Spatial organization of transmembrane receptor signalling
This review by Dikic and Acker-Palmer focuses on how receptor clustering and membrane microdomains impact signalling specificity and integration.
Keywords: membrane microdomains, receptor clustering, signal integration
Abstract
The spatial organization of transmembrane receptors is a critical step in signal transduction and receptor trafficking in cells. Transmembrane receptors engage in lateral homotypic and heterotypic cis-interactions as well as intercellular trans-interactions that result in the formation of signalling foci for the initiation of different signalling networks. Several aspects of ligand-induced receptor clustering and association with signalling proteins are also influenced by the lipid composition of membranes. Thus, lipid microdomains have a function in tuning the activity of many transmembrane receptors by positively or negatively affecting receptor clustering and signal transduction. We review the current knowledge about the functions of clustering of transmembrane receptors and lipid–protein interactions important for the spatial organization of signalling at the membrane.
Introduction
Receptor-mediated signalling is a highly complex, evolutionary conserved mechanism that allows communication between cells and their environment. Efficiency, high precision and specificity are required to transmit only relevant signals to the appropriate target cells. To ensure transmission of even weak signals, the receptor and their associated complexes can be modified by dynamic and reversible post-translational modifications, which in turn promote amplification and diversification of signalling in the cell (Deribe et al, 2010). An additional level of complexity comes from the organization of receptors in higher-order clusters. Receptors do not function as individual signalling units, but tend to associate in multimolecular complexes that can accommodate up to hundreds of molecules. Examples include the chemotaxis receptors in bacteria, the epidermal growth factor receptors (EGFRs) and T-cell receptors (TCRs) in mammalian cells (Kentner and Sourjik, 2006; Deribe et al, 2009; Manz and Groves, 2010). Receptors associate in such complexes through direct interactions, indirectly through adaptor molecules or through specific interactions with lipid microdomains (Simons and Toomre, 2000; Seet et al, 2006). The multimolecular signalling clusters may consist of homotypic or heterotypic receptor associations, and may also involve different molecules located in the membranes from adjacent cells.
Advantages of receptor clustering include restricted diffusion of receptors at the membrane and amplification of the signal as a result of simultaneous activation of multiple receptors. In this review, typical examples of receptors in signalling clusters will be described. The importance of these associations in regulating signalling specificity and sensitivity will be discussed, stressing that receptor co-operativity is absolutely necessary for the integration of multiple signals and for the achievement of a coordinated cellular response.
Homotypic receptor clustering in cis
Many basic principles governing the organization and functional importance of receptor–receptor complexes come from the study of the largest subfamily of receptor tyrosine kinases (RTK), the Eph receptors (Ephs) and their ligands, the ephrins. Ephs and ephrins are mainly involved in the developmental processes and organ morphogenesis, regulating cellular processes such as cell attraction and repulsion, sorting and motility or cell survival and differentiation (Pasquale, 2005). The nine human isoforms of EphA receptors bind to five glycosylphosphatidylinositol (GPI)-linked ephrinA ligands, whereas five EphB receptors bind to three transmembrane ephrinB ligands. Ligand–receptor specificity is low within the classes and even some inter-class associations have also been shown for ephrinA5 binding to EphB2 and the three ephrinB ligands binding to EphA4 (Pasquale, 2005, 2008). Upon interaction of Ephs with their ephrin ligands, signalling cascades are initiated in both cells carrying either receptor or ligand resulting in a bidirectional mode of signalling (see below under section ‘Receptor associations in trans'). Once receptors and ligands from opposing cells come into contact, bidirectional downstream signalling occurs only after tetramerization of the trans-complex (Pasquale, 2005). Initially, pre-clustered ephrins form homooligomers, which bind the Ephs with a 1:1 stoichiometry upon cell–cell contact. Further clustering progresses by the assembly of Eph–ephrin dimers into tetrameric complexes, inducing conformational changes on both the receptor and the ligand (Himanen and Nikolov, 2003). The Eph tyrosine kinase domains can then trans-phosphorylate each other and promote forward signalling, whereas recruitment of Src-family kinases (SFK) to ephrinA/B ligands and phosphorylation of the ephrinBs initiate reverse signalling. In a unique way, uncommon for RTKs, the tetramers can be further clustered in higher-order assemblies regulating the mode and strength of signalling.
Even though the binding of Ephs to pre-clustered ephrins is of catalytic importance for multimeric clustering and signalling efficiency, it seems that Ephs also have the ability to associate in homotypic complexes through their extracellular (Himanen et al, 2010; Seiradake et al, 2010) or/and cytoplasmic (Lackmann et al, 1998; Wimmer-Kleikamp et al, 2004) domains. At low receptor concentration, pre-clustered ephrin ligands are required for initial receptor clustering. However, above a certain concentration threshold, free EphAs can cluster through interactions of their ectodomains, independently of ligand binding. Taking advantage of this mechanism, even minimal amounts of ephrins can function as ‘nucleation seeds' and can cluster a small number of Ephs, which can then initiate the recruitment of more receptors at the area of initial cell–cell contact and strengthen intercellular communication. In such a way, by co-operative hetero- and homomeric interactions, even weak signals can be amplified and coordinated to achieve efficient signalling (Himanen et al, 2010; Seiradake et al, 2010). Clustering of receptors that have an intrinsic enzymatic activity (such as the Ephs) is a direct mechanism to enhance downstream signalling. Nevertheless, receptor clustering seems to have additional functions. A constitutively active form of EphA4 that is phosphorylated without the need of ligand binding and stimulation, fails to regulate several developmental processes unless it becomes clustered by ephrinB (Egea et al, 2005). The reason for this might be the necessity of accumulation of downstream signalling effectors. A similar mechanism seems to be used by the ligand ephrin, which does not possess any kinase activity. It has been shown that ephrinBs require SFKs for their phosphorylation upon receptor engagement (Palmer et al, 2002). Activation of SFKs is achieved by auto-trans-phosphorylation. EphrinB clustering upon Eph-stimulation increases the local concentration of ephrin-recruited SFKs at the signalling foci, ensuring efficient SFK auto-activation and downstream signalling (Palmer et al, 2002).
Importantly, size and spatial patterning of signalling assemblies are not of random importance. On the contrary, these factors significantly contribute to the specificity of the signalling outcome, with small variations often resulting in opposite cellular responses. It is well known that only clustered or membrane-presented (and not free-soluble) ephrinB1 can induce phosphorylation of EphB1 (Davis et al, 1994). Moreover, different multimeric states of the Eph–ephrin complex (dimers, tetramers or higher-order multimers) result in distinct cellular responses. Even though dimeric-ephrinB1 can induce EphB1 phosphorylation, higher multimeric states of receptor complexes are necessary for the recruitment of downstream effectors and promotion of cell attachment (Stein et al, 1998). In accordance with this, EphB1 receptors have been successfully characterized as ‘ligand density sensors' that modulate integrin-mediated cell–matrix attachment according to the density of the ephrinB1 that they encounter (Huynh-Do et al, 1999). Even though the Ephs are phosphorylated after exposure to low-density ephrinB1 ligands, they are only able to induce αvβ3 integrin-driven cell attachment within a certain higher range of ephrinB1 density presented. Variations in the ephrinB1 concentration above or below this threshold result in the complete opposite phenotype, decreasing cell attachment. Application of the same principle could easily explain other examples of bimodal function of ephrinB1 acting as an attractant or repellant in retinal ganglion cell branching (McLaughlin et al, 2003) or of EphA–ephrinA signalling in promoting or inhibiting axonal growth (Hansen et al, 2004).
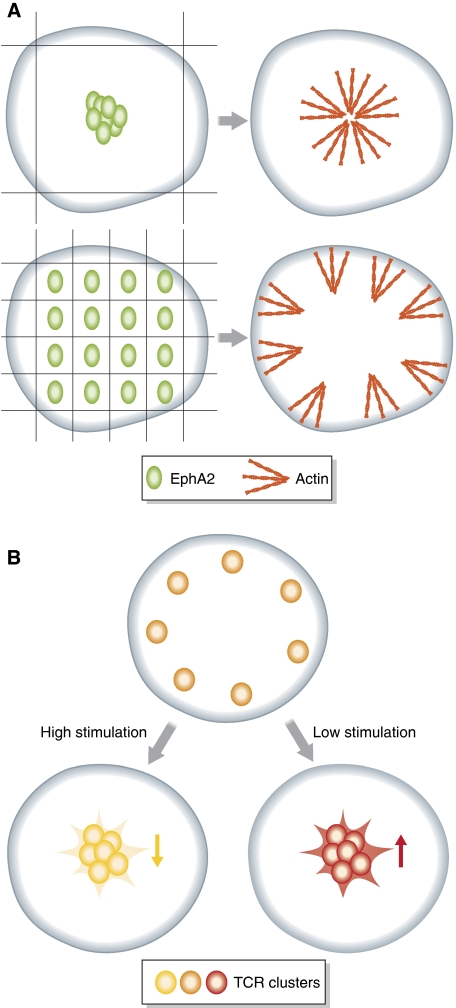
Further understanding of the mechanisms of multimeric receptor assembly was recently achieved by studies in which the size and shape of receptor complexes were not determined by the availability of the ligand, but was forced to desired configurations by mechanical artificial barriers (‘spatial mutation approach') (Groves, 2006). In an elegant study, Salaita et al (2010) confirmed the already described models and provided a direct insight on how the clustering pattern of Ephs at the cell surface is reflected on intracellular actin arrangements (Figure 1A). They geometrically constrained the distribution of ephrinA1 on synthetically engineered membranes and accordingly, modified the membrane patterning of EphA2 in a living cell. As expected, the mobility of ephrinA1 on the supported membranes defined to a great degree the transport and function of EphA2 receptor at the plasma membrane. Inability of ephrinA1 to freely diffuse and form microclusters had a direct impact on EphA2 forward signalling by decreasing the phosphorylation and degradation of the receptor. Interestingly, interference with the size and pattern of clusters formed by EphA2 did not affect the phosphorylation status of the receptor, but it had a strong impact on the intracellular distribution of different downstream effectors such as f-actin, as well as the amount of recruited ADAM2 to the EphA2–ephrinA1 clusters (Figure 1A). Moreover, applying the spatial mutation on a library of breast cancer cell lines revealed a strong correlation between the radial membrane transport of EphA2 and the invasiveness of the cells.
Figure 1.
Spatial distribution of membrane receptors. (A) The size and organization of EphA2 membrane clusters determines the distribution of downstream effectors. Under conditions of unrestricted membrane transport of EphA2 receptor (green), f-actin (red) accumulates in the periphery of activated ephrinA1–EphA2 clusters. Upon introduction of a spatial mutation that restricts EphA2 organization at the membrane, the distribution of f-actin shifted to the cell periphery (Salaita et al, 2010). (B) Re-distribution of T-cell receptors (TCRs) to the centre of the immunological synapse results in different signalling states depending on the stimulus strength. Upon high stimulation, transport of TCRs (yellow) to the centre of the synapse results in receptor deactivation and attenuation of signalling (pale yellow receptors). In contrast, under low-stimulation, the artificially-forced translocation of TCRs to the centre of the synapse enhances downstream signalling.
Interestingly, these results are highly reminiscent of how the differential spatial patterning of TCRs elicits different signalling outcomes at the immunological synapse. The building of the highly organized multimolecular structure of the immunological synapse is initiated by the recognition of antigen-presenting cells (APCs) from T cells. TCRs recognize and bind major histocompatibility complexes (MHCs) that are presenting specific antigenic peptides (Fooksman et al, 2010). The activated MHC–TCR complexes are then transferred with the help of the actin cytoskeleton to the centre of the synapse, in which hundreds of molecules can accumulate. At the same time, intercellular adhesion molecule complexes organize at the periphery of the synapse, broadening the contact area between the T cell and the APC (Manz and Groves, 2010).
The organization pattern of the TCRs in the synapse tightly regulates the signalling potential of the receptors, but at the same time the stimulus strength seems to determine the signalling outcome of the receptors clustered at the centre of the synapse. Under high-stimulation conditions, the transport of TCRs to the centre of the immunological synapse leads to an attenuation of the signalling by receptor dephosphorylation, inactivation and endocytosis (Lee et al, 2003) (Figure 1B). Blocking this translocation step by artificial barriers (‘spatial mutation approach') prolongs the presence of the receptors at the periphery of the synapse and results in a stronger T-cell response (Mossman et al, 2005) (Figure 1B). On the contrary, under low stimulation, the translocation of TCRs to the centre of the synapse helps enhancing the receptor signalling. Indeed, when the receptors are experimentally forced to occupy the centre of the synapse under conditions of low stimulation, the T-cell response is strongly enhanced (Cemerski et al, 2008) (Figure 1B). This example highlights the dual interplay between signalling regulation and receptor spatial organization: specific membrane arrangement of TCRs upon stimulation is essential for the efficiency of signalling, but at the same time the intensity of the signal seems to dictate how the positioning of the receptors in the synapse is perceived and ‘translated' by downstream signalling, such as phosphatases or the endocytic machinery.
Receptor clustering is not only determining signal specificity, but can also contribute to the increase in cellular sensitivity to external stimuli or can even have a purely mechanistic function, by enhancing the strength of cellular contacts to the extracellular matrix. For example, signalling sensitivity highly depends on the organization of receptor complexes on bacterial membranes upon chemotaxis. These macromolecular membrane associations consist of the chemotaxis receptors, adaptor molecules and downstream kinases. Interactions between the chemoreceptors are the critical determinant of cluster organization (Kentner and Sourjik, 2006). Thousands of molecules can accumulate in distinct signalling clusters, in a nucleation process possibly using trimers of receptor dimers as basic building blocks (Ames et al, 2002; Li and Hazelbauer, 2004). Following a yet-unclear mechanism, chemoreceptor clusters show an additional level of spatial organization, by preferentially localizing at the poles of the cell. Nevertheless, the important feature of these clusters is that receptors of different types co-exist in these associations and functionally interact in a highly orchestrated manner (Gestwicki and Kiessling, 2002; Sourjik, 2004; Studdert and Parkinson, 2004). This organization results in the formation of allosteric multimolecular complexes that function as one signalling network; multiple signals are perceived by the same complex because of its diverse content in receptor types. Conformational changes of stimulated receptors increase allosterically the sensitivity of other receptors for their ligands and, therefore, efficient signal transduction and amplification of weak signals are ensured (Sourjik, 2004; Sourjik and Berg, 2004). Another example of signalling enhancement by receptor clustering can be found on the integrin signalling system. Integrins are a family of α/β heterodimeric cell-surface receptors and the major mediator of cell attachment to the extracellular matrix. They are able to signal across the membrane in both directions and are often found in highly organized clusters on the cell surface (Arnaout et al, 2005). Receptor association seems to be mediated by ligand binding and is important for their function. But some integrins can form clusters independent of ligand stimulation (Li et al, 2004), possibly through association of their transmembrane domains (Li et al, 2003). Furthermore, it was shown that extensive receptor clustering enhances cell adhesion by increasing the contact area between the cell and the matrix and, therefore, the strength of binding (Hato et al, 1998; Chen and Moy, 2000). Cross-linked receptors bind the substrate in a co-operative manner and can resist more efficiently to detachment forces. In contrast, under a random distribution of individual receptors, the same forces would be unevenly exerted in fewer and weaker connections, increasing the risk of breaking. The application of modern microscopy and biophysical techniques has now provided mechanistic details on how weak associations mediated by single integrin molecules turn into strong adhesive cellular forces by receptor co-clustering and co-operativity (Gallant and García, 2007; Taubenberger et al, 2007).
Lipid microdomains in receptor organization and signalling
Ligand-induced receptor clustering in the membranes is also influenced by the lipid composition of membranes and a network of protein–lipid interactions. The theoretical number of cellular lipids is close to 200 000 species, including >100 000 glycosphingolipids, 9600 phospholipids, almost 70 000 mono-, di- and triglyceride variants, as well as numerous fatty-acid and sterol-based varieties (Yetukuri et al, 2008). This astounding number may give the impression of a crowded and randomly packed membrane, with little room for joint behaviour between lipids. However, the exact opposite is true. The lipids are meticulously organized when it comes to localization and concentration in the plasma membrane versus organellar membranes, composition in outer versus inner membrane bilayer, as well as in its planar distribution within the membrane leaflets (van Meer, 2005). These differences determine the functionality of the lipids.
Although the idea of an ordered membrane structure received attention already in the 1970s (Jain and White, 1977), the concept of membrane microdomains or so-called lipid rafts saw light when it was first shown that GPI-anchored proteins and glycosphingolipids are enriched in detergent-insoluble membrane fractions (Brown and Rose, 1992; Simons and Ikonen, 1997). Lipid rafts are now defined as microdomains in the plasma membrane enriched in cholesterol, sphingolipids and certain proteins. For the past couple of decades, however, it has been questioned whether lipid rafts are fact or artefact (Munro, 2003). It is widely accepted that the method commonly applied to determine whether a protein is lipid raft associated, detergent extraction, lacks a solid physical basis. At the same time, little or no consideration has been taken for the artefacts that can be induced by this method. One clear example of the effect of detergent extraction on the cell membrane was shown by microscopy, showing that the plasma membrane remained predominantly intact with a few large ‘holes' (Hao et al, 2001). This clearly does not correspond to the idea that detergent treatment solubilizes the membrane, leaving smaller microdomains intact. Another point that has caused debate is that the distribution of GPI-anchored raft proteins generally appears even across the plasma membrane when visualized by light microscopy, not in clustered units as expected (Mayor et al, 1994). Such observations have led to the speculation that the outer leaflet of the plasma membrane is ‘one big raft' with small regions of fluid lipids in between. However, by moving away from detergent extraction, the existence of nanoscale assemblies of raft proteins in the membrane has now been shown by different techniques (Lingwood and Simons, 2010). Single-particle tracking (Suzuki et al, 2007), fluorescence correlation spectroscopy (Lenne et al, 2006), high spatial and temporal-resolution fluorescence resonance energy transfer (Goswami et al, 2008) and high-resolution imaging (STED microscopy) (Eggeling et al, 2009) in living cells show temporal nanoclusters of GPI-anchored receptors.
As an attempt to fit old and new data in one lipid raft model, a revision of the classical view of lipid rafts as pre-existing structures in the plasma membrane (Simons and Ikonen, 1997) has been proposed (Hancock, 2006). The revised model proposes that small, unstable liquid-ordered domains are formed spontaneously. Larger, more stable domains can then form when proteins are recruited. The upper size of rafts is limited because larger rafts will be captured by endocytosis, disassembled in the endocytic pathway and returned to the plasma membrane as separate lipid and protein components (Hancock, 2006). Importantly, this model proposes that proteins have an active function in raft formation. The requirement for protein–protein interactions in surface distribution has been elegantly shown by two independent studies showing that the minimal lipid anchor of lymphocyte-specific protein tyrosine kinase (Lck) or Lyn fused to GFP, respectively, was insufficient for microdomain localization as seen with the full-length proteins (Douglass and Vale, 2005; Larson et al, 2005). Overall, recent work supports the lipid raft model, and the focus is now being shifted towards understanding the function of lipid rafts in certain processes rather than questioning their existence.
Lipid rafts have a function in various cellular processes, including endocytosis and signal transduction (Simons and Toomre, 2000; Lajoie and Nabi, 2007). It has been suggested that lipid rafts may serve as signalling ‘hot spots' largely based on the observation that many known signalling molecules are enriched within them (Simons and Toomre, 2000; Foster et al, 2003). One critical constituent of lipid rafts is cholesterol. An important structural feature of this lipid is its asymmetrical geometry resulting in two distinct faces, a smooth α-face and a rough β-face. This quality allows cholesterol to interact with two discrete membrane molecules simultaneously; for example a sphingolipid through the α-face and a transmembrane protein through the β-face (Fantini and Barrantes, 2009). Sphingolipids can additionally interact with the receptor and affect its conformation, showing a tight regulation between transmembrane receptors and their lipid environment.
One family of transmembrane receptors associated with lipid rafts constitute the G-protein-coupled receptors (GPCRs). The mechanism for raft association is still not clear for all the GPCRs, as there is no one general sorting signal. For some of the receptors, however, the activity status and the raft localization seem to be coupled. For example, the affinity state of human oxytocin receptor depends on structural features in cholesterol (Gimpl et al, 2000), suggesting that cholesterol interaction regulates receptor activation. Similarly, it is mainly the active version of the δ-opioid receptor that is found enriched in lipid rafts (Alves et al, 2005) (Figure 2A). A possible explanation for this could be the conformational change induced by activation, leading to a longer version of the receptor with a larger hydrophobic region. According to the hydrophobic matching hypothesis (Jensen and Mouritsen, 2004), this version of the receptor will be redistributed into the thicker sphingomyelin-enriched bilayer. Other proposed mechanisms for raft localization of GPCRs include modifications by fatty acids and interaction with caveolin, a protein found in a subpopulation of lipid rafts, caveolea (Chini and Parenti, 2004).
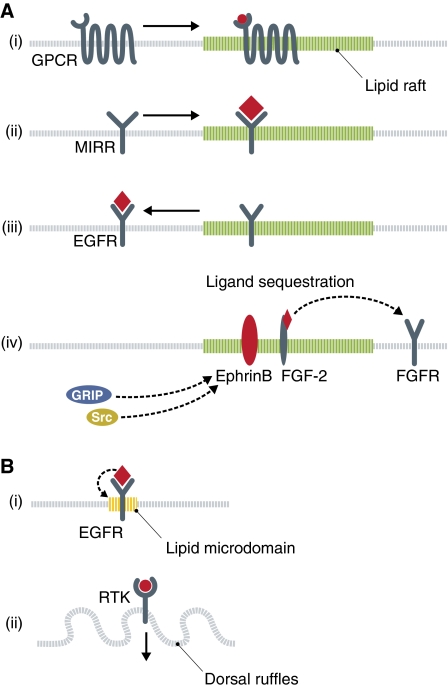
Figure 2.
Lipid microdomains in receptor organization and signalling. (A) Lipid rafts (green) can serve to recruit ligand (red) stimulated receptors, such as (i) G-protein-coupled receptors (GPCRs) or (ii) multichain immune recognition receptors (MIRRs) and thus focus the downstream signalling complexes. (iii) The receptor tyrosine kinase (RTK) epidermal growth factor receptor (EGFR) is localized to rafts in resting cells and is transported out of the raft upon ligand binding. (iv) A third function for rafts in signal transduction is to sequester receptor ligands. EphrinB is a transmembrane ligand associated with rafts, and serves to recruit cytoplasmic effector molecules to this domain. The FGF-2 ligand is kept in rafts by glypican-1 and away from the non-raft-associated fibroblast growth factor receptor (FGFR). (B) Other microdomains in the membrane can also serve to spatially arrange transmembrane receptors. (i) Upon ligand stimulation, the EGFR induces the formation of a local lipid domain rich in phosphatidic acid (yellow), which also contains an elevated number of EGFRs. (ii) Dorsal ruffles can be induced by stimulation of RTKs. These domains may serve both as signalling platforms and as internalization sites.
RTKs make up a second family of transmembrane receptors associated with lipid rafts. The EGFR shuttles in and out of lipid rafts, and it has been shown that interactions between the extracellular receptor region and the GM1 ganglioside participate in targeting the protein to rafts (Miljan et al, 2002). Interestingly, in contrast to the GPCRs mentioned above, it is the inactive form of EGFR that is associated with lipid rafts, and the receptor moves out of the raft in response to EGF (Mineo et al, 1999; Roepstorff et al, 2002) (Figure 2A). EphrinB ligands are also localized within rafts, inducing the recruitment of a downstream signalling complex to these domains (Brückner et al, 1999; Palmer et al, 2002) (Figure 2A). An interesting twist to the regulation of fibroblast growth factor receptor (FGFR) signalling was recently reported. The receptor is localized in non-raft membrane in both its active and inactive state, whereas the ligand FGF-2 is sequestered in lipid rafts by the heparin sulphate proteoglycan glypican-1, preventing receptor binding and thus allowing skeletal muscle differentiation (Gutiérrez and Brandan, 2010) (Figure 2A).
Rafts appear to have an important function in immune cell activation. Cells of the innate and adaptive immune systems express multichain immune recognition receptors (MIRRs), which respond to the presence of foreign macromolecules. These receptors include TCRs, B-cell receptors and certain receptors for the Fc regions of antibodies. In resting cells, MIRRs show no or very limited association with rafts. Upon ligand stimulation and receptor oligomerization, however, the receptors translocate into rafts (Cherukuri et al, 2001) (Figure 2A). Similar to certain GPCRs, it is likely that the recruitment is due to a conformational change in the receptor, which results in a high affinity for the raft environment. In the rafts, the receptors associate with SFKs and initiate downstream signalling events.
In membranes, receptor activation may also induce changes in the lipid microenvironment. The EGFR is a single transmembrane protein organized into oligomers at the plasma membrane in a cholesterol-dependent manner. Upon ligand binding, the tyrosine kinase domain is activated and induces downstream effects including hydrolysis of plasma membrane phosphatidylcholine by phospholipase D2 to produce phosphatidic acid (PA) and choline (Ariotti et al, 2010). This creates a local microdomain rich in PA, which also contains an elevated number of EGFRs (Figure 2B). As the EGFR can bind to acidic lipids in the plasma membrane, it is likely that the receptor interacts with newly synthesized PA to form this protein–lipid complex (Ariotti et al, 2010).
Stimulation of RTKs with platelet-derived growth factor, epidermal growth factor or hepatocyte growth factor (HGF) induces dorsal ruffles or ‘waves' in the plasma membrane (Mellström et al, 1988; Dowrick et al, 1993; Shinohara et al, 2002; Orth et al, 2006). Activated EGFR and Met RTK are recruited into these ruffles and internalized (Orth et al, 2006; Abella et al, 2010) (Figure 2B). Active Met shows a prolonged localization to dorsal ruffles, suggesting that these structures may serve as signalling platforms. Interestingly, internalization of Met from the dorsal ruffles also enhances their initial, but not total, degradation (Abella et al, 2010). The ruffles may, therefore, serve to focus downstream signalling, but at the same time more efficiently ‘turn off' the signal.
Heterotypic receptor clustering
Efficiency in signalling and most important in specificity is not only achieved by the membrane microdomains and homotypic clustering of receptors, but is also enhanced by the co-operative spatial accumulation of different receptor types. The co-existence of different receptors in the same signalling complex determines the molecular and cellular context that each receptor is facing and modulates accordingly its signalling outcome. Co-operative receptors can have agonist or antagonistic functions, activate each other without the need of their ligands, change each others ligand sensitivity (see above chemotaxis receptor clustering) or can simply influence each others membrane targeting and trafficking. Merging signalling pathways by receptor cross-talk can serve as a mechanism to efficiently integrate multiple environmental signals to one common signalling pathway, enabling direct interpretation of external stimuli by the cellular machineries.
Known for their function as guidance cues during development, semaphorins are a group of receptors that often require multiple co-receptors to exhibit their function. Semaphorins associate mainly with plexins, but their signalling might require their interaction with neuropilins (Npns) or the Ig superfamily cell adhesion molecules (IgCAMs). These different associations further determine the outcome of the semaphorin signalling (Zhou et al, 2008). For instance, Sema3s do not interact directly with plexinA receptors, but first bind to Npn-1 or Npn-2 to get incorporated in Npn–plexinA holoreceptor complexes and induce signal transduction through the plexins. Whether the outcome of this signalling event will lead to repulsive or attractive axonal guidance actions further depends on the final recruitment of either IgCAM L1 or NrCAM to the multimeric receptor complex (Castellani et al, 2000; Falk et al, 2005). Sema 3E alone can bind plexinD1 and mediate endothelial or axonal cell repulsion. However, when Npn-1 joins the complex, Sema 3E signalling is translated into cell attraction (Chauvet et al, 2007). Similarly, Sema 7A regulates cell adhesion differentially by binding to different receptors–plexinC1 (Walzer et al, 2005) or to integrins (Scott et al, 2008). This necessity for heteromeric signalling complexes in regulating semaphorin function is not restricted to its known co-receptors, but also includes members from other signalling pathways. Sema4D binding to plexinB1 increases the kinase activity of the RTKs Met and Erb2, mediating cell migration and metastasis (Giordano et al, 2002; Conrotto et al, 2005) or inducing cell migration and growth-cone collapse (Swiercz et al, 2004, 2008), respectively. Similarly, plexinA1 needs to form holoreceptor complexes with VEGFR2 to promote Sema6D-mediated signalling during cardiac development (Toyofuku et al, 2004).
Following similar principles, it has been realized in the recent years that Ephs and ephrins also cross-talk physically with other receptors to mediate many of their biological functions (Figure 3). For example, Ephs have been heavily implicated in cancer development and progression and their positive or negative contribution to these events highly depends on their functional association with known oncogenic growth factor receptors. Thus, EphA receptors can function as tumour suppressors when they are solely activated by their ephrin ligands (Pasquale, 2010), but can turn into potent tumour enhancers, through their associations with oncogenic receptors, such as the members of the EGF receptor family. In a transgenic mouse breast cancer model, Erb2 was shown to physically and functionally interact with EphA2, inducing EphA2 phosphorylation in the absence of ephrin ligands (Brantley-Sieders et al, 2008). Most importantly, the EphA2–Erb2 association was required for the maximal activation of the Erb2-downstream signalling cascade and seemed to account for the observed tumour proliferation and metastatic potential in Erb2-overexpressing mice. Conversely, in an earlier study, the activated EGFR and EGFRvIII (an oncogenic mutant form of EGFR) were shown to associate with EphA2, but this association led to a reduction of the EGF-induced cell migration (Larsen et al, 2007).
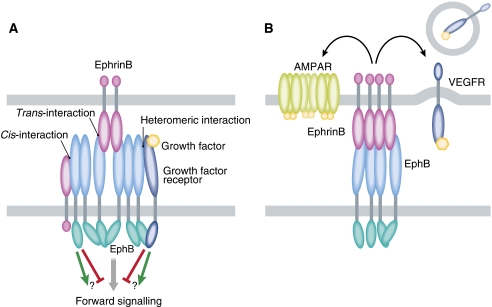
Figure 3.
Receptor–receptor complexes regulate signalling specificity and receptor trafficking. (A) Eph receptors (Ephs) are clustered on the membrane through trans-interactions with pre-clustered ephrin ligands or through homomeric interactions of their extracellular or intracellular domains. Clustering is necessary for the receptor activation and signalling. The downstream signalling of activated Ephs can be further modulated by interactions with ephrin ligands in cis or by heteromeric associations with other receptor types. (B) EphrinB2 differentially regulates the trafficking of AMPA and VEGF receptors. Serine phosphorylation of ephrinB2 promotes AMPAR stabilization at the cellular membrane of neurons, whereas ephrinB2 positively regulates VEGFR endocytosis through its PDZ-binding domain.
In glioma cells, the physical association of EphA4 and FGFR1 seems to be responsible for the observed enhancement of the FGFR-downstream signalling through the MAPK, Akt and Rac1/cdc42 pathways (Fukai et al, 2008). The increased cell proliferation and migration under conditions of EphA4 overexpression and FGF stimulation, does not seem to require ephrin ligands, as was shown for FGFR3 and EphA4 (Yokote et al, 2005). Owing to their physical interaction, the two receptors can trans-phosphorylate each other and activate the MAPK pathway. A similar principle of a functional cross-talk between EphA2 and the HGF-receptor c-Met could explain the activation of EphA2 and its positive impact on mammary epithelial proliferation and branching, upon HGF stimulation (Vaught et al, 2009).
Functional interactions between receptors that have antagonistic functions can provide an elegant fine-tuning mechanism of signalling regulation. Both ephrinB1 and the FGFR regulate retinal fate in Xenopus embryos. EphrinB1 promotes the retinal progenitor cell movement into the eye through its association with the scaffold protein Dishevelled (Dsh). The ability of FGFR to interact with ephrinB1 and induce its phosphorylation upon FGF stimulation disrupts the ephrinB1–Dsh interaction and results in suppression of the retinal fate (Chong et al, 2000; Lee et al, 2009). Similarly, activation of FGFR1 in EphB2-expressing cells interferes and blocks the ephrinB1-mediated cell repulsion and segregation, by increasing the steady-state phosphorylation of EphB2 and inhibiting the MAPK pathway in a feedback signalling cascade (Poliakov et al, 2008). At the immunological synapse, the presence of CD28 clusters surrounding the centred accumulation of TCRs promotes TCR signalling, but when CD28 molecules are experimentally forced to co-localize at the centre of the synapse with TCRs, cell signalling is decreased (Shen et al, 2008).
The formation of new synapses and the modulation of their activity primarily depend on the number of neurotransmitter receptors and their functional status. Modulation of synaptic morphogenesis and activity by the cross-talk of the Eph/ephrin bidirectional signalling with the NMDA and AMPA receptors constitute a perfect example on how receptor-coordinated function efficiently intermingles different signalling pathways in one developmental process. Mice lacking all three EphB isoforms expressed in the nervous system exhibit abnormal spine formation as well as low levels of NMDA and AMPA receptor clustering (Henkemeyer et al, 2003). Dalva et al (2000) showed that eprhinB1-mediated stimulation promotes the interaction of EphB2 with the NR1 subunit of the NMDA receptor and increases the clustering of NMDA receptors on dendritic membranes as well as the number of newly formed synapses on cultured neurons. Further evidence on the EphB2 and NMDAR co-operation comes from the facts that EphB2 can cluster NMDA receptors and promote spine formation (Contractor et al, 2002) and can enhance NMDA receptor phosphorylation (Takasu et al, 2002). Conversely, genetic depletion of EphB2 results in reduced NMDA-mediated currents and reduced synaptic localization of the NR1 subunit in neurons (Henderson et al, 2001). In addition, EphB2 co-clusters with AMPA receptors at synapses and regulates their activity, modulating in such a way spine development and mossy fibre long-term potentiation (Contractor et al, 2002; Kayser et al, 2006). But also reverse signalling by ephrinB ligands modulates the function of synaptic scaffold proteins or the trafficking of neurotransmitter receptors during synaptic plasticity and function. EphrinB2 modulates the trafficking of AMPA receptors—and, therefore, their activity—in a process that requires the bridging PDZ-containing protein Glutamate receptor-interacting protein1 and the serine phosphorylation of the ephrinB2 tail (Essmann et al, 2008) (Figure 3B). AMPA receptors become stabilized at the synaptic membrane by ephrinB ligands and lack of the ephrinB results in a constitutive internalization of AMPA receptors, which leads to impaired synaptic transmission (Essmann et al, 2008). Interestingly, recent work has revealed a novel cross-talk of ephrinB2 with the VEGF receptors. EphrinB2 associates physically with VEGFR2 (Sawamiphak et al, 2010) and VEGFR3 (Wang et al, 2010) at the membrane regulating the trafficking of these vascular receptors (Figure 3B). VEGFR2 and VEGFR3 endocytosis is required for the function of these receptors and ephrinB2 emerges now as a major controlling co-receptor needed to regulate the VEGFR signalling cascades during developmental and tumour angiogenesis (Sawamiphak et al, 2010) as well as lymphangiogenesis (Wang et al, 2010).
Receptor associations in trans
Receptor–receptor associations are not restricted in one membrane plane, but can adapt spatial configurations that serve intercellular communication. Receptors can be located on the membranes of different cells and associate in a trans-configuration, driving signalling cascades in both cells, in a process known as bidirectional signalling. The mode of receptor–receptor spatial organization in bidirectional signalling is imposed by the nature of their function as the receptors have to be spatially confined at the interfaces of cell–cell contact. However, receptor associations in cis are still possible and can interfere with the functions of the trans-complexes, so additional regulation on the spatial domain is required to ensure appropriate distributions between cis- and trans-assemblies. From the following examples, it becomes apparent that intercellular interactions among receptors involved in bidirectional signalling occur in a highly orchestrated manner and require mechanisms that discriminate them from identical receptor associations that take place in cis.
As previously discussed, Ephs and their ephrin ligands participate in intercellular signal transduction events. Activation of Ephs (forward signalling) results in auto-phosphorylation of their cytoplasmic kinase domain, further phosphorylation of downstream effectors and association with other protein adaptors, whereas ephrin activation (reverse signalling) also involves the phosphorylation of their cytoplasmic tails and interaction with multiple proteins. Eph and ephrin downstream signalling mediate cell proliferation, survival and differentiation, but mainly cell adhesion, shape and motility through cytoskeleton rearrangements. As these actions require the coordinate response of both signalling cells, it is not surprising that some of the molecules that are activated downstream of both receptor and ligand are common, such as the SFKs (Palmer et al, 2002; Knöll and Drescher, 2004) or the Rho-family GTPases (Noren and Pasquale, 2004). Nevertheless, a detailed proteomic and computational study revealed recently that the downstream signalling networks activated in the two participating cells upon Eph/ephrin ligand binding are distinct, involving either different molecules or the same molecules, but regulated in opposite manners (Jørgensen et al, 2009). Cell repulsion and cell sorting is then achieved by this asymmetric and differential activation of downstream cascades in the two cell populations. Another interesting outcome from this work is the discrepancies observed in the cellular responses when the bidirectional signalling is transformed to unidirectional. Stimulation of EphB2-expressing cells with either soluble ephrinB1 molecules or membrane-bound ephrins that lack their cytoplasmic tail results in a different pattern of downstream signalling compared with the one induced by transmembrane wild-type ephrins. Removal of the cytoplasmic tail on the ephrinBs leads to an impairment of ligand endocytosis (Zimmer et al, 2003) that will thereby increase the concentration of ligands and will influence the balance and activation on the receptor side. This unbalanced situation leads to changes in the cellular behaviour outcome and can for example convert cellular repulsion into adhesion (Zimmer et al, 2003). Therefore, bidirectional signalling is not a cell-autonomous process—the functional bridge built by the interaction of receptors in trans regulates cellular responses depending on the molecular status of the co-signalling cell.
An interesting aspect concerning the spatial organization of receptor complexes involved in bidirectional signalling is the discrimination between trans- and cis-receptor associations and signalling. Both Ephs and ephrins have their own downstream pathways that can often induce complete opposite responses. For example, in neuronal axon targeting, EphA forward signalling results in growth-cone collapse and cell repulsion, whereas ephrinA signalling promotes axonal growth and attraction (Egea and Klein, 2007). An intriguing complication on how bidirectional signalling is regulated raises from the fact that both molecules are co-expressed on the growth cones of developing neurons. The work of Marquardt et al (2005) provided useful insight into this issue showing that both molecules manage to keep their signalling activities separate by segregating in distinct membrane microdomains thereby preventing their cis-association. The disruption of the membrane organization of co-expressed EphAs and ephrinAs using chimeric proteins results in massive cis-associations among receptors and ligands and a complete disturbance of the signalling patterns exhibited by the endogenous proteins. In another example of bidirectional signalling, the signalling outcome of Semaphorin3A can lead to cell attraction or repulsion depending on whether its interaction with the neuronal adhesion molecule L1-CAM and Neuropilin 1 occurs in cis- or trans-configuration (Castellani et al, 2002). Further complemented by additional studies that suggest the necessity of cis-interactions between EphA3 and ephrinA5 in regulating the developmental targeting of retinal axons (Hornberger et al, 1999; Carvalho et al, 2006), not only the absolute spatial distribution of receptor complexes, but also its relative positioning to other signalling clusters seem to be a necessary mechanism ensuring specificity in bidirectional signalling.
Another signalling pathway requiring direct cell–cell contact is the Notch signalling cascade. This evolutionary highly conserved pathway functions with an impressive degree of spatial and temporal specificity and it is of fundamental importance in a range of developmental processes, promoting cell differentiation and embryonic patterning. The members of the Notch signalling pathway are transmembrane proteins and consist of the Notch receptors and the members of the DSL-family Delta and Serrate/Jagged that act as ligands. In brief, the canonical signalling involves binding of Notch to its ligand, cleavage of Notch and the release of the Notch intracellular domain (NICD), in the cytosol. NICD is then translocated to the nucleus and regulates transcription of various developmental genes including these of Notch itself and its ligands, determining this way the fate of the activated cells (Bray, 2006). As with the previous examples, interactions between Notch and Delta ligands can occur both in cis and in trans leading to differential cellular outcomes. The Delta ligands have been shown to activate Notch when the proteins are located in opposing cells, but can inhibit its signalling when they interact in cis (Jacobsen et al, 1998; Miller et al, 2009). A recent study using advanced microscopy and modelling techniques shows that the coordinated action of cis- and trans-signalling might actually regulate the cellular fate determination mediated by Notch during development. Notch function specializes in amplifying small molecular differences between cells to promote differentiation. Sprinzak et al (2010) show that the strength of the Notch response to trans-Delta (activation) depends on the amount of presented ligand, but the response to cis-Delta (inhibition) occurs at a certain concentration threshold and does not depend on trans-Delta. They, therefore, suggest that on the multicellular level, this mechanism can be applied to read-out and subsequently amplify pre-existing, intercellular molecular differences and promote differential development of the interacting cells by creating boundaries and establishing lateral inhibition patterns.
Conclusions and future perspectives
Our understanding on signalling transduction mechanisms has been accelerated in the last decade. We have now a fairly broad knowledge about mechanisms regulating ligand–receptor interactions at the molecular and structural levels as well as the signalling cascades activated downstream of specific receptors. Studying the assembly and function of receptor complexes will advance significantly our understanding on receptor-mediated signalling and it is our next challenge to try to elucidate this extra level of complexity in receptor-mediated signal transduction. Which are the mechanisms that cluster or segregate receptors? What modes of receptor–receptor or receptor–lipid associations are conserved and important for receptor function? How can the activation of a single receptor be translated in different cellular responses depending on a differential organization and activation in space and time? Or vice versa, how can the cell coordinate the cross-talk among different receptors to achieve one single cellular response?
The study of this higher level of complexity achieved by the cross-talk of receptors or receptor–lipid interactions is of particular importance during the development of therapeutical strategies in disease. From this perspective, it is interesting to note that lipid rafts are more abundant in cancer cells than in normal cells, because of a generally elevated level of saturated fatty acids and cholesterol in these cells (Siddiqui et al, 2007). The increased level of raft lipids has been proposed to alter the lipid raft structure and consequently its protein composition (Rakheja et al, 2005). As major signalling proteins in large part are regulated by lipid rafts, one can easily imagine that such changes will have drastic effects on the cell. Indeed, it has been suggested that this may enhance cancer cell survival by promoting growth, escaping immune surveillance or preventing apoptosis (Rakheja et al, 2005). How to translate this knowledge into new therapeutics is still unresolved. It has been shown that cholesterol depletion of cancer cells renders them sensitive to apoptosis (Li et al, 2006). However, such an approach would be unspecific, and, therefore, limits its application in therapy. An alternative and promising strategy is to alter the lipid raft structure by controlling the uptake of dietary lipids (Siddiqui et al, 2007).
Other diseases such as obesity and insulin resistance also involve disordered lipid dynamics and membrane microdomains (Frühbeck et al, 2007). As briefly mentioned above, caveolea constitute a subgroup of lipid rafts, characterized by the presence of caveolin. The caveolin protein family is composed of three isoforms, caveolin-1, -2 and -3, with unique tissue distribution. Caveolin-1 knockout mice are lean and show insulin resistance and resistance to diet-induced obesity (Razani et al, 2002; Cohen et al, 2003). On the contrary, caveolin-3 knockout mice show increased body weight despite normal food intake, as well as insulin resistance (Capozza et al, 2005). These phenotypes may be relevant for human beings, as the human caveolin-1 gene is located in a chromosomal region associated with an obesity-related phenotype, and two mutations in the human insulin receptor associated with severe insulin resistance are located within the established caveolin-1-binding motif (Pérusse et al, 2005). Furthermore, a mutant mouse model unable to synthesize the GM3 ganglioside shows increased insulin sensitivity and is protected from fatty diet-induced insulin resistance (Yamashita et al, 2003). On the basis of this knowledge, novel therapeutic strategies are being proposed (Inokuchi, 2010).
Dysfunction in signal transduction pathways is often the main cause of diseases and cancer. The current therapeutic approaches target individual receptors and try to inhibit its ligand-mediated activation or the function of its downstream effectors. Such approaches seem ineffective when one considers the co-operative mode of action that most of the receptors exhibit in the cell. The signalling network built by clustering of multiple receptors complicates the manipulation of individual signalling pathways, as disrupting the signalling of one receptor type might trigger unpredictable reactions from other co-functioning signalling pathways leading, for example, to hyperactivation of redundant mechanisms that will result in a bypass of the particular targeted signalling pathway. Interfering with receptor clustering, either by preventing receptor–receptor associations or disrupting membrane lipid organization, might be an intelligent novel direction in receptor targeting.
Acknowledgments
We apologize to all scientists whose important contribution was not referenced in this review because of space limitations. Research in the ID laboratory is supported by the Deutsche Forschungsgemeinschaft and the Cluster of Excellence ‘Macromolecular Complexes' of the Goethe University Frankfurt (EXC115). Research in AA-P is supported by grants from the German Research Foundation (SPP1190, SFB 834 and AC180/2-2), the Cluster of Excellence ‘Macromolecular Complexes (CEF)' (EXC 115) at the University Frankfurt and programs LOEWE-OSF and LOEWE-NeFF from the Hessian government. IB is supported by the Cluster of ‘Cardio-Pulmonary System (ECCPS)' (EXC 147) at the Universities of Giessen and Frankfurt. SSS is supported by an EMBO long-term fellowship.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abella JV, Parachoniak CA, Sangwan V, Park M (2010) Dorsal ruffle microdomains potentiate Met RTK signalling and downregulation. J Biol Chem (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves ID, Salamon Z, Hruby VJ, Tollin G (2005) Ligand modulation of lateral segregation of a G-protein-coupled receptor into lipid microdomains in sphingomyelin/phosphatidylcholine solid-supported bilayers. Biochemistry 44: 9168–9178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames P, Studdert CA, Reiser RH, Parkinson JS (2002) Collaborative signaling by mixed chemoreceptor teams in Escherichia coli. Proc Natl Acad Sci USA 99: 7060–7065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti N, Liang H, Xu Y, Zhang Y, Yonekubo Y, Inder K, Du G, Parton RG, Hancock JF, Plowman SJ (2010) EGFR activation remodels the plasma membrane lipid environment to induce nanocluster formation. Mol Cell Biol 30: 3795–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout MA, Mahalingam B, Xiong JP (2005) Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol 21: 381–410 [DOI] [PubMed] [Google Scholar]
- Brantley-Sieders DM, Zhuang G, Hicks D, Fang WB, Hwang Y, Cates JMM, Coffman K, Jackson D, Bruckheimer E, Muraoka-Cook RS, Chen J (2008) The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest 118: 64–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689 [DOI] [PubMed] [Google Scholar]
- Brückner K, Labrador JP, Scheiffele P, Herb A, Seeburg PH, Klein R (1999) EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron 22: 511–524 [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK (1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68: 533–544 [DOI] [PubMed] [Google Scholar]
- Capozza F, Combs TP, Cohen AW, Cho YR, Park SY, Schubert W, Williams TM, Brasaemle DL, Jelicks LA, Scherer PE, Kim JK, Lisanti MP (2005) Caveolin-3 knockout mice show increased adiposity and whole body insulin resistance, with ligand-induced insulin receptor instability in skeletal muscle. Am J Physiol Cell Physiol 288: C1317–C1331 [DOI] [PubMed] [Google Scholar]
- Carvalho RF, Beutler M, Marler KJM, Knöll B, Becker-Barroso E, Heintzmann R, Ng T, Drescher U (2006) Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci 9: 322–330 [DOI] [PubMed] [Google Scholar]
- Castellani V, Angelis ED, Kenwrick S, Rougon G (2002) Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J 21: 6348–6357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani V, Chédotal A, Schachner M, Faivre-Sarrailh C, Rougon G (2000) Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron 27: 237–249 [DOI] [PubMed] [Google Scholar]
- Cemerski S, Das J, Giurisato E, Markiewicz MA, Allen PM, Chakraborty AK, Shaw AS (2008) The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity 29: 414–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet S, Cohen S, Yoshida Y, Fekrane L, Livet J, Gayet O, Segu L, Buhot MC, Jessell TM, Henderson CE, Mann F (2007) Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron 56: 807–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Moy VT (2000) Cross-linking of cell surface receptors enhances cooperativity of molecular adhesion. Biophys J 78: 2814–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherukuri A, Dykstra M, Pierce SK (2001) Floating the raft hypothesis: lipid rafts play a role in immune cell activation. Immunity 14: 657–660 [DOI] [PubMed] [Google Scholar]
- Chini B, Parenti M (2004) G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J Mol Endocrinol 32: 325–338 [DOI] [PubMed] [Google Scholar]
- Chong LD, Park EK, Latimer E, Friesel R, Daar IO (2000) Fibroblast growth factor receptor-mediated rescue of x-ephrin B1-induced cell dissociation in Xenopus embryos. Mol Cell Biol 20: 724–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AW, Razani B, Wang XB, Combs TP, Williams TM, Scherer PE, Lisanti MP (2003) Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol 285: C222–C235 [DOI] [PubMed] [Google Scholar]
- Conrotto P, Valdembri D, Corso S, Serini G, Tamagnone L, Comoglio PM, Bussolino F, Giordano S (2005) Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood 105: 4321–4329 [DOI] [PubMed] [Google Scholar]
- Contractor A, Rogers C, Maron C, Henkemeyer M, Swanson GT, Heinemann SF (2002) Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science 296: 1864–1869 [DOI] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME (2000) EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell 103: 945–956 [DOI] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD (1994) Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science 266: 816–819 [DOI] [PubMed] [Google Scholar]
- Deribe YL, Pawson T, Dikic I (2010) Post-translational modifications in signal integration. Nat Struct Mol Biol 17: 666–672 [DOI] [PubMed] [Google Scholar]
- Deribe YL, Wild P, Chandrashaker A, Curak J, Schmidt MHH, Kalaidzidis Y, Milutinovic N, Kratchmarova I, Buerkle L, Fetchko MJ, Schmidt P, Kittanakom S, Brown KR, Jurisica I, Blagoev B, Zerial M, Stagljar I, Dikic I (2009) Regulation of epidermal growth factor receptor trafficking by lysine deacetylase HDAC6. Sci Signal 2: ra84. [DOI] [PubMed] [Google Scholar]
- Douglass AD, Vale RD (2005) Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 121: 937–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowrick P, Kenworthy P, McCann B, Warn R (1993) Circular ruffle formation and closure lead to macropinocytosis in hepatocyte growth factor/scatter factor-treated cells. Eur J Cell Biol 61: 44–53 [PubMed] [Google Scholar]
- Egea J, Klein R (2007) Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol 17: 230–238 [DOI] [PubMed] [Google Scholar]
- Egea J, Nissen UV, Dufour A, Sahin M, Greer P, Kullander K, Mrsic-Flogel TD, Greenberg ME, Kiehn O, Vanderhaeghen P, Klein R (2005) Regulation of EphA 4 kinase activity is required for a subset of axon guidance decisions suggesting a key role for receptor clustering in Eph function. Neuron 47: 515–528 [DOI] [PubMed] [Google Scholar]
- Eggeling C, Ringemann C, Medda R, Schwarzmann G, Sandhoff K, Polyakova S, Belov VN, Hein B, von Middendorff C, Schönle A, Hell SW (2009) Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457: 1159–1162 [DOI] [PubMed] [Google Scholar]
- Essmann CL, Martinez E, Geiger JC, Zimmer M, Traut MH, Stein V, Klein R, Acker-Palmer A (2008) Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat Neurosci 11: 1035–1043 [DOI] [PubMed] [Google Scholar]
- Falk J, Julien F, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Püschel AW, Sanes JR, Castellani V (2005) Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron 48: 63–75 [DOI] [PubMed] [Google Scholar]
- Fantini J, Barrantes FJ (2009) Sphingolipid/cholesterol regulation of neurotransmitter receptor conformation and function. Biochim Biophys Acta 1788: 2345–2361 [DOI] [PubMed] [Google Scholar]
- Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristán C, Victora GD, Zanin-Zhorov A, Dustin ML (2010) Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol 28: 79–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster LJ, Hoog CLD, Mann M (2003) Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA 100: 5813–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühbeck G, López M, Diéguez C (2007) Role of caveolins in body weight and insulin resistance regulation. Trends Endocrinol Metab 18: 177–182 [DOI] [PubMed] [Google Scholar]
- Fukai J, Yokote H, Yamanaka R, Arao T, Nishio K, Itakura T (2008) EphA4 promotes cell proliferation and migration through a novel EphA4-FGFR1 signaling pathway in the human glioma U251 cell line. Mol Cancer Ther 7: 2768–2778 [DOI] [PubMed] [Google Scholar]
- Gallant ND, García AJ (2007) Quantitative analyses of cell adhesion strength. Methods Mol Biol 370: 83–96 [DOI] [PubMed] [Google Scholar]
- Gestwicki JE, Kiessling LL (2002) Inter-receptor communication through arrays of bacterial chemoreceptors. Nature 415: 81–84 [DOI] [PubMed] [Google Scholar]
- Gimpl G, Burger K, Politowska E, Ciarkowski J, Fahrenholz F (2000) Oxytocin receptors and cholesterol: interaction and regulation. Exp Physiol 85 (Spec No): 41S–49S [DOI] [PubMed] [Google Scholar]
- Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM (2002) The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol 4: 720–724 [DOI] [PubMed] [Google Scholar]
- Goswami D, Gowrishankar K, Bilgrami S, Ghosh S, Raghupathy R, Chadda R, Vishwakarma R, Rao M, Mayor S (2008) Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell 135: 1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves JT (2006) Spatial mutation of the T cell immunological synapse. Curr Opin Chem Biol 10: 544–550 [DOI] [PubMed] [Google Scholar]
- Gutiérrez J, Brandan E (2010) A novel mechanism of sequestering fibroblast growth factor 2 by glypican in lipid rafts, allowing skeletal muscle differentiation. Mol Cell Biol 30: 1634–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF (2006) Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol 7: 456–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MJ, Dallal GE, Flanagan JG (2004) Retinal axon response to ephrin-as shows a graded, concentration-dependent transition from growth promotion to inhibition. Neuron 42: 717–730 [DOI] [PubMed] [Google Scholar]
- Hao M, Mukherjee S, Maxfield FR (2001) Cholesterol depletion induces large scale domain segregation in living cell membranes. Proc Natl Acad Sci USA 98: 13072–13077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hato T, Pampori N, Shattil SJ (1998) Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin alphaIIb beta3. J Cell Biol 141: 1685–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JT, Georgiou J, Jia Z, Robertson J, Elowe S, Roder JC, Pawson T (2001) The receptor tyrosine kinase EphB2 regulates NMDA-dependent synaptic function. Neuron 32: 1041–1056 [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Itkis OS, Ngo M, Hickmott PW, Ethell IM (2003) Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. J Cell Biol 163: 1313–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen JP, Nikolov DB (2003) Eph receptors and ephrins. Int J Biochem Cell Biol 35: 130–134 [DOI] [PubMed] [Google Scholar]
- Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, Rajashankar KR, Mensinga A, Lackmann M, Nikolov DB, Dhe-Paganon S (2010) Architecture of Eph receptor clusters. Proc Natl Acad Sci USA 107: 10860–10865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger MR, Dütting D, Ciossek T, Yamada T, Handwerker C, Lang S, Weth F, Huf J, Wessel R, Logan C, Tanaka H, Drescher U (1999) Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron 22: 731–742 [DOI] [PubMed] [Google Scholar]
- Huynh-Do U, Stein E, Lane AA, Liu H, Cerretti DP, Daniel TO (1999) Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through alphavbeta3 and alpha5beta1 integrins. EMBO J 18: 2165–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi J (2010) Membrane microdomains and insulin resistance. FEBS Lett 584: 1864–1871 [DOI] [PubMed] [Google Scholar]
- Jacobsen TL, Brennan K, Arias AM, Muskavitch MA (1998) Cis-interactions between Delta and Notch modulate neurogenic signalling in Drosophila. Development 125: 4531–4540 [DOI] [PubMed] [Google Scholar]
- Jain MK, White HB (1977) Long-range order in biomembranes. Adv Lipid Res 15: 1–60 [DOI] [PubMed] [Google Scholar]
- Jensen M, Mouritsen OG (2004) Lipids do influence protein function-the hydrophobic matching hypothesis revisited. Biochim Biophys Acta 1666: 205–226 [DOI] [PubMed] [Google Scholar]
- Jørgensen C, Sherman A, Chen GI, Pasculescu A, Poliakov A, Hsiung M, Larsen B, Wilkinson DG, Linding R, Pawson T (2009) Cell-specific information processing in segregating populations of Eph receptor ephrin-expressing cells. Science 326: 1502–1509 [DOI] [PubMed] [Google Scholar]
- Kayser MS, McClelland AC, Hughes EG, Dalva MB (2006) Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci 26: 12152–12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner D, Sourjik V (2006) Spatial organization of the bacterial chemotaxis system. Curr Opin Microbiol 9: 619–624 [DOI] [PubMed] [Google Scholar]
- Knöll B, Drescher U (2004) Src family kinases are involved in EphA receptor-mediated retinal axon guidance. J Neurosci 24: 6248–6257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackmann M, Oates AC, Dottori M, Smith FM, Do C, Power M, Kravets L, Boyd AW (1998) Distinct subdomains of the EphA3 receptor mediate ligand binding and receptor dimerization. J Biol Chem 273: 20228–20237 [DOI] [PubMed] [Google Scholar]
- Lajoie P, Nabi IR (2007) Regulation of raft-dependent endocytosis. J Cell Mol Med 11: 644–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen AB, Pedersen MW, Stockhausen MT, Grandal MV, van Deurs B, Poulsen HS (2007) Activation of the EGFR gene target EphA2 inhibits epidermal growth factor-induced cancer cell motility. Mol Cancer Res 5: 283–293 [DOI] [PubMed] [Google Scholar]
- Larson DR, Gosse JA, Holowka DA, Baird BA, Webb WW (2005) Temporally resolved interactions between antigen-stimulated IgE receptors and Lyn kinase on living cells. J Cell Biol 171: 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Mood K, Battu G, Ji YJ, Singh A, Daar IO (2009) Fibroblast growth factor receptor-induced phosphorylation of ephrinB1 modulates its interaction with Dishevelled. Mol Biol Cell 20: 124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, Kanagawa O, Markiewicz M, Allen PM, Dustin ML, Chakraborty AK, Shaw AS (2003) The immunological synapse balances T cell receptor signaling and degradation. Science 302: 1218–1222 [DOI] [PubMed] [Google Scholar]
- Lenne PF, Wawrezinieck L, Conchonaud F, Wurtz O, Boned A, Guo XJ, Rigneault H, He HT, Marguet D (2006) Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. EMBO J 25: 3245–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Hazelbauer GL (2004) Cellular stoichiometry of the components of the chemotaxis signaling complex. J Bacteriol 186: 3687–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Bennett JS, Degrado WF (2004) Structural basis for integrin alphaIIbbeta3 clustering. Biochem Soc Trans 32: 412–415 [DOI] [PubMed] [Google Scholar]
- Li R, Mitra N, Gratkowski H, Vilaire G, Litvinov R, Nagasami C, Weisel JW, Lear JD, DeGrado WF, Bennett JS (2003) Activation of integrin alphaIIbbeta3 by modulation of transmembrane helix associations. Science 300: 795–798 [DOI] [PubMed] [Google Scholar]
- Li YC, Park MJ, Ye SK, Kim CW, Kim YN (2006) Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol 168: 1107–1118; quiz 1404–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K (2010) Lipid rafts as a membrane-organizing principle. Science 327: 46–50 [DOI] [PubMed] [Google Scholar]
- Manz BN, Groves JT (2010) Spatial organization and signal transduction at intercellular junctions. Nat Rev Mol Cell Biol 11: 342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Shirasaki R, Ghosh S, Andrews SE, Carter N, Hunter T, Pfaff SL (2005) Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell 121: 127–139 [DOI] [PubMed] [Google Scholar]
- Mayor S, Rothberg KG, Maxfield FR (1994) Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science 264: 1948–1951 [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Hindges R, Yates PA, O'Leary DDM (2003) Bifunctional action of ephrin-B1 as a repellent and attractant to control bidirectional branch extension in dorsal-ventral retinotopic mapping. Development 130: 2407–2418 [DOI] [PubMed] [Google Scholar]
- Mellström K, Heldin CH, Westermark B (1988) Induction of circular membrane ruffling on human fibroblasts by platelet-derived growth factor. Exp Cell Res 177: 347–359 [DOI] [PubMed] [Google Scholar]
- Miljan EA, Meuillet EJ, Mania-Farnell B, George D, Yamamoto H, Simon HG, Bremer EG (2002) Interaction of the extracellular domain of the epidermal growth factor receptor with gangliosides. J Biol Chem 277: 10108–10113 [DOI] [PubMed] [Google Scholar]
- Miller AC, Lyons EL, Herman TG (2009) cis-Inhibition of Notch by endogenous Delta biases the outcome of lateral inhibition. Curr Biol 19: 1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo C, Gill GN, Anderson RG (1999) Regulated migration of epidermal growth factor receptor from caveolae. J Biol Chem 274: 30636–30643 [DOI] [PubMed] [Google Scholar]
- Mossman KD, Campi G, Groves JT, Dustin ML (2005) Altered TCR signaling from geometrically repatterned immunological synapses. Science 310: 1191–1193 [DOI] [PubMed] [Google Scholar]
- Munro S (2003) Lipid rafts: elusive or illusive? Cell 115: 377–388 [DOI] [PubMed] [Google Scholar]
- Noren NK, Pasquale EB (2004) Eph receptor-ephrin bidirectional signals that target Ras and Rho proteins. Cell Signal 16: 655–666 [DOI] [PubMed] [Google Scholar]
- Orth JD, Krueger EW, Weller SG, McNiven MA (2006) A novel endocytic mechanism of epidermal growth factor receptor sequestration and internalization. Cancer Res 66: 3603–3610 [DOI] [PubMed] [Google Scholar]
- Palmer A, Zimmer M, Erdmann KS, Eulenburg V, Porthin A, Heumann R, Deutsch U, Klein R (2002) EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase. Mol Cell 9: 725–737 [DOI] [PubMed] [Google Scholar]
- Pasquale EB (2005) Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol 6: 462–475 [DOI] [PubMed] [Google Scholar]
- Pasquale EB (2008) Eph-ephrin bidirectional signaling in physiology and disease. Cell 133: 38–52 [DOI] [PubMed] [Google Scholar]
- Pasquale EB (2010) Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer 10: 165–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérusse L, Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Snyder EE, Bouchard C (2005) The human obesity gene map: the 2004 update. Obes Res 13: 381–490 [DOI] [PubMed] [Google Scholar]
- Poliakov A, Cotrina ML, Pasini A, Wilkinson DG (2008) Regulation of EphB2 activation and cell repulsion by feedback control of the MAPK pathway. J Cell Biol 183: 933–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakheja D, Kapur P, Hoang MP, Roy LC, Bennett MJ (2005) Increased ratio of saturated to unsaturated C18 fatty acids in colonic adenocarcinoma: implications for cryotherapy and lipid raft function. Med Hypotheses 65: 1120–1123 [DOI] [PubMed] [Google Scholar]
- Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H, Christ GJ, Edelmann W, Lisanti MP (2002) Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol 22: 2329–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepstorff K, Thomsen P, Sandvig K, van Deurs B (2002) Sequestration of epidermal growth factor receptors in non-caveolar lipid rafts inhibits ligand binding. J Biol Chem 277: 18954–18960 [DOI] [PubMed] [Google Scholar]
- Salaita K, Nair PM, Petit RS, Neve RM, Das D, Gray JW, Groves JT (2010) Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science 327: 1380–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A (2010) Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature 465: 487–491 [DOI] [PubMed] [Google Scholar]
- Scott GA, McClelland LA, Fricke AF (2008) Semaphorin 7a promotes spreading and dendricity in human melanocytes through beta1-integrins. J Invest Dermatol 128: 151–161 [DOI] [PubMed] [Google Scholar]
- Seet BT, Dikic I, Zhou MM, Pawson T (2006) Reading protein modifications with interaction domains. Nat Rev Mol Cell Biol 7: 473–483 [DOI] [PubMed] [Google Scholar]
- Seiradake E, Harlos K, Sutton G, Aricescu AR, Jones EY (2010) An extracellular steric seeding mechanism for Eph-ephrin signaling platform assembly. Nat Struct Mol Biol 17: 398–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Thomas VK, Dustin ML, Kam LC (2008) Micropatterning of costimulatory ligands enhances CD4+ T cell function. Proc Natl Acad Sci USA 105: 7791–7796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Terada Y, Iwamatsu A, Shinohara A, Mochizuki N, Higuchi M, Gotoh Y, Ihara S, Nagata S, Itoh H, Fukui Y, Jessberger R (2002) SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature 416: 759–763 [DOI] [PubMed] [Google Scholar]
- Siddiqui RA, Harvey KA, Zaloga GP, Stillwell W (2007) Modulation of lipid rafts by Omega-3 fatty acids in inflammation and cancer: implications for use of lipids during nutrition support. Nutr Clin Pract 22: 74–88 [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387: 569–572 [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39 [DOI] [PubMed] [Google Scholar]
- Sourjik V (2004) Receptor clustering and signal processing in E. coli chemotaxis. Trends Microbiol 12: 569–576 [DOI] [PubMed] [Google Scholar]
- Sourjik V, Berg HC (2004) Functional interactions between receptors in bacterial chemotaxis. Nature 428: 437–441 [DOI] [PubMed] [Google Scholar]
- Sprinzak D, Lakhanpal A, Lebon L, Santat LA, Fontes ME, Anderson GA, Garcia-Ojalvo J, Elowitz MB (2010) Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature 465: 86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein E, Lane AA, Cerretti DP, Schoecklmann HO, Schroff AD, Etten RLV, Daniel TO (1998) Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev 12: 667–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studdert CA, Parkinson JS (2004) Crosslinking snapshots of bacterial chemoreceptor squads. Proc Natl Acad Sci USA 101: 2117–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki KGN, Fujiwara TK, Sanematsu F, Iino R, Edidin M, Kusumi A (2007) GPI-anchored receptor clusters transiently recruit Lyn and G alpha for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J Cell Biol 177: 717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Offermanns S (2004) Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J Cell Biol 165: 869–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiercz JM, Worzfeld T, Offermanns S (2008) ErbB-2 and met reciprocally regulate cellular signaling via plexin-B1. J Biol Chem 283: 1893–1901 [DOI] [PubMed] [Google Scholar]
- Takasu MA, Dalva MB, Zigmond RE, Greenberg ME (2002) Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science 295: 491–495 [DOI] [PubMed] [Google Scholar]
- Taubenberger A, Cisneros DA, Friedrichs J, Puech PH, Muller DJ, Franz CM (2007) Revealing early steps of alpha2beta1 integrin-mediated adhesion to collagen type I by using single-cell force spectroscopy. Mol Biol Cell 18: 1634–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, Kikutani H (2004) Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev 18: 435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G (2005) Cellular lipidomics. EMBO J 24: 3159–3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaught D, Chen J, Brantley-Sieders DM (2009) Regulation of mammary gland branching morphogenesis by EphA2 receptor tyrosine kinase. Mol Biol Cell 20: 2572–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer T, Galibert L, Comeau MR, Smedt TD (2005) Plexin C1 engagement on mouse dendritic cells by viral semaphorin A39R induces actin cytoskeleton rearrangement and inhibits integrin-mediated adhesion and chemokine-induced migration. J Immunol 174: 51–59 [DOI] [PubMed] [Google Scholar]
- Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U, Barberis A, Benjamin LE, Mäkinen T, Nobes CD, Adams RH (2010) Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465: 483–486 [DOI] [PubMed] [Google Scholar]
- Wimmer-Kleikamp SH, Janes PW, Squire A, Bastiaens PIH, Lackmann M (2004) Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J Cell Biol 164: 661–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, Kono M, Tsuji S, Daniotti JL, Werth N, Sandhoff R, Sandhoff K, Proia RL (2003) Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci USA 100: 3445–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetukuri L, Ekroos K, Vidal-Puig A, Oresic M (2008) Informatics and computational strategies for the study of lipids. Mol Biosyst 4: 121–127 [DOI] [PubMed] [Google Scholar]
- Yokote H, Fujita K, Jing X, Sawada T, Liang S, Yao L, Yan X, Zhang Y, Schlessinger J, Sakaguchi K (2005) Trans-activation of EphA4 and FGF receptors mediated by direct interactions between their cytoplasmic domains. Proc Natl Acad Sci USA 102: 18866–18871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Gunput RAF, Pasterkamp RJ (2008) Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci 33: 161–170 [DOI] [PubMed] [Google Scholar]
- Zimmer M, Palmer A, Köhler J, Klein R (2003) EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol 5: 869–878 [DOI] [PubMed] [Google Scholar]