JC, Amaral ME, Picardi PK, Calegari VC, Romanatto T, Bermudez-Echeverry M, Chiavegatto S, Saad MJ, Velloso LA. Inducible-NOS but not neuronal-NOS participate in the acute effect of TNF-alpha on hypothalamic insulin-dependent inhibition of food intake. FEBS Lett. 2006;580:4625–4631. doi: 10.1016/j.febslet.2006.07.042. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Bluthe RM, May MJ, Dantzer R, Parnet P. Inactivation of the cerebral NFkappaB pathway inhibits interleukin-1beta-induced sickness behavior and c-Fos expression in various brain nuclei. Neuropsychopharmacology. 2005;30:1492–1499. doi: 10.1038/sj.npp.1300755. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, André C, Moreau M, Lestage J, Cantanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiat. 2008a Jan 15; doi: 10.1038/sj.mp.4002148. in press DOI 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW. Regulation of IGF-I function by proinflammatory cytokines: At the interface of immunology and endocrinology. Cell. Immunol. 2008b doi: 10.1016/j.cellimm.2007.09.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palin K, Bluthe RM, McCusker RH, Moos F, Dantzer R, Kelley KW. TNFalpha-induced sickness behavior in mice with functional 55 kD TNF receptors is blocked by central IGF-I. J Neuroimmunol. 2007;187:55–60. doi: 10.1016/j.jneuroim.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates, second edition (Deluxe) edn. Academic Press; New York, NY: 2001. [Google Scholar]
- Pradillo JM, Romera C, Hurtado O, Cardenas A, Moro MA, Leza JC, Davalos A, Castillo J, Lorenzo P, Lizasoain I. TNFR1 upregulation mediates tolerance after brain ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:193–203. doi: 10.1038/sj.jcbfm.9600019. [DOI] [PubMed] [Google Scholar]
- Repici M, Borsello T. JNK pathway as therapeutic target to prevent degeneration in the central nervous system. Adv Exp Med Biol. 2006;588:145–155. doi: 10.1007/978-0-387-34817-9_13. [DOI] [PubMed] [Google Scholar]
- Repici M, Centeno C, Tomasi S, Forloni G, Bonny C, Vercelli A, Borsello T. Time-course of c-Jun N-terminal kinase activation after cerebral ischemia and effect of D-JNKI1 on c-Jun and caspase-3 activation. Neuroscience. 2007;150:40–49. doi: 10.1016/j.neuroscience.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Strle K, Broussard SR, McCusker RH, Shen WH, LeCleir JM, Johnson RW, Freund GG, Dantzer R, Kelley KW. C-jun N-terminal kinase mediates tumor necrosis factor-alpha suppression of differentiation in myoblasts. Endocrinology. 2006;147:4363–4373. doi: 10.1210/en.2005-1541. [DOI] [PubMed] [Google Scholar]
- Tilstra J, Rehman KK, Hennon T, Plevy SE, Clemens P, Robbins PD. Protein transduction: identification, characterization and optimization. Biochem Soc Trans. 2007;35:811–815. doi: 10.1042/BST0350811. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumour necrosis factor receptors: structure and function. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- Wadia JS, Dowdy SF. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv Drug Deliv Rev. 2005;57:579–596. doi: 10.1016/j.addr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Waetzig V, Czeloth K, Hidding U, Mielke K, Kanzow M, Brecht S, Goetz M, Lucius R, Herdegen T, Hanisch UK. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia. 2005;50:235–246. doi: 10.1002/glia.20173. [DOI] [PubMed] [Google Scholar]
- Yang L, Lindholm K, Konishi Y, Li R, Shen Y. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J Neurosci. 2002;22:3025–3032. doi: 10.1523/JNEUROSCI.22-08-03025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]