Abstract
Atrial fibrillation is the most common clinical cardiac arrhythmia. It is often initiated by ectopic beats arising from the pulmonary veins and atria. While pulmonary vein myocytes most likely contribute to atrial ectopic beats initiating atrial fibrillation, emerging evidence suggests the existence of other cell populations that may also contribute to atrial arrhythmias. In addition to sinus node-like and intestinal Cajal-like cells, we recently characterized a novel, melanocyte-like cell population in murine and human hearts that may contribute to atrial arrhythmogenic triggers in mice. Murine cardiac melanocyte-like cells are electrically excitable, and express adrenergic and muscarinic receptors. Adult mice lacking the gene encoding dopachrome tautomerase (Dct) are susceptible to atrial arrhythmias, and Dct is expressed by both murine and human cardiac melanocytes. While Dct-expressing cells are present in human hearts in regions from which atrial arrhythmias often arise, the contribution of these cells to clinical atrial arrhythmias remains to be determined.
Keywords: atrial fibrillation, autonomic tone, ectopic foci, melanocyte-like cells
Atrial fibrillation (AF) is the most common clinical arrhythmia and is associated with significant morbidity [1–4]. While most individuals with AF have some underlying cardiac disease, approximately 30–45% with paroxysmal AF and 20–25% with persistent AF are younger patients with no structural heart disease [5,6]. Just over 10 years ago, Haissaguerre et al. recognized that ectopic beats arising from the pulmonary veins (PVs) could act as initiators or ‘arrhythmogenic triggers’ of AF [7]. This seminal discovery led to the development and use of PV isolation as an effective, empiric therapy for drug-refractory AF. However, PV isolation is associated with complications and risks, including PV reconnection, which reduces its efficacy [8–10]. On the other hand, antiarrhythmic drug therapy for AF is often ineffective and associated with burdensome side effects [11–13]. Although recent clinical insights have improved our understanding of how AF is initiated, our relative inability to treat or cure AF is rooted in a lack of understanding of the cellular and basic mechanisms that underlie atrial arrhythmogenesis. Therefore, a better appreciation of the cellular basis of AF is likely to positively impact the effectiveness of future therapies for this common disease.
The focus of this article is on the cell populations in the PVs and atria that may contribute to clinical atrial arrhythmias. For several reasons, most of the basic research evidence connecting a specific cell population to the initiation of atrial arrhythmias must come from animal studies; however, the existence of analogous cell populations in similar anatomic distributions within human hearts provides a rationale for speculating that these findings may have clinical relevance. Along these lines, we recently discovered a novel population of cells in the PVs and atria that we have shown contribute to atrial arrhythmias in murine models [14]. These cells express markers of the melanocyte lineage, and therefore we have devised the term ‘cardiac melanocytes’ to describe them. Cardiac melanocytes are found in the PVs and posterior atria of murine and human hearts (Figure 1), and exist in groups near the PV ostia of mice. We have also found that murine cardiac melanocytes are excitable and possess similar electrophysiological properties to atrial myocytes. Interestingly, deletion of the melanin synthesis enzyme dopachrome tautomerase (Dct), which is expressed by both human and murine cardiac melanocytes, prolongs repolarization and induces afterdepolarization in isolated cardiac melanocytes. Furthermore, mice engineered with genetic deletion of Dct (Dct-null) display spontaneous and inducible AF. However, the level of atrial arrhythmogenesis is reduced to that of wild-type animals when melanocyte-like cells are prevented from being expressed in the heart, suggesting that this cell population contributes to the genesis of atrial arrhythmias in mouse models. Furthermore, murine cardiac melanocytes express adrenergic and muscarinic receptors, and are in close proximity to autonomic nerve terminals. When challenged with a muscarinic agonist, murine models lacking cardiac melanocytes have fewer induced atrial arrhythmias than wild-type littermate mice that express cardiac melanocytes. These findings suggest a potential connection may exist between Dct-expressing cells, atrial ectopy-initiating arrhythmias and autonomic dysregulation.
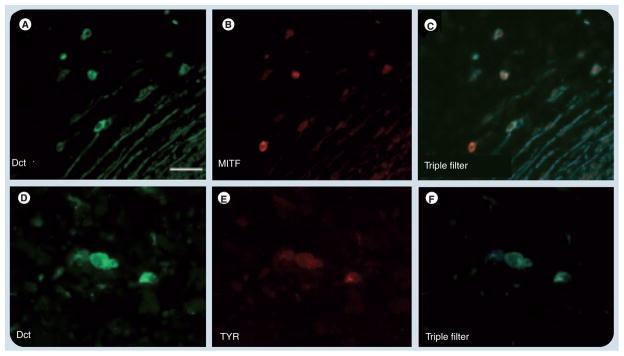
Figure 1. Immunofluorescent analysis of dopachrome tautomerase-positive cells in the human atrium.
Sections from a cadaveric human posterior atrium costained by immunofluorescence for (A & D) Dct, (B) MITF and (E) TYR. (C & F) Triple filter images for Dct and each marker are shown on the right. Dct, KIT and TYR are well-characterized melanocyte markers. Scale bar: 50 μm.
Dct: Dopachrome tautomerase; MITF: Microphthalmia-associated transcription factor; TYR: Tyrosinase.
Ectopic triggers initiate atrial arrhythmias
Since publication of the initial report by Haissaguerre et al. in 1998, multiple studies have confirmed that ectopic foci originating from the thoracic veins contribute to the initiation of clinical AF [15–17]. However, comparatively little is known about the cellular source and mechanism by which thoracic veins initiate atrial arrhythmias. Debate still exists concerning the mechanisms underlying atrial arrhythmia triggers. Those proposed include enhanced automaticity, triggered activity [18,19] and micro-re-entry from within the myocardial sleeves of thoracic veins [20,21]. Accumulating evidence suggests that PV myocardial cells themselves contribute to arrhythmogenic triggers [22,23], and that these specialized myocytes are highly excitable in the setting of altered autonomic tone [18,19]. It has been suggested that the unique electrophysiological properties of PV cardiomyocytes [24,25], coupled with the dense autonomic innervation of the PVs [26], produce a highly arrhythmogenic region within the posterior left atria around the PVs. However, while PV cardiomyocytes probably do contribute to atrial arrhythmias, these cells are not likely to be the only ones involved with initiating atrial arrhythmias.
In this regard, there are recent reports of other cell types in the PVs that may contribute to PV ectopy [27–29]. In addition, evidence continues to grow supporting the contribution of autonomic efflux to atrial arrhythmogenesis. Therefore, this article presents a brief overview of what is currently known about cell populations in the PVs with regards to atrial arrhythmias, in addition to our present understanding of how autonomic tone contributes to AF triggers. This overview is followed by a discussion regarding cardiac melanocytes and how their discovery may expand our understanding of the cellular basis of AF.
Cells other than myocytes in the PVs contribute to AF
Most investigations to date have focused upon the contribution of PV cardiomyocytes to atrial arrhythmia initiation. While PV cardiomyocytes probably do contribute to arrhythmogenic triggers, there are recent reports of other cell populations in the PVs that may also play a role in atrial arrhythmias [14,27–29]. In addition, foci outside the PVs are known to initiate atrial arrhythmias [30–33], and the unique electrophysiologic properties of PV cardiomyocytes cannot account for the origin of triggers outside of the PVs. Thus, it is important to consider the contribution of other cell populations to atrial arrhythmias. In fact, a better understanding of the basic electrophysiology and contribution of these other cells to atrial arrhythmias may reveal new information underlying arrhythmogenic mechanisms and help explain the source of non-PV triggers.
Several years ago, Perez-Lugones et al. identified sinus node-like cells within human PVs that were associated with AF [29]. These cells were identified by their pale cytoplasm and positive response to periodic acid-Schiff (PAS) staining within cadaveric samples. Based upon the attached clinical histories for each sample, the authors found that samples from four patients with a history of AF had sinus node-like cells in the PVs. However, no such cells could be detected in the PVs of five transplant hearts from patients without AF. While the authors of this study were clear the connection of sinus node-like cells to clinical atrial arrhythmias required further investigation, studies in canine models have provided some evidence for the contribution of PAS-positive cells in the PVs to atrial arrhythmias. In a study by Chuo et al., the authors induced voltage-independent triggered ectopy in Langendorf-perfused canine hearts by applying ryanodine and isoproterenol, which induced atrial arrhythmias [20]. The authors then identified PAS-positive cells clustered in the endocardium of the PV muscular sleeves from one preparation near the region from which they mapped frequent focal discharges, while few PAS-positive cells could be found in another preparation from a heart with no focal discharges.
More recently, Morel et al. and Gherghiceanu et al. have demonstrated the presence of interstitial Cajal-like cells (ICCs) within the muscular sleeves of PVs [27,28]. Morel et al. described a rough correlation between ICCs in the PVs and clinical AF, where two out of three patients with detectable PV ICCs had documented AF. The report by Gherghiceanu et al. described the presence of ICCs in human PVs with no analysis of their connection to arrhythmia. However, neither of these studies characterized the electrophysiological properties of ICCs or explored mechanisms by which they may contribute to atrial arrhythmias. This is partially due to the fact that ICCs are well characterized within enteric smooth muscle and are known to mediate gut motility in response to autonomic efflux [34,35]. Therefore, the assumption that ICCs within human PVs probably exhibit similar electrophysiologic behavior leading to PV ectopy is reasonable. However, in the gut, ICCs mediate very slow electrical responses (in the order of seconds), which are too sluggish to explain the rapid response time of PV ectopy that triggers AF (which is on the scale of milliseconds). While the contribution of ICCs to atrial arrhythmias will require further validation, it is intriguing to consider the possibility that ICCs in the heart may contribute to atrial arrhythmias either as a source of triggered activity (as they appear to in the intestines), or they may increase arrhythmogenicity by contributing to zones of slowed conduction and promoting local re-entry. In either case, these reports highlight the potential contribution of noncardiomyocytes to atrial arrhythmogenesis, and open the way for exploring the contribution of other cell populations to atrial arrhythmias, such as cardiac melanocytes.
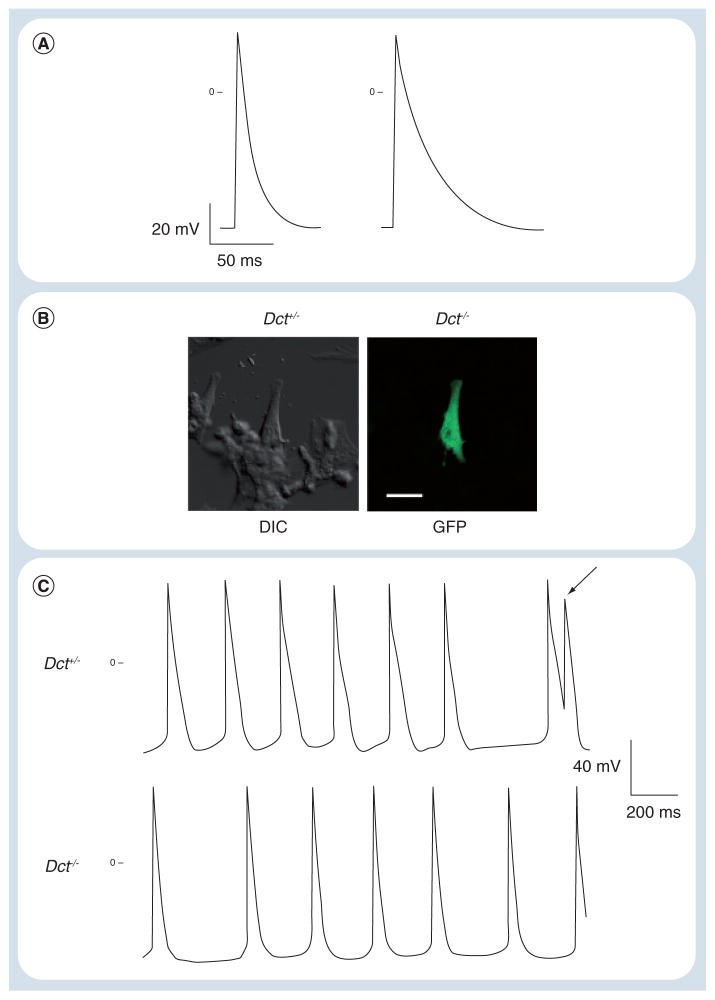
Interestingly, we have found that isolated murine cardiac melanocytes are electrically excitable and generate action potentials with a similar time course to that of murine atrial myocytes (Table 1 & Figure 2A). However, murine cardiac melanocytes isolated from Dct-null mice displayed dramatically prolonged action potential duration (Table 1 & Figure 2A). With prolonged repolarization it was not surprising that Dct-null murine cardiac melanocytes also displayed triggered activity with frequent afterdepolarizations (Figure 1C). Furthermore, single-cell transcriptional profiling of murine cardiac melanocytes showed that these cells express many of the same voltage-dependent ion channels and calcium-handling proteins as atrial myocytes in the presence of Dct (Table 2). However, it will be interesting to examine the effects of Dct deletion upon transcript expression by comparing the transcriptional profile of Dct-null and wild-type cardiac melanocytes. Still, it appears that murine cardiac melanocytes possess the electrical properties and ability to trigger afterdepolarizations in the absence of Dct (analogous to ectopic foci), that are consistent with those required for the generation of atrial arrhythmia triggers.
Table 1.
Action potential parameters.
| Parameter | Cardiac melanocytes | Atrial myocytes | ||
|---|---|---|---|---|
| Dct+/−(n = 7) | Dct−/−(n = 7) | Dct+/−(n = 8) | Dct−/−(n = 8) | |
| APD90 (ms) | 17.0 ± 3.8 | 75.5 ± 22.2†§¶ | 15.2 ± 1.0‡ | 14.6 ± 1.72‡ |
| APD50 (ms) | 9.2 ± 4.0 | 19.3 ± 8.0†§¶ | 5.7 ± 1.0‡ | 5.2 ± 1.5‡ |
| dV/dt (mV/ms) | 37.8 ± 14.7 | 35.7 ± 12.0 | 30.1 ± 8.1 | 34.0 ± 8.7 |
| Vr (mV) | −59.3 ± 8.9 | −65.7 ± 12.9 | −61.1 ± 9.2 | −62.3 ± 12.8 |
Data are presented as the mean ± standard deviation and statistical significance was assessed using one-way analysis of variance followed by the least significant difference procedure.
p < 0.05 compared with Dct+/− melanocytes.
p < 0.05 compared with Dct−/− melanocytes.
p < 0.05 compared with Dct+/− myocytes.
p < 0.05 compared with Dct−/− myocytes.
APD50: Action potential duration at 50% repolarization; APD90: Action potential duration at 90% repolarization; dV/dt: Action potential upstroke rate of rise; Vr: Resting membrane potential.
Reproduced with permission from [14].
Figure 2. Dopachrome tautomerase-null cardiac melanocytes demonstrate prolonged action potential duration and afterdepolarizations.
(A) Current clamp recordings from cardiac Dct+/− and Dct−/− cells demonstrated increased action potential duration in the absence of Dct. Resting potential of the Dct+/− cell was −62 mV, while the Dct−/− cell had a resting potential of −60 mV. (B) Representative cardiac melanocyte from a Dct+/−, GFP-positive mouse visualized by DIC and fluorescent microscopy for GFP. Scale bar: 10 μm. (C) Spontaneous action potential recordings from Dct−/− and Dct+/− cardiac melanocytes. Within the trace from the Dct−/− cell, note the presence of an early afterdepolarization (arrow) arising from phase 3 of the preceding action potential. The trace from a Dct+/− cell is included for comparison.
Dct: Dopachrome tautomerase; DIC: Differential interference contrast; GFP: Green fluorescent protein.
Reproduced with permission from [14].
Table 2.
Select ion channel and calcium-handling transcripts in cardiac dopachrome tautomerase-expressing cells.
| Symbol | Genbank name | Description | Fold change |
|---|---|---|---|
| Pln | AK002622 | Phospholamban | 5.93 |
| Cacna1c | NM_009781 | Calcium channel, voltage dependent, L-type, α1C subunit | 2.49 |
| Atp2b1 | BI080417 | ATPase, Ca2+ transporting, plasma membrane 1 | 2.67 |
| Ryr2 | NM_023868 | Ryanodine receptor 2, cardiac | 2.31 |
| Gjc1 | NM_008122 | Gap junction protein, γ1 | 2.30 |
| Atp2a2 | AA245637 | ATPase, Ca2+ transporting, cardiac muscle 2 | 1.88 |
| Clic4 | BB814844 | Chloride intracellular channel 4 | 1.82 |
| Vdac2 | BC003731 | Voltage-dependent anion channel 2 | 1.74 |
| Cav3 | NM_007617 | Caveolin 3 | 1.60 |
| Kctd18 | BB257241 | Potassium channel tetramerization domain | 1.58 |
| Atp1a1 | BC025618 | ATPase, Na+/K+ transporting, α1 polypeptide | 1.53 |
| Kcmf1 | BG071725 | Potassium channel modulatory factor 1 | 1.52 |
| Scn5a | BB516098 | Sodium channel, voltage gated, type V, α | 1.14 |
| Kcnk3 | BF467278 | Potassium channel, subfamily K, member 3 | 1.11 |
| Kcnj1 | NM_019659 | Potassium inwardly rectifying channel, subfamily J, member 1 | 1.08 |
Single-cell transcriptional profiling was performed with RNA harvested from five Dct-positive cells and five atrial myocytes isolated from Dct+/− hearts. Expression data were normalized to the mean of all transcript assays for each cell type and displayed as fold change above the norm of transcripts assayed in atrial myocytes. The GeneSpring analysis program was used to identify genes categorized as ion channel transcripts and the table shows transcripts with >1.0-fold expression above normal.
Dct: Dopachrome tautomerase.
Reproduced with permission from [14].
We targeted the deletion of Dct because it is strongly expressed by both murine and human cardiac melanocytes, and in cutaneous melanocytes Dct aids in reducing reactive oxygen species (ROS) [36]. ROS have been implicated in promoting atrial arrhythmias, and in several preparations reactive species are known to modify voltage-dependent channels [37–39], which may result in prolonged repolarization and increased ectopy. While we do not know the exact mechanism by which Dct influences cellular repolarization and excitability in cardiac melanocytes, we have found evidence for increased ROS in the atria of Dct-null mice by the presence of swollen mitochondria with loss of their matrices [14], so it is possible that the oxidative modification of ion channels in these cells is one mechanism by which their repolarization and excitability is altered.
The relationship of cardiac melanocytes to atrial arrhythmias was further supported by the observation that Dct-null mice display both spontaneous and inducible atrial arrhythmias, while wild-type littermate mice do not have arrhythmias (Figure 3). The fact that Dct-null mice have atrial arrhythmias and cardiac melanocytes from Dct-null mice have afterdepolarizations, which could trigger atrial arrhythmias, suggests, but does not prove, that these cells may be a source of arrhythmogenic triggers. Therefore, to better define the relationship between atrial arrhythmias and cardiac melanocytes, we sought a model whereby cardiac melanocytes could be selectively removed or ‘ablated’ from the heart to prove their causality with atrial arrhythmias. Interestingly, cardiac melanocytes express the cell surface receptor c-kit, which has been shown to be important for the survival and migration of cells descending from the melanoblast lineage [40,41]. Mice with mutations in c-kit (also known as the W locus, where two of the more common mutations are known as W and Wv) display various levels of coat color defects depending upon the severity of the c-kit mutation they harbor [42,43]. When we examined the hearts of c-kit mutant mice we found that they did not express cardiac melanocytes (Figure 4), and when we studied the offspring of c-kit mutant mice crossed with Dct-null mice, we found that these animals no longer had inducible atrial arrhythmias (Table 3). While c-kit has pleiotropic effects outside of those upon cardiac melanocytes, these findings strongly suggest that cardiac melanocytes contribute to the genesis of atrial arrhythmias in mice. Interestingly, in the mouse heart, approximately the same number of cardiac melanocytes are expressed in the right atria as are in the PVs and left atria. While the right atrium has not been shown to contribute significantly to clinical AF, our findings from the c-kit mutant mice confirm cardiac melanocytes do contribute to AF. However, the exact distribution of cardiac melanocytes in the human heart remains to be confirmed, as well as their contribution to AF.
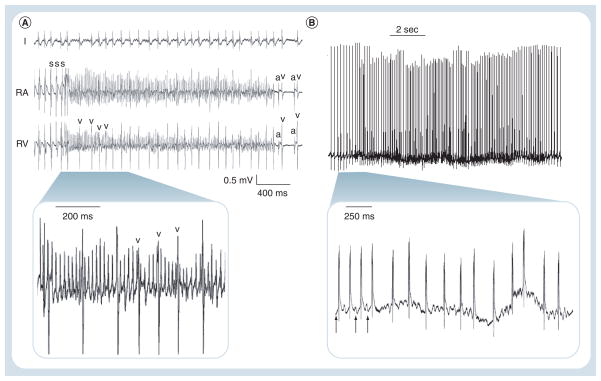
Figure 3. Dopachrome tautomerase-null mice have inducible and spontaneous atrial arrhythmias.
(A) Recordings from I, RA and RV show an atrial tachyarrhythmia induced by burst S in a Dct−/− animal. Irregular intervals between v beats are denoted. Sinus beats are shown on termination of the episode with normal a and v electrograms. Inset demonstrates magnified view of denoted area. (B) Real-time telemetry recorded in an awake Dct−/− mouse with an implantable monitor demonstrates spontaneous AF characterized by abrupt onset of a rapid irregularly irregular rhythm with no discrete p-waves. The inset shows a faster timescale of a few beats of sinus rhythm (arrows), followed by the onset of AF.
a: Atrial; AF: Atrial fibrillation; Dct: Dopachrome tautomerase; I: ECG lead I; RA: Right atrium; RV: Right ventricle; s: Stimulation; v: Ventricular.
Reproduced with permission from [14].
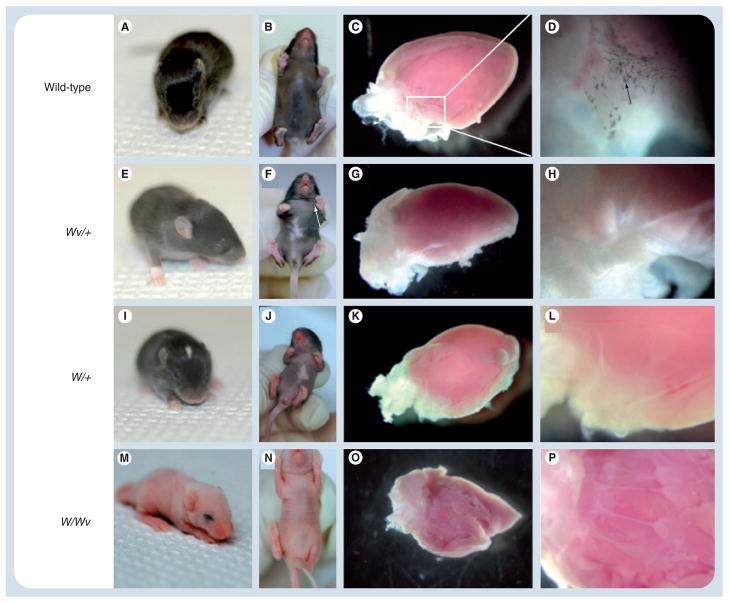
Figure 4. Dermal and cardiac pigmentation in W/Wv mice.
Shown are representative hearts from (A–D) wild-type, (E–H) Wv/+, (I–L) W/+ and (M–P) W/Wv mouse littermates. (A & B) The wild-type mouse demonstrated normal dermal pigmentation and (C & D) the presence of cardiac pigmentation. Tricuspid valve at (C) low and (D) high power; black arrow denotes pigmented cells in a valve leaflet. (E, F, I, J, M & N) Wv/+, W/+, and W/Wv mice showed abnormal dermal pigmentation, (G, H, K, L, O & P) as well as an absence of pigmented cells within the valves. Immunohistochemical staining with an anti-Dct antibody similarly showed labeling within wild-type mouse hearts, but cardiac staining was absent in Wv/+, W/+ and W/Wv mice (not shown). Original magnification ×40 (C, G, K & O) and ×200 (D, H, L & P).
Reproduced with permission from [14].
Table 3.
Baseline invasive electrophysiology intervals.
| Parameter | Dct−/− (n = 11) | Dct+/− (n = 12) | W/Wv (n = 10) | Dct−/−, W/Wv (n = 10) |
|---|---|---|---|---|
| Episodes AT | 27 | 2* | 2* | 5* |
| Episodes VT | 7 | 10 | 8 | 9 |
| ATCL (ms) | 32.2 ± 7.8 | 76.5 | 46.9 | 38.4 ± 6.7 |
| AT duration (ms) | 781 ± 417 | 356 | 112 | 178 ± 112 |
| Age (days) | 56.7 ± 4.8 | 58.4 ± 6.4 | 59.7 ± 5.8 | 62.0 ± 7.9 |
| Weight (g) | 21.2 ± 3.7 | 21.4 ± 2.8 | 22.0 ± 2.4 | 21.8 ± 2.6 |
Data are presented as the mean ± standard deviation and tests of statistical difference were computed using one-way analysis of variance followed by the least significant difference procedure for tachycardia duration, cycle length, animal weight and age. The number of arrhythmic episodes were assumed to have a Poisson distribution and the Kolmogorov–Smirnov test was used to assess statistical significance between groups. Statistical significance for mean AT duration was determined by the Mann–Whitney test.
p < 0.05 compared with Dct−/−.
AT: Atrial tachycardia; ATCL: Atrial tachycardia cycle length; Dct: Dopachrome tautomerase; VT: Ventricular tachycardia.
Modified with permission from [14].
Autonomic variation contributes to AF initiation via cardiac melanocytes
It has been known for some time that the atria and PVs contain enriched autonomic innervation [44,45], and autonomic output is a significant contributing factor to the initiation and maintenance of atrial arrhythmias [46–49]. Past studies have suggested that a predominance of either vagal or sympathetic tone is largely responsible for initiating AF [50,51]. In this regard, AF may be thought of as two clinical forms: one induced predominantly during sleep (vagotonic) and the other provoked by exertion or emotion (adrenergic) [52]. The distinction between vagotonic and adrenergic-mediated AF has important implications for treating clinical AF, since vagotonic AF is less likely to originate from the PVs and responds more poorly to PV isolation than does adrenergic-mediated AF [53]. However, reducing overall autonomic influences via ablation of the ganglionated plexi appears to improve the incidence of both vagotonic and adrenergic-mediated AF [54,55].
The fact that adrenergic-mediated AF responds better to PV isolation is probably because this form of AF is initiated primarily by ectopy arising from the PVs. In this regard, Patterson et al. have shown in canine models that the underlying mechanism of PV ectopy initiating AF is calcium-mediated triggered activity [56], as have Hirose and Laurtia [18]. However, what is not well known is the cellular origin of these triggered beats that initiate adrenergic-mediated AF. In this regard, the discovery of cardiac melanocytes may help to fill this missing piece in our understanding of the dual autonomic nature of AF. Given that cardiac melanocytes with prolonged action potential duration can trigger phase 3 afterdepolarizations (Figure 2), in the setting of increased adrenergic tone it is possible that these cells contribute to calcium-mediated triggers that arise from the PVs. Along these lines, a recent report by Genade et al. suggests melatonin mediates receptor-dependent protection against ischemia–reperfusion injury in the rodent heart by blunting the effects of adrenergic stimulation [57]. Therefore, cardiac melanocytes may respond more vigorously to adrenergic stimulation and initiate calcium-mediated triggers in a setting where melatonin levels are reduced. On the other hand, the antiadrenergic effects of melatonin may actually be able to prevent initiation of AF triggers, if these findings are eventually validated in the clinical setting.
While the evidence supporting the contribution of autonomic variation to atrial arrhythmogenesis is relatively extensive, more importantly, any theories proposed to explain the cellular basis of atrial arrhythmias must account for the contribution of autonomic influences. In this regard, we have found that murine cardiac melanocytes express both adrenergic and muscarinic receptors, and appear in close approximation with sympathetic and parasympathetic nerve endings (Figure 5). Furthermore, we found that wild-type mice that express cardiac melanocytes have 44% more atrial arrhythmia episodes than the offspring of c-kit mutant mice that lack cardiac melanocytes when they are challenged with the muscarinic agonist carbachol (25 μg/kg intraperitoneal), which mimics increased parasympathetic tone (64 vs 36 episodes; Table 4).
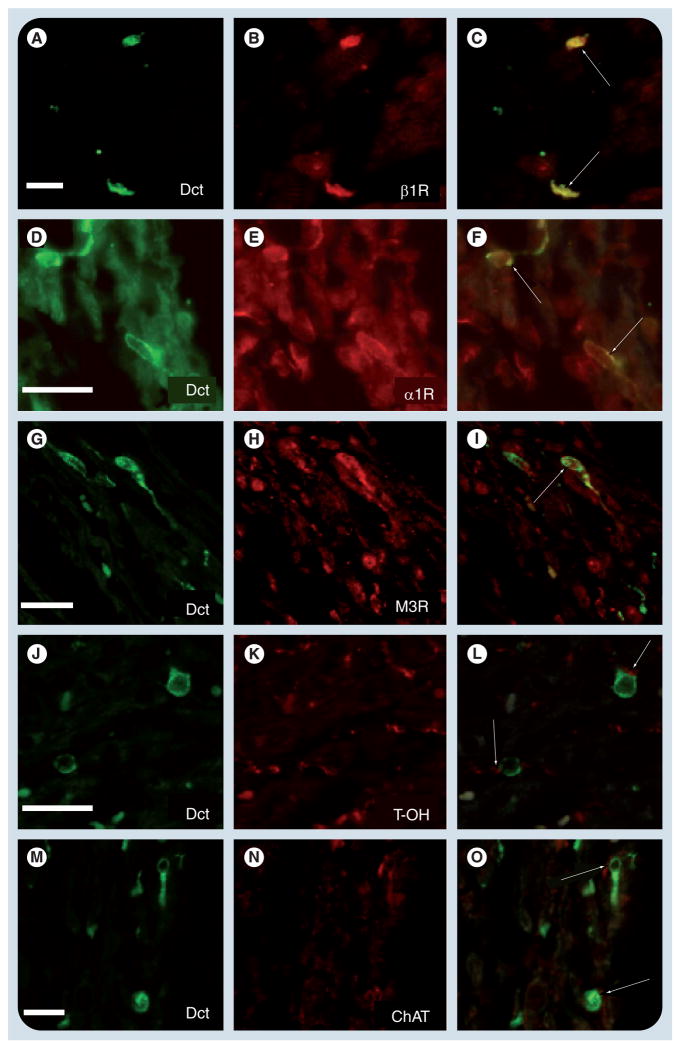
Figure 5. Dopachrome tautomerase-expressing cells coexpress adrenergic and muscarinic receptors.
Immunohistochemistry within the adult mouse atrium using an antibody to Dct demonstrates Dct-positive cells with characteristic morphology (A, D, G, J & M). Sections were costained with antibodies to (B) β1R, (E) α1R, (H) M3R, (K) T-OH and (N) ChAT. Merged images of Dct and each respective antibody are shown, demonstrating coexpression of Dct with (C) β1R, (F) α1R and (I) M3R receptors (arrows). In addition, presumptive nerve terminals that express (L) T-OH and (O) ChAT were seen in close proximity to Dct-expressing cells (arrows). Scale bars: 20 μm.
α1R: α1-adrenergic receptor; β1R: β1-adrenergic receptor; ChAT: Choline acetyltransferase; Dct: Dopachrome tautomerase; M3R: Muscarinic receptor subtype 3; T-OH: Tyrosine hydroxylase.
Reproduced with permission from [14].
Table 4.
Electrophysiology effects of muscarinic stimulation on cardiac melanocytes.
| Parameter | Wild-type mice (n = 8) | W/Wv mice (n = 8) | ||
|---|---|---|---|---|
| Baseline | Carbachol | Baseline | Carbachol | |
| Episodes AT | 2 | 64†§¶ | 4 | 36†‡§ |
| ATCL (ms) | 36.4 | 26.7 ± 8.7 | 35.5 ± 6.2 | 25.0 ± 8.2 |
| AT duration (s) | 0.19 | 29.7 ± 3.5†§ | 0.23 ± 0.15‡¶ | 27.8 ± 2.9†§ |
| Age (days) | 54.9 ± 5.2 | 54.9 ± 5.2 | 56.2 ± 5.4 | 56.2 ± 5.4 |
| Weight (g) | 22.8 ± 2.9 | 22.8 ± 2.9 | 21.6 ± 2.7 | 21.6 ± 2.7 |
Data analysis was performed as described in Table 3.
p < 0.05 compared with wild-type.
p < 0.05 compared with wild-type + carbachol.
p < 0.05 compared with W/Wv.
p < 0.05 compared with W/Wv + carbachol.
AT: Atrial tachycardia; ATCL: Atrial tachycardia cycle length.
Modified with permission from [14].
In the future, it will be interesting to determine how autonomic stimulation influences the electrical excitability of cardiac melanocytes, and specifically the effects of adrenergic stimulation upon triggered activity and arrhythmogenesis. Simple experiments could be performed using the patch clamp technique on isolated murine cardiac melanocytes to investigate the influence of pharmacologic stimulation upon action potential duration and afterdepolarizations. Similarly, dual optical voltage and calcium transient mapping studies could be performed in ex vivo atrial preparations to assess the effects of pharmacological stimulation upon triggered activity and atrial arrhythmia initiation.
Expert commentary & five-year view
While melanocyte-like cells are present in human hearts, the electrophysiological properties of these cells in patients and their contribution to clinical atrial arrhythmias will need to be validated. It is likely that within the next 5 years interest in cardiac melanocytes will increase and their physiology and contribution to clinical atrial arrhythmias will be explored. If the contribution of cardiac melanocytes to clinical atrial arrhythmias is confirmed, then the potential for reducing or curing atrial arrhythmias by specifically targeting cardiac melanocytes opens up a whole new realm in the therapeutic armatorium against this disease. The promise of this tremendous opportunity, to treat and/or cure a very common disease that has thus far proven to be quite difficult to quell, is likely to stimulate the curiosity of many groups with diverse interests and expertise. Not only is it likely that the role of cardiac melanocytes in clinical atrial arrhythmias will be explored and deciphered in the foreseeable future, but that innovative individuals will devise novel technologies to identify these cells in the beating heart, and specifically modify or eliminate them to treat atrial arrhythmias.
Key issues.
The exact source and mechanism by which ectopic foci arise that initiate atrial fibrillation remain unclear.
Present-day therapies for atrial fibrillation have dramatically improved but are still plagued by side effects, complications and reoccurrence of the arrhythmia.
Melanocyte-like cells are a novel cell population that reside in the pulmonary veins and atria of humans and mice.
Murine melanocyte-like cells are electrically excitable and contribute to the initiation of atrial arrhythmias in response to increased autonomic tone.
The contribution of human melanocyte-like cells to atrial arrhythmias remains to be confirmed.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
This work was supported by grants from the NIH (grant no. K08-HL074108-05), the WW Smith Charitable Trust and the Gunther Fund for Cardiovascular Research. The author is a named inventor on US patents pending to treat atrial arrhythmias by targeting melanocyte-like cells. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Carnes CA, Chung MK, Nakayama T, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–E38. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 2.Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation. 2003;108:711–716. doi: 10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 3.Friberg J, Buch P, Scharling H, Gadsbphioll N, Jensen GB. Rising rates of hospital admissions for atrial fibrillation. Epidemiology. 2003;14:666–672. doi: 10.1097/01.ede.0000091649.26364.c0. [DOI] [PubMed] [Google Scholar]
- 4.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 5.Brand FN, Abbott RD, Kannel WB, Wolf PA. Characteristics and prognosis of lone atrial fibrillation. 30-year follow-up in the Framingham Study. J Am Med Assoc. 1985;254:3449–3453. [PubMed] [Google Scholar]
- 6.Levy S, Maarek M, Coumel P, et al. Characterization of different subsets of atrial fibrillation in general practice in France: the ALFA study. The College of French Cardiologists. Circulation. 1999;99:3028–3035. doi: 10.1161/01.cir.99.23.3028. [DOI] [PubMed] [Google Scholar]
- 7••.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. Landmark report that first identified and demonstrated that ectopic beats originating from the pulmonary veins initiate clinical atrial fibrillation. [DOI] [PubMed] [Google Scholar]
- 8•.Cappato R, Calkins H, Chen SA, et al. Up-dated worldwide survey on the methods, efficacy and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–38. doi: 10.1161/CIRCEP.109.859116. Most comprehensive survey to date of outcomes and complications associated with catheter ablation for atrial fibrillation. [DOI] [PubMed] [Google Scholar]
- 9.Dhruvakumar S, Gerstenfeld EP. Complications associated with catheter ablation of atrial fibrillation. Minerva Cardioangiol. 2007;55:353–368. [PubMed] [Google Scholar]
- 10.Sauer WH, McKernan ML, Lin D, Gerstenfeld EP, Callans DJ, Marchlinski FE. Clinical predictors and outcomes associated with acute return of pulmonary vein conduction during pulmonary vein isolation for treatment of atrial fibrillation. Heart Rhythm. 2006;3:1024–1028. doi: 10.1016/j.hrthm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Bellandi F, Simonetti I, Leoncini M, et al. Long-term efficacy and safety of propafenone and sotalol for the maintenance of sinus rhythm after conversion of recurrent symptomatic atrial fibrillation. Am J Cardiol. 2001;88(6):640–645. doi: 10.1016/s0002-9149(01)01806-9. [DOI] [PubMed] [Google Scholar]
- 12.Kochiadakis GE, Marketou ME, Igoumenidis NE, et al. Amiodarone, sotalol, or propafenone in atrial fibrillation: which is preferred to maintain normal sinus rhythm? Pacing Clin Electrophysiol. 2000;23:1883–1887. doi: 10.1111/j.1540-8159.2000.tb07044.x. [DOI] [PubMed] [Google Scholar]
- 13.Singh BN, Singh SN, Reda DJ, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352(18):1861–1872. doi: 10.1056/NEJMoa041705. [DOI] [PubMed] [Google Scholar]
- 14••.Levin MD, Lu MM, Peterenko NB, et al. Melanocyte-like cells in the heart and pulmonary veins contribute to atrial arrhythmia triggers. J Clin Invest. 2009;119:3420–3436. doi: 10.1172/JCI39109. First report to identify melanocyte-like cells in human hearts and demonstrate that they contribute to atrial arrhythmias in murine models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen SA, Hsieh MH, Tai TC, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins. Circulation. 1999;100:1879–1886. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- 16.Pappone C, Rosanio S, Oreto G, et al. Circumferential radiofrequency ablation of pulmonary vein ostia: a new anatomic approach. Circulation. 2000;102:2619–2628. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- 17.Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–1081. doi: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 18.Hirose M, Laurita KR. Calcium-mediated triggered activity is an underlying cellular mechanism of ectopy originating from the pulmonary vein in dogs. Am J Physiol Heart Circ Physiol. 2007;292:H1861–H1867. doi: 10.1152/ajpheart.00826.2006. [DOI] [PubMed] [Google Scholar]
- 19.Patterson E, Po S, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Chou C-C, Nihei M, Zhou S, et al. Intracellular calcium dynamics and anisotropic reentry in isolated canine pulmonary veins and left atrium. Circulation. 2005;111:2889–2897. doi: 10.1161/CIRCULATIONAHA.104.498758. [DOI] [PubMed] [Google Scholar]
- 21.Arora R, Verheule S, Scott L, et al. Arrhythmogenic substrate of the pulmonary veins assessed by high-resolution optical mapping. Circulation. 2003;107:1816–1821. doi: 10.1161/01.CIR.0000058461.86339.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seol CA, Kim J, Kim WT, et al. Simulation of spontaneous action potentials of cardiomyocytes in pulmonary veins of rabbits. Prog Biophys Mol Biol. 2008;96:132–151. doi: 10.1016/j.pbiomolbio.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Wongcharoen W, Chen YC, Chen YJ, et al. Effects of a Na+/Ca2+ exchanger inhibitor on pulmonary vein electrical activity and ouabain-induced arrhythmogenicity. Cardiovasc Res. 2006;70:497–508. doi: 10.1016/j.cardiores.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Melnyk P, Ehrlich JR, Pourrier M, Villeneuve L, Cha TJ, Nattel S. Comparison of ion channel distribution and expression in cardiomyocytes of canine pulmonary veins versus left atrium. Circulation. 2005;65:104–116. doi: 10.1016/j.cardiores.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Ehrlich JR, Cha TJ, Zhang L, et al. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. J Physiol. 2003;551:801–813. doi: 10.1113/jphysiol.2003.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan AY, Chen P-S, Chen LS, Fishbein MC. Autonomic nerves in pulmonary veins. Heart Rhythm. 2007;4:S57–S60. doi: 10.1016/j.hrthm.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morel E, Meyronet D, Thivolet-Bejuy F, Chevalier P. Identification and distribution of interstitial Cajal cells in human pulmonary veins. Heart Rhythm. 2008;5:1063–1067. doi: 10.1016/j.hrthm.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 28.Gherghiceanu M, Hinescu ME, Andrei F, et al. Interstitial Cajal-like cells (ICLC) in myocardial sleeves of human pulmonary veins. J Cell Mol Med. 2008;12:1777–1781. doi: 10.1111/j.1582-4934.2008.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Lugones A, McMahon JT, Ratliff NB, et al. Evidence of specialized conduction cells in human pulmonary veins of patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14:803–809. doi: 10.1046/j.1540-8167.2003.03075.x. [DOI] [PubMed] [Google Scholar]
- 30.Gerstenfeld EP, Callans DJ, Dixit S, Zado E, Marchlinski FE. Incidence and location of focal atrial fibrillation triggers in patients undergoing repeat pulmonary vein isolation: implications for ablation strategies. J Cardiovasc Electrophysiol. 2003;14:685–690. doi: 10.1046/j.1540-8167.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- 31.Kistler PM, Roberts-Thomson KC, Haqqani HM, et al. P-wave morphology in focal atrial tachycardia: development of an algorithm to predict the anatomic site of origin. J Am Coll Cardiol. 2006;48:1010–1017. doi: 10.1016/j.jacc.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 32.Hillock RJ, Kalman JM, Roberts-Thomson KC, Haqqani H, Sparks PB. Multiple focal atrial tachycardias in a healthy adult population: characterization and description of successful radiofrequency ablation. Heart Rhythm. 2007;4:435–438. doi: 10.1016/j.hrthm.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 33.Kistler PM, Sanders P, Hussin A, et al. Focal atrial tachycardia arising from the mitral annulus: electrocardiographic and electrophysiologic characterization. J Am Coll Cardiol. 2003;41:2212–2219. doi: 10.1016/s0735-1097(03)00484-4. [DOI] [PubMed] [Google Scholar]
- 34.Lee HT, Hennig GW, Fleming NW, et al. Septal interstitial cells of Cajal conduct pacemaker activity to excite muscle bundles in human jejunum. Gastroenterology. 2007;133:907–917. doi: 10.1053/j.gastro.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- 36.Dudley SC, Jr, Hoch NE, McCann LA, et al. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266–1273. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- 37.Campbell DL, Stamler JS, Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and S-nitrosothiols. J Gen Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W, Ma J, Zhang P, Luo A. Redox reaction modulates transient and persistent sodium current during hypoxia in guinea pig ventricular myocytes. Pflugers Arch. 2007;454:461–475. doi: 10.1007/s00424-007-0219-1. [DOI] [PubMed] [Google Scholar]
- 39.Lu Z, Abe J, Taunton J, et al. Reactive oxygen species-induced activation of p90 ribosomal S6 kinase prolongs cardiac repolarization through inhibiting outward K+ channel activity. Circ Res. 2008;103:269–278. doi: 10.1161/CIRCRESAHA.107.166678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cable J, Jackson IJ, Steel KP. Mutations at the W locus affect survival of neural crestderived melanocytes in the mouse. Mech Dev. 1995;50:139–150. doi: 10.1016/0925-4773(94)00331-g. [DOI] [PubMed] [Google Scholar]
- 41.Steel KP, Davidson DR, Jackson IJ. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development. 1992;115:1111–1119. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- 42.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 43.Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W ) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;7:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 44•.Ardell JL. Structure and function of the mammalian intrinsic cardiac neurons. In: Armour JA, Ardell JL, editors. Neurocardiology. Oxford University Press; NY, USA: 1994. Excellent overview of the anatomy and distribution of autonomic nerves in the atria and pulmonary veins. [Google Scholar]
- 45.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 46.Scherlag BJ, Yamanashi WS, Patel U, Lazzara R, Jackman WM. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J Am Coll Cardiol. 2005;45:1575–1580. doi: 10.1016/j.jacc.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 47.Lin J, Scherlag BJ, Lu Z, et al. Inducibility of atrial and ventricular arrhythmias along the ligament of Marshall: role of autonomic factors. J Cardiovasc Electrophysiol. 2008;19:955–962. doi: 10.1111/j.1540-8167.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 48.Zimmermann M, Kalusche D. Fluctuation in autonomic tone is a major determinant of sustained atrial arrhythmias in patients with focal ectopy originating from the pulmonary veins. J Cardiovasc Electrophysiol. 2001;12:285–291. doi: 10.1046/j.1540-8167.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- 49.Lu Z, Scherlag BJ, Lin J, et al. Autonomic mechanism for initiation of rapid firing from atria and pulmonary veins: evidence by ablation of ganglionated plexi. Cardiovasc Res. 2009;84:245–252. doi: 10.1093/cvr/cvp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coumel P, Attuel P, Lavallee J, Flammang D, Leclercq JF, Slama R. The atrial arrhythmia syndrome of vagal origin. Arch Mal Coeur Vaiss. 1978;71:645–656. [PubMed] [Google Scholar]
- 51.Doshi RN, Wu T-J, Yashima M, et al. Relation between ligament of Marshall and adrenergic atrial tachyarrhythmia. Circulation. 1999;100:876–883. doi: 10.1161/01.cir.100.8.876. [DOI] [PubMed] [Google Scholar]
- 52.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J. 1994;15:9–16. doi: 10.1093/eurheartj/15.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 53.Oral H, Chugh A, Scharf C, et al. Pulmonary vein isolation for vagotonic, adrenergic, and random episodes of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:402–408. doi: 10.1046/j.1540-8167.2004.03432.x. [DOI] [PubMed] [Google Scholar]
- 54.Pappone C, Santinelli V, Manguso F, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 55.Lemola K, Chartier D, Yeh YH, et al. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation. 2008;117:470–477. doi: 10.1161/CIRCULATIONAHA.107.737023. [DOI] [PubMed] [Google Scholar]
- 56.Patterson E, Lazzara R, Szabo B, et al. Sodium–calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 57.Genade S, Genis A, Ytehus K, Huisamen B, Lochner A. Melatonin receptor-mediated protection against myocardial ischaemia/reperfusion injury: role of its anti-adrenergic actions. J Pineal Res. 2008;45:449–458. doi: 10.1111/j.1600-079X.2008.00615.x. [DOI] [PubMed] [Google Scholar]