An intimate liaison: spatial organization of the endoplasmic reticulum–mitochondria relationship
This review from the Scorrano lab describes how regions of close physical apposition of the ER and mitochondrial membranes result in cross-talk between the two organelles and have a role in determining cellular decisions.
Keywords: apoptosis, Ca2+ signalling, ER-mitochondria juxtaposition, lipid biosynthesis, mitofusin-2
Abstract
Organelle localization is often crucial to properly modulate cellular functions and signalling cascades. For example, the distribution of organelles in axons is crucial for their function and is dysregulated in several diseases. Similarly, relative positioning of two or more organelles is also important to perform certain specialized processes. Perhaps, the best-known form of interorganellar organization is that between endoplasmic reticulum (ER) and mitochondria. Close communication between these two compartments has been observed for a long time. Recent evidence suggests that this is the basis for a bidirectional communication regulating a number of physiological processes ranging from mitochondrial energy and lipid metabolism to Ca2+ signalling and cell death. The recent discovery of some of the molecular mediators of the tethering already allowed to extend the function of this paradigmatic spatial organization to previously unexpected functions, and will foster future research to explore it in cellular signalling cascades as well as in disease.
Introduction
Compartmentalization is one of the key features of cellular signalling. The requirement for spatial and temporal limitation of amplifying second messengers stems from their pleiotropic nature. One single messenger, like Ca2+, can control cell proliferation or death: the outcome is determined by the strength, the localization, the duration and the pattern of the signal. Organelles are key participants in the amplification of signalling cascades, either because they regulate the production or the release of crucial second messengers, or because they are strategically located in the cytoplasm at the sites of signal propagation. One particular aspect that is gaining further consideration is the impact of relative organelle positioning on organellar and cellular function (Figures 1, 2, 3).
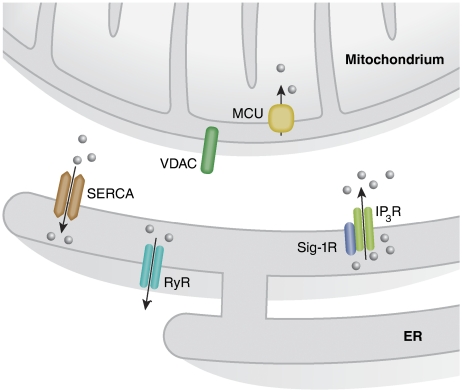
Figure 1.
Local Ca2+ signalling at the ER–mitochondria interface. The proteins involved in Ca2+ signalling between ER and mitochondria are shown. Ca2+ release from the ER occurs mainly through IP3R and RyR enriched at the regions of the ER in close contact with mitochondria. These receptors account for the formation of microdomains of high Ca2+ concentration that are needed to activate the transport of the ion into the mitochondrial matrix through the MCU. Finally, the recently identified Sig-1R is able to modulate the activity of the IP3R and thus Ca2+ transmission from the ER to mitochondria.
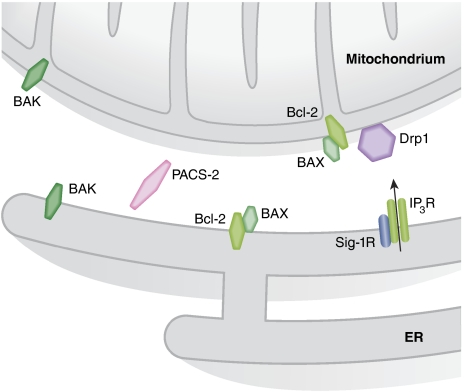
Figure 2.
Apoptotic signalling at the ER–mitochondria interface. Cross-talk between ER and mitochondria has a major function in the decision whether the cell should live or die. Bcl-2 family members can control apoptosis by controlling indirectly the amount of ER-releasable Ca2+ that can reach mitochondria. Drp1 has a dual function in controlling apoptosis. On one side, recruitment of Drp1 to mitochondria upon sustained Ca2+ release from the ER can protect from cell death by fragmenting the mitochondrial network and impeding the propagation of the fatal Ca2+ wave. On the other hand, mobilization of Drp1 to mitochondria can also trigger mitochondrial cristae remodelling, facilitating cytochrome c mobilization and subsequent apoptosis. Also, the recently identified Sig-1R has a bivalent function in apoptosis. Sig-1R promotes Ca2+ transmission to mitochondria through the IP3R thus maintaining mitochondrial metabolism in conditions of ER Ca2+ depletion. However, excessive Ca2+ transfer to mitochondria risks to expose this organelle to Ca2+ overload and subsequent dysfunction. In this regard, the truncated version of SERCA-1 S1T, expressed upon ER stress, promotes Ca2+ transfer to mitochondria leading to Ca2+ overload.
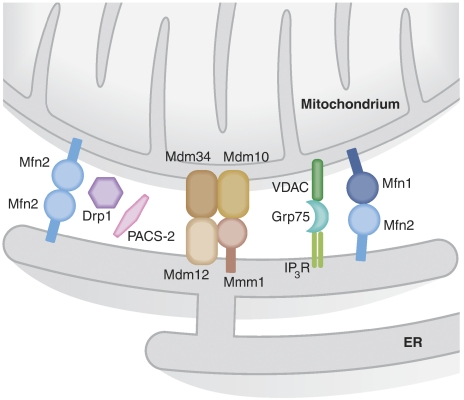
Figure 3.
Tethers between ER and mitochondria. The molecular bridges that regulate the close contacts between ER and mitochondria are shown. PACS-2 and Drp1 indirectly controls the distance between the two organelles by impinging on mitochondrial morphology and distribution. A more direct function in linking ER and mitochondria has been suggested for the complex composed by the IP3R on the ER, the cytosolic chaperone Grp75 and the mitochondrial anion channel VDAC. Further, ERMES, a multimeric complex formed by the mitochondrial proteins Mdm34 and Mdm10 and Mmm1 and Mdm12 on the ER appears to regulate ER–mitochondria tethering in yeast. Importantly, the dynamin-related GTPase Mfn2 on the ER forms homo–heterodimers with Mfn1 or Mfn2 on mitochondria to keep the tight contacts between the two organelles.
Like localization, relative position of organelles in the cytoplasm is not random. Often their reciprocal position is precisely organized in order to allow the exchange of different components: this is the case of the endoplasmic reticulum (ER)-Golgi and Golgi stack relationships or of the nucleus-vacuole tethering in yeast. This organization has precise consequences on integrated signalling cascades, as well as on the function of the individual organelles. The interorganellar organization, which is perhaps best characterized from a functional point of view, is that between ER and mitochondria: we have already learned a great deal on its function in Ca2+ signalling, cell metabolism and death.
Here, we will review our current understanding of the molecular basis and of the functional consequence of the specialized relative spatial organization of mitochondria and ER in cellular signalling cascades. Before going into the details of the functional consequences of mitochondria–ER connection, we should consider that the relative juxtaposition of these two very dynamic organelles is likely to be influenced by their morphology. We will therefore briefly describe the key-known regulators of mitochondrial and ER shape, for some of which a function in ER–mitochondria tethering has been demonstrated.
Mitochondrial shape
Early studies by George Palade and Fritjof Sjostrand revealed that mitochondria possess two membranes, an outer mitochondrial membrane (OMM) and a highly convoluted inner membrane (IMM), folded into a series of ridges called cristae (Palade, 1952; Sjostrand, 1953). More recent electron tomography (ET) studies extended these earlier observations, showing that cristae are enlarged cisternae or sacs, with narrow, tubular connections to the peripheral surface of the inner boundary membrane. These observations implicate the existence of three compartments in mitochondria, formed by the matrix, the intermembrane space and the interior of the cristae (Perkins et al, 1997; Frey and Mannella, 2000; Scorrano and Korsmeyer, 2003; Frezza et al, 2006).
Mitochondrial shape in living cells is very heterogeneous and can range from small spheres to interconnected tubules (Bereiter-Hahn and Voth, 1994). Mitochondria of rat cardiac muscle and diaphragm skeletal muscle appear as isolated ellipses or tubules in embryonic stages but then reorganize into reticular networks in the adult (Bakeeva et al, 1981). The dynamics of the mitochondrial network is well depicted by the continuous movement of mitochondria. Occasionally, two mitochondrial units encounter each other and eventually fuse (Bereiter-Hahn and Voth, 1994). On the other hand, mitochondrial tubules can undergo fission and give rise to two or more mitochondrial units. It is important to note that mitochondrial fusion and fission are complicated processes, being mitochondria bound by two membranes, thus, any mechanism of fusion and fission has to take into account the coordinate fusion–fission of four lipid bilayers.
The first mediator of mitochondrial fusion identified was the Drosophila melanogaster Fuzzy onions 1 protein (Fzo1p), a large transmembrane guanosine triphosphatase required for the formation of the large mitochondrial derivative during spermatogenesis (Hales and Fuller, 1997). In mammals, two Fzo1p homologues, Mitofusin (Mfn)1 and Mfn2 are widely expressed in many tissues (Rojo et al, 2002; Santel et al, 2003; Eura et al, 2003). Mfn1 and Mfn2 display high (81%) homology, similar topologies and both reside in the OMM (Rojo et al, 2002; Chen et al, 2003; Santel et al, 2003). IMM fusion is mediated by another GTPase, Opa1. During mitochondrial fusion, Mfn1 and Mfn2 are believed to dock two juxtaposed mitochondria through their coiled-coil domains (Koshiba et al, 2004). Mfn1 has a higher GTPase activity than Mfn2, although its affinity for GTP is lower (Ishihara et al, 2004). In agreement with this, Mfn1 exhibits a higher capacity in inducing fusion (Ishihara et al, 2004) and participates in Opa1-mediated mitochondrial fusion, as opposed to Mfn2 (Cipolat et al, 2004).
The two proteins Fis1 and dynamin-related protein 1 (Drp1) are required for mitochondrial fission in mammals. Drp1 exists largely in a cytosolic pool, but a fraction is found at spots on mitochondria at sites of constriction (Labrousse et al, 1999; Smirnova et al, 2001). Fis1, on the other hand, is evenly distributed on the surface of the OMM (James et al, 2003) and is thought to recruit Drp1 on punctuate structures on mitochondria during mitochondrial fission in yeast (Yoon et al, 2003). In mammalian cells, as opposed to yeast, the mechanism of Drp1 translocation to mitochondria seems to be independent of the presence of Fis1 (Lee et al, 2004; Wasiak et al, 2007) and is regulated by post-translational modification, like phosphorylation at several different Serine residues (Taguchi et al, 2007; Cereghetti et al, 2008). Once on mitochondria, Drp1 is then stabilized by sumoylation (Harder et al, 2004; Braschi et al, 2009; Zunino et al, 2009).
Several other players besides these ‘core'-shaping proteins have been shown to participate in the regulation of mitochondrial morphology. Further details can be found in recent and comprehensive reviews (Zorzano et al, 2010).
ER shape
The ER is a continuous membrane-bound compartment present in all eukaryotic cells occupying >10% of the total cell volume (Baumann and Walz, 2001; Voeltz et al, 2002). Despite the continuity of the ER network, morphologically defined compartments can be distinguished: the nuclear envelope and the peripheral ER comprising the ribosome-bound rough ER and the ribosome-free smooth ER (Baumann and Walz, 2001; Voeltz et al, 2002; Goetz and Nabi, 2006).
Although the nuclear envelope separates the cytoplasm from the nuclear lumen in interphase cells (Baumann and Walz, 2001), the peripheral ER is evenly distributed throughout the whole cellular volume. In general, the peripheral ER consists of sheet-like cisternae and a polygonal array of tubules connected by three-way junctions, the thickness of the sheets and the diameter of the tubules varies typically between 60–100 nm (Voeltz and Prinz, 2007), indicating that an active mechanism maintains this shape. In fact, interaction between the ER and microtubule (MT)-binding proteins seems to have a function in shaping the ER in mammalian cells. The interaction through motor proteins could permit the extension along stationary MT. In the case of non-motor proteins, this interaction would mediate the movement of the ER attached to motile or polymerizing MT (Vedrenne and Hauri, 2006), as it has been suggested for cytoskeleton-linking membrane protein 63 kDa (CLIMP63), VAP-B/Nir3 couple and p22 (Klopfenstein et al, 1998; Andrade et al, 2004; Amarilio et al, 2005).
CLIMP63 is an integral ER protein localized exclusively on the peripheral ER. Anchoring of the ER to MT by CLIMP63 seems to be required for maintaining the spatial distribution of the ER network (Klopfenstein et al, 1998). Regarding p22, this myristoylated EF-hand containing protein, binds MT in a Ca2+-dependent manner, providing a link between ER morphology and Ca2+ (Andrade et al, 2004). Moreover, in vitro studies show that branching ER tubules can be created in a system containing solely giant unilamellar lipid vesicles, MT and MT motor proteins (Koster et al, 2003; Leduc et al, 2004). Despite this evidence, the function of MT in regulating ER morphology remains controversial. In fact, ER tubules can be generated in vitro from oocyte-derived light microsomes and this occurs in the absence of MT (Dreier and Rapoport, 2000). This in vitro ER tubulation assay was used to identify two classes of proteins required for the shaping of the ER: the reticulons and DP1/Yop1 (Voeltz et al, 2006). These integral ER proteins are enriched in ER tubules and excluded from sheets and nuclear envelope. It is thought that they adopt a hairpin topology on the cytoplasmic leaflet of the ER membrane, occupying more space in the outer than in the inner leaflet and causing the ER membrane to curve and tubulate, forming the typical ER tubules (Voeltz et al, 2006). More recently, a different class of proteins, the dynamin-related membrane GTPases atlastins, has been shown to also control morphology of the ER by promoting the branching of the tubules (Hu et al, 2009; Orso et al, 2009). On the other hand, Snapp et al (2003) have suggested that ER shape results from the weak interaction between cytosolic domains of ER integral proteins during formation of stacked ER cisternae within cells.
Turning back to the function of Ca2+ in regulating ER morphology, increases in concentration of this ion are known to cause ER fragmentation (Subramanian and Meyer, 1997; Ribeiro et al, 2000; Terasaki et al, 2001). This can occur through the action of the EF-hand containing protein p22 (Andrade et al, 2004), as mentioned above, or through Drp1 (Pitts et al, 1999). However, how the different regulators of ER shape cooperate to generate the branched and tubular network remains an open question.
The ER/mitochondria liaison: an historical overview
The first proposal of the existence of a close contact between the membrane of the ER and mitochondrial outer membrane (OMM) dates back to the 1960s by several independent groups (Ruby et al, 1969). Subcellular fractionation studies identified ER membranes co-purifying with mitochondrial fractions (Lewis and Tata, 1973). Electron microscopy (EM) observations suggested even a continuity between OMM and ER membrane, establishing a direct communication between the cisternal space of the ER and the mitochondrial intermembrane space (Ruby et al, 1969; Franke and Kartenbeck, 1971; Morre et al, 1971). Modern EM techniques reveal that mitochondria are surrounded by tubules of the ER that lie preferentially within 200 nm of distance (Wang et al, 2000), whereas wide-field digital 3D deconvolution microscopy indicates that as much as 20% of the mitochondrial surface is in direct contact with the ER (Rizzuto et al, 1998). Finally, ET and high-resolution three-dimensional ET shows the existence of physical linkers between the two organelles. The size of these bridges appears to vary between 10 and 25 nm in length (Perkins et al, 1997; Marsh et al, 2001; Csordas et al, 2006). The specific ER region that interacts with mitochondria has been christened mitochondria-associated membrane (MAM) by Jean Vance when she identified for the first time an important function for the intimate relationship between the two compartments in the exchange of phospholipids (Vance, 1990). The functional importance of the contact sites is further substantiated by a ‘quasi-synaptic' mechanism transmission of Ca2+ between the two organelles (Rizzuto et al, 1993, 1998; Csordas et al, 1999) and by the crucial function of this process during apoptosis (Szalai et al, 1999; Scorrano et al, 2003).
How ER and mitochondria collaborate to produce lipids
The physiological function of the close apposition between ER and mitochondria started to become evident when its function in phospholipid synthesis was discovered. Lipids are poorly soluble in water and most of them are predicted not to move efficiently across the hydrophilic cytosol by diffusion (Daum and Vance, 1997). Thus, a mechanism that exchanges phospholipids between membranes of different organelles must exist. For many years, this was thought to be performed by phospholipid exchange proteins (Voelker, 2005). Alternatively, interorganellar lipid transfer could be mediated by vesicles. In the latter model, a vesicle buds from the donor membrane, crosses the cytosol and fuses with the acceptor membrane where it delivers the lipid cargo. However, a more recent hypothesis indicates that the most likely mechanism of interorganellar lipid transfer involves the direct contact between the donor and the acceptor membrane eliminating the need of the energetically unfavourable transfer of lipids through the hydrophilic cytosol (Voelker, 2003). In agreement with his view, phosphatidylserine (PtdSer) synthase-1 and -2, the enzymes involved in the synthesis of PtdSer, and PtdSer are enriched in MAMs as opposed to the bulk ER. On the other hand, PtdSer decarboxylase, the enzyme that catalyses the conversion of PtdSer to phosphatidylethanolamine (PtdEtn), is associated to the external leaflet of the inner mitochondrial membrane. PtdEtn, synthesized in mitochondria, can shuttle back to the MAMs, where it is further processed into phosphatidylcholine by PtdEtn methyltransferase (Cui et al, 1993). Altogether, this suggests the exchange of phospholipids and existence of a close physical interaction between the ER and mitochondria (Vance, 1990; Stone and Vance, 2000). In addition to the function in phospholipid homeostasis, MAMs have been implicated in the metabolism of cholesterol and its metabolites. First, the ER is the main site of synthesis and storage of cholesterol and several enzymes involved in the metabolism of cholesterol derivatives, such as diacylglycerol acyltransferase and acyl-coenzymeA:cholesterol acyltransferase, are highly enriched in MAMs (Rusinol et al, 1994). However, cytochrome P450scc, the enzyme required for conversion of cholesterol to pregnenolone is localized exclusively on mitochondria (Thomson, 2003). Although it has not been clarified yet how cholesterol is imported into the mitochondria, one possibility is that this occurs at contact sites with the ER. Finally, MAMs might also be implicated in the metabolism and trafficking of sphingolipids (Hayashi et al, 2009). Upon exposure to a fluorescently labelled analogue of ceramide, sphingolipids are trafficked through the ER to mitochondria (Lipsky and Pagano, 1983). Further, MAMs and mitochondria participate together in the metabolism of ceramide, a metabolite of sphingolipids with assigned functions in cell cycle, differentiation and apoptosis (Bionda et al, 2004).
Altogether, strong evidence supports the idea that the interface between ER and mitochondria has a major function in controlling the metabolism of different classes of lipids. In agreement with this view, studies in yeast indicate that disrupting the close contact sites between the two organelles affects the exchange of phospholipids (Kornmann et al, 2009).
The protein liaison: dual targeting to mitochondria and ER
Most of the proteins are specifically targeted to a single organelle, yet in some cases the same transcript can be found on different compartments, like ER and mitochondria. Most of the proteins that are delivered to two different organelles result either from two different genes, two mRNAs deriving from the same gene or two translation initiations on the same mRNA; in each case, the translation product differs by the presence or absence of distinct targeting signals (Danpure, 1995). More recent discoveries show that additionally the same translation product can be sent to two different compartments.
To target a protein to an organelle, the transcript has to be both ‘recognized' and imported into the specific compartment; implying that dual targeting results either from promiscuity or competition of the targeting signal (Karniely and Pines, 2005). The targeting of proteins with accessible (either one ambiguous or two different) targeting signals depends on the relative affinity of the receptors. Dual targeting is also achieved if during translation, a subpopulation of proteins suffers a post-translational modification that renders the targeting sequence inaccessible as it happens for mammalian NADH cytochrome b5 reductase, which is double targeted to both mitochondria and ER because of the presence of a myristoylation site on the N-terminus of the protein. In the absence of myristoylation, the protein is recognized by the signal recognition particle (SRP) and is targeted to the ER. However, myristoylation on a glycine residue on the N-terminal of the transcript diminishes the affinity of the protein for the SRP and the nascent chain remains attached to free ribosomes, becoming available for post-translational targeting to the OMM (Colombo et al, 2005). Several members of the cytochrome P450 family are also dually localized on mitochondria and ER. As for NADH cytochrome b5 reductase, the targeting signal of cytochrome P450 2B1 to the ER can be ‘masked' by a post-translational modification, phosphorylation (Anandatheerthavarada et al, 1999). In the case of cytochrome P450 1A1, alternative targeting is achieved by endoproteolytic cleavage that removes the ER targeting sequence (Addya et al, 1997).
Several members of the B-cell leukaemia-2 (Bcl-2) family similarly are localized both on ER and on mitochondria, such as Bcl-2 (Annis et al, 2001) itself, Bax and Bak (Gajkowska et al, 2001; Nutt et al, 2002). However, very little is known on how Bcl-2 family proteins reach the two different organelles.
Finally, it is important to note that alternative localization of proteins either on ER and mitochondria may have different functions (in the case of Bcl-2 family proteins, differently localized proteins regulate apoptosis through different mechanisms). Preferential targeting of proteins to either one compartment or the other can be regulated by the enzymatic activity (such as phosphorylation, myristoylation,…) of the cell, as exemplified for cytochrome p450 2b1.
The Ca2+ liaison
The ER is the main Ca2+ store of the mammalian cell, although it has been known for a long time that mitochondria as well are able to accumulate Ca2+. In response to cytosolic Ca2+ transients not exceeding concentrations of 1–3 μM, mitochondrial Ca2+ concentrations rise almost simultaneously to values above 10 μM (Rizzuto and Pozzan, 2006). This is unexpected considering the low affinity to Ca2+ of the mitochondrial Ca2+ uniporter (Rizzuto et al, 1993, 1998). Moreover, Ca2+ released by the ER into the cytosol in response to inositol-1,4,5-triphosphate (IP3) is transferred to mitochondria much more efficiently than Ca2+ elevations induced by leakage of Ca2+ from this organelle (Rizzuto et al, 1993; Hajnoczky et al, 1995). These observations led to the proposal by Rizzuto and Pozzan of the existence of close contact points between ER and mitochondria, enriched in IP3 and Ryanodine receptors. Upon cell stimulation, the release of high concentrations of Ca2+ at contact sites between the two organelles leads to the formation of microdomains of high Ca2+ concentration that are crucial for efficient Ca2+ uptake by mitochondria (Figure 1) (Rizzuto et al, 1993, 1998).
Ca2+ transmission from the ER to mitochondria, and thus contact between the two organelles, has a crucial function in regulating mitochondrial metabolism. Mitochondrial Ca2+ levels regulate the activity of several enzymes of the tricarboxylic acid and of the electron transport chain, such as α-ketoglutarate, isocitrate dehydrogenases and pyruvate dehydrogenase (Bernardi, 1999). In addition to enzymes involved in mitochondrial metabolism, Ca2+ also controls the phosphorylation status and thus the activity of mitochondrial metabolite transporters, manganese superoxide dismutase and other enzymes (Hayashi et al, 2009).
Paradoxically, excessive Ca2+ can also be detrimental for mitochondria. Indeed, the entry of Ca2+ into the mitochondrial matrix is an energetically expensive process, associated with a transient depolarization of the IMM and therefore with a transiently lower driving force for ATP synthase (Duchen, 2000). Finally, exaggerated accumulation of Ca2+ in mitochondria can lead to overload and impairment of mitochondrial function because of opening of the permeability transition pore, an inner mitochondrial membrane channel that allows the diffusion of ions and other small molecules along their gradients, ultimately leading to dissipation of the mitochondrial potential and in certain cases to cell death (Bernardi, 1999; Hajnoczky et al, 2006).
If one considers the dual function of Ca2+ ions as mediators of life and death, then a tight control over the distance between ER and mitochondria would be required to regulate vital mitochondrial functions. However, little is known on how physiological stimuli control the distance between ER and mitochondria and therefore Ca2+ transfer between the two organelles. In an attempt to gain further insight into this mechanism, a pioneer work shows that the chaperone Sigma-1 receptor (Sig-1R) could have a function in controlling the sustained Ca2+ signalling at the ER–mitochondria interface upon ER Ca2+ depletion (Hayashi and Su, 2007). In basal conditions, Sig-1R forms a complex with the ER chaperone immunoglobulin heavy-chain binding protein (in B lymphocytes) (BIP). As soon as ER Ca2+ stores are depleted or the Sig-1R is activated by binding to its ligands, the receptor dissociates from BIP and modulates the activity of the IP3R, leading to prolonged Ca2+ signalling into mitochondria (Figure 1), a mechanism that could impact dramatically on Ca2+-dependent mitochondrial functions as well as on interorganellar Ca2+ signalling and cell survival. It is tempting to speculate that different physiological processes might use specialized ‘molecular tools' to regulate communication between ER and mitochondria according to cellular needs.
How does the Ca2+ liaison lead to death?
If one considers that Ca2+ homeostasis participates actively in different forms of cell death and that mitochondria are main effectors during this process, it is not surprising that the cross-talk between ER and mitochondria contributes in several circumstances to take the decision whether the cell should live or die. The first indication was given by the demonstration that IP3-induced Ca2+ mobilization from the ER to mitochondria has a function in apoptosis (Szalai et al, 1999). Interestingly, the anti-apoptotic protein Bcl-2 decreases the steady-state Ca2+ content of the ER, resulting in a reduced amount of agonist-releasable Ca2+ and in a diminution of cytosolic and mitochondrial Ca2+ response (Pinton et al, 2000, 2001). Thus, by diminishing ER Ca2+ levels, Bcl-2 is able to protect from Ca2+-dependent apoptotic stimuli (Pinton et al, 2001). Moreover, knocking out the pro-apoptotics Bax and Bak leads to a dramatic reduction of the steady-state Ca2+ concentration in the ER, rendering the knock-out cells more resistant to apoptosis (Figure 2) (Scorrano et al, 2003). Again, the amount of Ca2+ that can be released into the cytosol (and thus the amount of Ca2+ that reaches mitochondria) rather than effective ER Ca2+ concentration is relevant for the transmission of the cell death signal to mitochondria.
As mentioned above, phospho-acidic cluster protein (PACS)-2 is also involved in the regulation of apoptosis. In response to apoptotic stimuli, PACS-2 induces Bid translocation to mitochondria initiating the apoptotic cascade (Simmen et al, 2005).
Interestingly, during apoptosis, the mitochondria-shaping protein Drp1 is recruited to mitochondria where it protects from Ca2+-dependent death by inducing fragmentation of the mitochondrial network and consequently by blocking the transmission of potentially fatal Ca2+ waves along this organelle (Szabadkai et al, 2004). However, contrasting evidence indicates that cell death activation by death receptors leads to the cleavage of BAP31 by caspases-8 and its translocation to mitochondria. Translocation of BAP31 accompanied by ER Ca2+ release mediates mitochondrial fragmentation and recruitment of Drp1 to mitochondria. Finally, Drp1 on mitochondria may lead to mitochondrial cristae remodelling and contribute to the apoptotic response (Breckenridge et al, 2003; Germain et al, 2005). Despite the inconsistency of the above-mentioned data, both observations imply that certain paradigms of apoptosis are regulated by the cross-talk between mitochondria and ER (Figure 2).
A more indirect but still very interesting approach to elucidate how distance between ER and mitochondria regulates cell function was taken by Hajnoczky and colleagues. The use of synthetic linkers or limited proteolyses, which either increase or decrease artificially the distance between ER and mitochondria, demonstrates that altering the distance between the two organelles leads inevitably to cell dysfunction. In fact, decreasing the space between ER and mitochondria leads to mitochondrial Ca2+ overload. On the other hand, an increase in the distance between the two compartments puts at risk the Ca2+-dependent regulation of mitochondrial metabolism by removing Ca2+ transmission between them (Csordas et al, 2006) and consequently cell viability. In extreme cases, both conditions can trigger cell death, further substantiating the view that the distance between the two organelles needs to be tightly regulated. On one side, the Sig-1R is able to sense Ca2+ concentrations in the ER and control the amount of releasable Ca2+ that can be tunnelled to mitochondria in order to maintain mitochondrial metabolism and protect the cell from energy depletion (Hayashi and Su, 2007). On the other hand, enhanced Ca2+ transmission from the ER to mitochondria during ER stress might also have a function in the onset of apoptosis. For instance, a truncated version of the Sarcoendoplasmic reticulum Ca2+-ATPase 1 (S1T) expressed upon ER stress appears to increase ER Ca2+ leak and promote Ca2+ transfer to mitochondria. The subsequent mitochondrial Ca2+ overload promotes apoptosis (Chami et al, 2008). In a similar manner, gangliosides can also have a function in linking ER stress to mitochondrial apoptosis (Sano et al, 2009). GM1-ganglioside accumulation at MAMs can influence the activity of the IP3R by directly interacting with the channel. This binding promotes an increased Ca2+ release from the ER. Ca2+ is then tunnelled to the mitochondria promoting Ca2+ overload and activating the mitochondrial apopototic cascade (Sano et al, 2009). Finally, ER and mitochondria participate together in the metabolism of ceramides. As it has been proposed that ceramides are able to initiate the apoptotic pathway by forming channels in the OMM (Siskind, 2005), it is tempting to speculate that MAMs are at the cross-road also for the action of ceramide during apoptosis.
Structural basis for ER–mitochondrial tethering
Although over the last 20 years substantial evidence accumulated on the cellular importance of communication between ER and mitochondria, the field has gained little insight onto the structural basis for tethering between the two organelles. One crucial question is how the intimate liaison is maintained despite considering the continuous movement and reorganization of both organelles in the cell. However, live imaging studies support that also the tethering regions are continuously formed and disrupted in a dynamic process (Rizzuto et al, 1998). It is therefore not surprising that both the mitochondria-shaping protein Drp1 (Pitts et al, 1999) and PACS-2 (Simmen et al, 2005) can have a function in regulating contacts between ER and mitochondria. PACS-2, mainly localized at the ER, regulates juxtaposition of the two compartments through BAP31-dependent fission and perinuclear clustering of mitochondria (Simmen et al, 2005). In the same way, Drp1 could alter tethering by causing fragmentation of mitochondria (Figure 3) (Pitts et al, 1999; Szabadkai et al, 2004). Interestingly, ionomycin-induced increases in cytosolic Ca2+ also seem to disrupt vicinity between ER and mitochondria by an unknown mechanism involving autocrine motility factor receptor (Wang et al, 2000; Goetz et al, 2007). However, the function of these proteins appear to be indirect, resulting from alterations in mitochondrial morphology and distribution in the cell. In other words, they do not take direct part in the bridges of proteinaceous origin directly linking ER to mitochondria whose existence has been shown in EM of ER–mitochondria contact sites (Perkins et al, 1997; Marsh et al, 2001; Csordas et al, 2006). The nature of these bridges remained largely elusive. One hypothesis is that the IP3R on the ER and the voltage-dependent anion channel (VDAC)1 on the OMM are physically coupled through the chaperone glucose-regulated protein 75 kDa (GRP75) (Figure 3) (Szabadkai et al, 2006). Albeit IP3R might regulate contact points between the two organelles, recent work by the group of Hajnoczky demonstrates that an IP3R-independent tether exists (Csordas et al, 2006).
The first direct ER–mitochondria tether to be discovered was Mfn2 (de Brito and Scorrano, 2008). This mitochondria-shaping protein localizes not only on mitochondria, as previously believed, but it is also highly enriched in the MAM fraction and retrieved, albeit to a lower extent, at the ER. Expression of Mfn2 exclusively on the ER or on mitochondria indicates that Mfn2 needs to be localized on the ER to maintain the shape and continuity of this organelle. These surprising results suggest that Mfn2 has a crucial function on the ER–mitochondria interface. The use of advanced imaging techniques and a novel in vitro interaction assay demonstrated that Mfn2 is required on the ER to maintain the close contact points between the two organelles (Figure 3). Moreover, Mfn2 on the ER forms large complexes comprising Mfn2 or Mfn1 on mitochondria that could represent the bridges identified in EM experiments. In other words, while mitochondrial Mfn2 regulates shape of this organelle, ER-associated Mfn2 is required for ER morphology and its tethering to mitochondria (de Brito and Scorrano, 2008). The finding that Mfn2 participates in forming a structural linker between ER and mitochondrial, constituted a valuable genetic tool to demonstrate the functional consequence of the altered ER–mitochondria distance on mitochondrial Ca2+ uptake (Rizzuto et al, 1993, 1998). For the first time, using a loss of function model, the increased distance between ER and mitochondria was unequivocally linked to reduced uptake of Ca2+ by mitochondria during physiological IP3-mediated Ca2+ signalling. This constituted the first experimental proof of the ‘Ca2+ microdomain' theory proposed by Pozzan and coworkers in the nineties (Rizzuto et al, 1993, 1998). More recently, the microdomain theory has been reconfirmed in the laboratory of Tullio Pozzan by taking advantage of the use of recombinant Ca2+ sensors (Giacomello et al, 2010).
A completely different approach, based on synthetic biology, was used to screen for the nature of the tethers in yeast. A synthetic linker that inserts in both the OMM and in the membrane of the ER was used to rescue a library of mutagenized yeast cells, thereby identifying the mutations whose viability is rescued by the synthetic bridge. This elegant approach revealed that Mdm12, an OMM protein, is required for maintenance of the contact points between the two organelles in yeast. Further, Mdm12 is part of a tethering complex, the ‘ER–mitochondria encounter complex' (ERMES), which consists of outer mitochondrial and ER membrane proteins and appears essential to keep the contact points between the two organelles in yeast (Figure 3) (Kornmann et al, 2009). Even tough this study provides a first exciting glance for the identification of the tethering complex, it needs to be extended to mammalian cells to identify meaningful tethering components. Indeed, yeast is not the best experimental system as the only crucial ER–mitochondria process that is conserved is phospholipid biosynthesis. On the other hand, ER is not the main Ca2+ store in Saccharomyces cerevisiae, and yeast mitochondria do not posses the Ca2+ uniporter. Therefore, the existence of specialized tethers for phospholipid transfer is likely and confirmed by the lack of conservation in higher eukaryotes of the components of the ERMES complex. It is worth mentioning that synthetic linkers have already been applied successfully in mammalian cells (Csordas et al, 2006; Komatsu et al, 2010). In particular, a chemically inducible dimerization probe where one of the dimerization partners was targeted to the OMM while the other localized to the ER membrane allows efficient tethering upon addition of the chemical dimerizer (Komatsu et al, 2010). This strategy would be particularly interesting to screen for novel components of the mammalian tethering complex and to dynamically study the function of the tethering, avoiding the problems arising from the stable expression of tethers that reduce the distance in a permanent manner.
A more obvious regulator of juxtaposition between ER and mitochondria is the cytoskeletal network (Soltys and Gupta, 1992). In fact, both ER and mitochondria bind to MTs and actin filaments (Sturmer et al, 1995; Ebneth et al, 1998), implicating that the cytoskeleton could provide a scaffold that stabilizes the contact points between the compartments. As a matter of fact, a recently identified protein, trichoplein/mitostatin, which binds to keratins and other intermediate filaments, is highly enriched in MAMs, and negatively regulates the juxtaposition between mitochondria and ER in an Mfn2-dependent manner (Cerqua, Anesti, Baffa, Dimmer and Scorrano, unpublished data). These result open the possibility that intermediate filaments participate in the tethering. In addition, mitostatin is an oncosuppressor, deleted in a variety of solid tumours (Vecchione et al, 2009), placing the ER–mitochondria interface also in the stage of neoplastic transformation.
Concluding remarks
We are only at the beginning of our understanding of the details of how ER and mitochondria interact. Several indirect mechanisms such as binding to the cytoskeletal scaffold or alterations in organelle morphology and positioning probably have a function. However, more direct regulators of this interaction are emerging, and it remains to be elucidated how these tethers function. Another open question is how are these tethers regulated in response to diverse cellular cues; is increased distance between ER and mitochondria privileged in response to intense Ca2+ release from the ER? Does the need for phospholipid synthesis encourage a more intimate contact between the two compartments? These are all open questions that need to be addressed in the future.
In conclusion, current evidence supports the view that the multiple aspects of interorganellar signalling between ER and mitochondria, as well as the functions of these organelles, are regulated by their relative spatial organization. The importance of the spatial organization is confirmed by the evidence that its impairment severely affects this balance and can initiate cell death.
Acknowledgments
LS is a Senior Telethon Scientist of the Dulbecco-Telethon Institute. This study was supported by Swiss National Foundation Grant 31-118171, OncoSuisse, Telethon Italy. OMdB was supported by a FCT Portugal Doctoral Fellowship.
Footnotes
The authors declare that they have no conflict of interest.
References
- Addya S, Anandatheerthavarada HK, Biswas G, Bhagwat SV, Mullick J, Avadhani NG (1997) Targeting of NH2-terminal-processed microsomal protein to mitochondria: a novel pathway for the biogenesis of hepatic mitochondrial P450MT2. J Cell Biol 139: 589–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarilio R, Ramachandran S, Sabanay H, Lev S (2005) Differential regulation of endoplasmic reticulum structure through VAP-Nir protein interaction. J Biol Chem 280: 5934–5944 [DOI] [PubMed] [Google Scholar]
- Anandatheerthavarada HK, Biswas G, Mullick J, Sepuri NB, Otvos L, Pain D, Avadhani NG (1999) Dual targeting of cytochrome P4502B1 to endoplasmic reticulum and mitochondria involves a novel signal activation by cyclic AMP-dependent phosphorylation at ser128. EMBO J 18: 5494–5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade J, Pearce ST, Zhao H, Barroso M (2004) Interactions among p22, glyceraldehyde-3-phosphate dehydrogenase and microtubules. Biochem J 384: 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annis MG, Zamzami N, Zhu W, Penn LZ, Kroemer G, Leber B, Andrews DW (2001) Endoplasmic reticulum localized Bcl-2 prevents apoptosis when redistribution of cytochrome c is a late event. Oncogene 20: 1939–1952 [DOI] [PubMed] [Google Scholar]
- Bakeeva LE, Chentsov YS, Skulachev VP (1981) Ontogenesis of mitochondrial reticulum in rat diaphragm muscle. Eur J Cell Biol 25: 175–181 [PubMed] [Google Scholar]
- Baumann O, Walz B (2001) Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int Rev Cytol 205: 149–214 [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J, Voth M (1994) Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech 27: 198–219 [DOI] [PubMed] [Google Scholar]
- Bernardi P (1999) Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev 79: 1127–1155 [DOI] [PubMed] [Google Scholar]
- Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D (2004) Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J 382: 527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschi E, Zunino R, McBride HM (2009) MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep 10: 748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC (2003) Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol 160: 1115–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghetti GM, Stangherlin A, Martins de BO, Chang CR, Blackstone C, Bernardi P, Scorrano L (2008) Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA 105: 15803–15808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chami M, Oules B, Szabadkai G, Tacine R, Rizzuto R, Paterlini-Brechot P (2008) Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol Cell 32: 641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160: 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, Martins de BO, Dal ZB, Scorrano L (2004) OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA 101: 15927–15932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo S, Longhi R, Alcaro S, Ortuso F, Sprocati T, Flora A, Borgese N (2005) N-myristoylation determines dual targeting of mammalian NADH-cytochrome b5 reductase to ER and mitochondrial outer membranes by a mechanism of kinetic partitioning. J Cell Biol 168: 735–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G (2006) Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174: 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Thomas AP, Hajnoczky G (1999) Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J 18: 96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Vance JE, Chen MH, Voelker DR, Vance DE (1993) Cloning and expression of a novel phosphatidylethanolamine N-methyltransferase. A specific biochemical and cytological marker for a unique membrane fraction in rat liver. J Biol Chem 268: 16655–16663 [PubMed] [Google Scholar]
- Danpure CJ (1995) How can the products of a single gene be localized to more than one intracellular compartment? Trends Cell Biol 5: 230–238 [DOI] [PubMed] [Google Scholar]
- Daum G, Vance JE (1997) Import of lipids into mitochondria. Prog Lipid Res 36: 103–130 [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610 [DOI] [PubMed] [Google Scholar]
- Dreier L, Rapoport TA (2000) In vitro formation of the endoplasmic reticulum occurs independently of microtubules by a controlled fusion reaction. J Cell Biol 148: 883–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR (2000) Mitochondria and calcium: from cell signalling to cell death. J Physiol 529(Pt 1): 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E (1998) Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer's disease. J Cell Biol 143: 777–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eura Y, Ishihara N, Yokota S, Mihara K (2003) Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem (Tokyo) 134: 333–344 [DOI] [PubMed] [Google Scholar]
- Franke WW, Kartenbeck J (1971) Outer mitochondrial membrane continuous with endoplasmic reticulum. Protoplasma 73: 35–41 [DOI] [PubMed] [Google Scholar]
- Frey TG, Mannella CA (2000) The internal structure of mitochondria. Trends Biochem Sci 25: 319–324 [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Martins de BO, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De SB, Scorrano L (2006) OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126: 177–189 [DOI] [PubMed] [Google Scholar]
- Gajkowska B, Motyl T, Olszewska-Badarczuk H, Godlewski MM (2001) Expression of BAX in cell nucleus after experimentally induced apoptosis revealed by immunogold and embedment-free electron microscopy. Cell Biol Int 25: 725–733 [DOI] [PubMed] [Google Scholar]
- Germain M, Mathai JP, McBride HM, Shore GC (2005) Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J 24: 1546–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, Pozzan T (2010) Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell 38: 280–290 [DOI] [PubMed] [Google Scholar]
- Goetz JG, Genty H, St-Pierre P, Dang T, Joshi B, Sauve R, Vogl W, Nabi IR (2007) Reversible interactions between smooth domains of the endoplasmic reticulum and mitochondria are regulated by physiological cytosolic Ca2+ levels. J Cell Sci 120: 3553–3564 [DOI] [PubMed] [Google Scholar]
- Goetz JG, Nabi IR (2006) Interaction of the smooth endoplasmic reticulum and mitochondria. Biochem Soc Trans 34: 370–373 [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha RS, Yi M (2006) Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium 40: 553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP (1995) Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82: 415–424 [DOI] [PubMed] [Google Scholar]
- Hales KG, Fuller MT (1997) Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell 90: 121–129 [DOI] [PubMed] [Google Scholar]
- Harder Z, Zunino R, McBride H (2004) Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol 14: 340–345 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, Su TP (2009) MAM: more than just a housekeeper. Trends Cell Biol 19: 81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131: 596–610 [DOI] [PubMed] [Google Scholar]
- Hu J, Shibata Y, Zhu PP, Voss C, Rismanchi N, Prinz WA, Rapoport TA, Blackstone C (2009) A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell 138: 549–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Eura Y, Mihara K (2004) Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci 117: 6535–6546 [DOI] [PubMed] [Google Scholar]
- James DI, Parone PA, Mattenberger Y, Martinou JC (2003) hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem 278: 36373–36379 [DOI] [PubMed] [Google Scholar]
- Karniely S, Pines O (2005) Single translation—dual destination: mechanisms of dual protein targeting in eukaryotes. EMBO Rep 6: 420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein DR, Kappeler F, Hauri HP (1998) A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J 17: 6168–6177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T, Kukelyansky I, McCaffery JM, Ueno T, Varela LC, Inoue T (2010) Organelle-specific, rapid induction of molecular activities and membrane tethering. Nat Methods 7: 206–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P (2009) An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325: 477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC (2004) Structural basis of mitochondrial tethering by mitofusin complexes. Science 305: 858–862 [DOI] [PubMed] [Google Scholar]
- Koster G, VanDuijn M, Hofs B, Dogterom M (2003) Membrane tube formation from giant vesicles by dynamic association of motor proteins. Proc Natl Acad Sci USA 100: 15583–15588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM (1999) C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell 4: 815–826 [DOI] [PubMed] [Google Scholar]
- Leduc C, Campas O, Zeldovich KB, Roux A, Jolimaitre P, Bourel-Bonnet L, Goud B, Joanny JF, Bassereau P, Prost J (2004) Cooperative extraction of membrane nanotubes by molecular motors. Proc Natl Acad Sci USA 101: 17096–17101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ (2004) Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15: 5001–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JA, Tata JR (1973) A rapidly sedimenting fraction of rat liver endoplasmic reticulum. J Cell Sci 13: 447–459 [DOI] [PubMed] [Google Scholar]
- Lipsky NG, Pagano RE (1983) Sphingolipid metabolism in cultured fibroblasts: microscopic and biochemical studies employing a fluorescent ceramide analogue. Proc Natl Acad Sci USA 80: 2608–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh BJ, Mastronarde DN, Buttle KF, Howell KE, McIntosh JR (2001) Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc Natl Acad Sci USA 98: 2399–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morre DJ, Merritt WD, Lembi CA (1971) Connections between mitochondria and endoplasmic reticulum in rat liver and onion stem. Protoplasma 73: 43–49 [DOI] [PubMed] [Google Scholar]
- Nutt LK, Pataer A, Pahler J, Fang B, Roth J, McConkey DJ, Swisher SG (2002) Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. J Biol Chem 277: 9219–9225 [DOI] [PubMed] [Google Scholar]
- Orso G, Pendin D, Liu S, Tosetto J, Moss TJ, Faust JE, Micaroni M, Egorova A, Martinuzzi A, McNew JA, Daga A (2009) Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature 460: 978–983 [DOI] [PubMed] [Google Scholar]
- Palade GE (1952) The fine structure of mitochondria. Anat Rec 114: 427–451 [DOI] [PubMed] [Google Scholar]
- Perkins G, Renken C, Martone ME, Young SJ, Ellisman M, Frey T (1997) Electron tomography of neuronal mitochondria: three-dimensional structure and organization of cristae and membrane contacts. J Struct Biol 119: 260–272 [DOI] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di VF, Pozzan T, Rizzuto R (2000) Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J Cell Biol 148: 857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, Di VF, Pozzan T, Rizzuto R (2001) The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J 20: 2690–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts KR, Yoon Y, Krueger EW, McNiven MA (1999) The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol Biol Cell 10: 4403–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro CM, McKay RR, Hosoki E, Bird GS, Putney JW Jr (2000) Effects of elevated cytoplasmic calcium and protein kinase C on endoplasmic reticulum structure and function in HEK293 cells. Cell Calcium 27: 175–185 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T (1993) Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262: 744–747 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280: 1763–1766 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pozzan T (2006) Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev 86: 369–408 [DOI] [PubMed] [Google Scholar]
- Rojo M, Legros F, Chateau D, Lombes A (2002) Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci 115: 1663–1674 [DOI] [PubMed] [Google Scholar]
- Ruby JR, Dyer RF, Skalko RG (1969) Continuities between mitochondria and endoplasmic reticulum in the mammalian ovary. Z Zellforsch Mikrosk Anat 97: 30–37 [DOI] [PubMed] [Google Scholar]
- Rusinol AE, Cui Z, Chen MH, Vance JE (1994) A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J Biol Chem 269: 27494–27502 [PubMed] [Google Scholar]
- Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, Forte M, d'Azzo A (2009) GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol Cell 36: 500–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel A, Frank S, Gaume B, Herrler M, Youle RJ, Fuller MT (2003) Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci 116: 2763–2774 [DOI] [PubMed] [Google Scholar]
- Scorrano L, Korsmeyer SJ (2003) Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun 304: 437–444 [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ (2003) BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300: 135–139 [DOI] [PubMed] [Google Scholar]
- Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, Feliciangeli SF, Hung CH, Crump CM, Thomas G (2005) PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J 24: 717–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ (2005) Mitochondrial ceramide and the induction of apoptosis. J Bioenerg Biomembr 37: 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrand FS (1953) Electron microscopy of mitochondria and cytoplasmic double membranes. Nature 171: 30–32 [DOI] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12: 2245–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapp EL, Hegde RS, Francolini M, Lombardo F, Colombo S, Pedrazzini E, Borgese N, Lippincott-Schwartz J (2003) Formation of stacked ER cisternae by low affinity protein interactions. J Cell Biol 163: 257–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys BJ, Gupta RS (1992) Interrelationships of endoplasmic reticulum, mitochondria, intermediate filaments, and microtubules--a quadruple fluorescence labeling study. Biochem Cell Biol 70: 1174–1186 [DOI] [PubMed] [Google Scholar]
- Stone SJ, Vance JE (2000) Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J Biol Chem 275: 34534–34540 [DOI] [PubMed] [Google Scholar]
- Sturmer K, Baumann O, Walz B (1995) Actin-dependent light-induced translocation of mitochondria and ER cisternae in the photoreceptor cells of the locust Schistocerca gregaria. J Cell Sci 108(Pt 6): 2273–2283 [DOI] [PubMed] [Google Scholar]
- Subramanian K, Meyer T (1997) Calcium-induced restructuring of nuclear envelope and endoplasmic reticulum calcium stores. Cell 89: 963–971 [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R (2006) Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175: 901–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R (2004) Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell 16: 59–68 [DOI] [PubMed] [Google Scholar]
- Szalai G, Krishnamurthy R, Hajnoczky G (1999) Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J 18: 6349–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K (2007) Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282: 11521–11529 [DOI] [PubMed] [Google Scholar]
- Terasaki M, Runft LL, Hand AR (2001) Changes in organization of the endoplasmic reticulum during Xenopus oocyte maturation and activation. Mol Biol Cell 12: 1103–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson M (2003) Does cholesterol use the mitochondrial contact site as a conduit to the steroidogenic pathway? Bioessays 25: 252–258 [DOI] [PubMed] [Google Scholar]
- Vance JE (1990) Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 265: 7248–7256 [PubMed] [Google Scholar]
- Vecchione A, Fassan M, Anesti V, Morrione A, Goldoni S, Baldassarre G, Byrne D, D'Arca D, Palazzo JP, Lloyd J, Scorrano L, Gomella LG, Iozzo RV, Baffa R (2009) MITOSTATIN, a putative tumor suppressor on chromosome 12q24.1, is downregulated in human bladder and breast cancer. Oncogene 28: 257–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedrenne C, Hauri HP (2006) Morphogenesis of the endoplasmic reticulum: beyond active membrane expansion. Traffic 7: 639–646 [DOI] [PubMed] [Google Scholar]
- Voelker DR (2003) New perspectives on the regulation of intermembrane glycerophospholipid traffic. J Lipid Res 44: 441–449 [DOI] [PubMed] [Google Scholar]
- Voelker DR (2005) Bridging gaps in phospholipid transport. Trends Biochem Sci 30: 396–404 [DOI] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA (2007) Sheets, ribbons and tubules—how organelles get their shape. Nat Rev Mol Cell Biol 8: 258–264 [DOI] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA (2006) A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124: 573–586 [DOI] [PubMed] [Google Scholar]
- Voeltz GK, Rolls MM, Rapoport TA (2002) Structural organization of the endoplasmic reticulum. EMBO Rep 3: 944–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Guay G, Pogan L, Sauve R, Nabi IR (2000) Calcium regulates the association between mitochondria and a smooth subdomain of the endoplasmic reticulum. J Cell Biol 150: 1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasiak S, Zunino R, McBride HM (2007) Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol 177: 439–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y, Krueger EW, Oswald BJ, McNiven MA (2003) The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol 23: 5409–5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzano A, Liesa M, Sebastian D, Segales J, Palacin M (2010) Mitochondrial fusion proteins: dual regulators of morphology and metabolism. Semin Cell Dev Biol (doi:10.1016/j.semcdb.2010.01.002) [DOI] [PubMed] [Google Scholar]
- Zunino R, Braschi E, Xu L, McBride HM (2009) Translocation of SenP5 from the nucleoli to the mitochondria modulates DRP1-dependent fission during mitosis. J Biol Chem 284: 17783–17795 [DOI] [PMC free article] [PubMed] [Google Scholar]