Abstract
Behavioral sensitization in mammals, including humans, is sensitive to factors such as administration route, testing environment, and pharmacokinetic confounds, unrelated to the drugs themselves, that are difficult to eliminate. Simpler animals less susceptible to these confounding influences may be advantageous substitutes for studying sensitization. We tested this hypothesis by determining if planarians display sensitization and cross-sensitization to cocaine and glutamate. Planarian hyperactivity was quantified as the number of C-like hyperkinesias during a 1-min drug exposure. Planarians exposed initially to cocaine (or glutamate) on day 1 were challenged with cocaine (or glutamate) after 2 or 6 days of abstinence. Acute cocaine or glutamate produced concentration-related hyperactivity. Cocaine or glutamate challenge after 2 and 6 days of abstinence enhanced the hyperactivity, indicating the substances produced planarian behavioral sensitization (pBS). Cross-sensitization experiments showed that cocaine produced greater hyperactivity in planarians previously exposed to glutamate than in glutamate-naïve planarians, and vice versa. Behavioral responses were pharmacologically selective because neither scopolamine nor caffeine produced pBS despite causing hyperactivity after initial administration, and acute GABA did not cause hyperactivity. Demonstration of pharmacologically-selective behavioral sensitization in planarians suggests these flatworms represent a sensitive in vivo model to study cocaine behavioral sensitization and to screen potential abuse-deterrent therapeutics.
Keywords: planaria, cocaine, glutamate, sensitization, addiction, invertebrate
Introduction
Behavioral sensitization (reverse tolerance) is a hallmark effect of cocaine in mammals (Narendran and Martinez, 2008). This phenomenon is an enhancement in hyperactivity which occurs during the repeated, intermittent exposure to cocaine and most other addictive drugs (Vanderschuren and Kalivas, 2000). In this process, an animal exposed to an abused drug for the first time displays hyperactivity. When the same animal is exposed repeatedly to the drug, undergoes a period of abstinence, and is then reintroduced to the drug, the animal displays an exaggeration in hyperactivity compared to the increase in activity produced by initial exposure. Behavioral sensitization is a progressive and enduring phenomenon which can persist for months after withdrawal from drug exposure. Cellular adaptations which mediate behavioral sensitization are hypothesized to underlie the intensification of drug craving and relapse into drug use (Nestby et al., 1997; Berridge and Robinson, 1998; Vanderschuren and Kalivas, 2000; Narendran and Martinez, 2008).
The physiological mechanisms underlying development and expression of cocaine-induced behavioral sensitization are not entirely clear, but a critical role for glutamate - the major excitatory neurotransmitter in the mammalian brain and a primary regulator of the synaptic plasticity underlying learning and memory - is established. Development of cocaine-induced behavioral sensitization in mammals is dependent on increased glutamate transmission at NMDA and AMPA receptors (Karler et al. 1989, 1994; Kalivas and Alesdatter 1993; Wolf and Jeziorski 1993; Gambarana et al. 1998; Li et al., 1997; Wanat and Bonci, 2008). Expression of cocaine-induced behavioral sensitization in mammals is also highly dependent on increased glutamate activity (Karler et al., 1994; Vanderschuren and Kalivas, 2000). Prior work indicates cocaine-exposed rats which undergo a period of cocaine abstinence display an increased level of extracellular glutamate in the brain reward center (nucleus accumbens) following administration of a cocaine challenge (Reid and Berger, 1996; Kalivas and Duffy, 1998). At least some degree of cross-sensitization exists between cocaine and glutamate because NMDA or AMPA receptor agonist administration produces a sensitized behavioral response in rats previously exposed to cocaine (Bell and Kalivas, 1996; Pierce et al., 1996). Related experiments have demonstrated that a single injection of amphetamine induces behavioral sensitization in rats exposed previously to glutamate itself or to pharmacological agents that increase extracellular glutamate (Aked et al., 2005). Taken together, these results indicate that the ability of cocaine to produce sensitized behavioral responses in mammals is highly dependent on increased glutamate signaling.
Behavioral sensitization is understudied in invertebrates with the notable exception of drosophila, which display sensitized responses during repeated cocaine administration (Heberlein et al., 2009; Quinn et al., 1974; Griffith et al., 1993; McClung and Hirsh, 1998; Andretic et al., 1999; Dimitrijevic et al., 2004). An attractive invertebrate for investigating the process of behavioral sensitization is Planaria. These flatworms possess a primitive, yet centralized, nervous system (cephalic ganglia and spinal processes); utilize mammalian-like neurotransmitter systems, including glutamate, dopamine, and serotonin; and display abstinence-related withdrawal to abused drugs that is highly dependent on increased glutamate activity (Raffa et al., 2008; Raffa and Valdez, 2001; Rawls et al., 2006, 2007, 2008; Venturini et al., 1989; Creti et al., 1992; Eriksson and Panula, 1994; Umeda et al., 2005). Most importantly, planarians display hyperactivity following acute cocaine exposure and express key substrates that mediate cocaine-induced behavioral sensitization in mammals (Palladini et al., 1996; Pagan et al., 2008; Rowlands and Pagán, 2008). The present study investigated the specific hypothesis that addictive substances produce detectable, quantifiable behavioral sensitization in planarians with the ultimate goal of developing a sensitive, and broadly applicable, methodology for studying behavioral sensitization. The effects of four substances were examined: cocaine; glutamate; caffeine, a psychoactive stimulant and adenosine receptor antagonist; and scopolamine, a muscarinic receptor antagonist. We now provide the first evidence that cocaine and glutamate produce behavioral sensitization, and display cross-sensitization, in planarians, the simplest animals to possess a body plan common to all vertebrates and most invertebrates.
Methods
Animals and drugs
Planarians (Dugesia dorotocephala) were purchased from Carolina Biological Supply (Burlington, NC, USA), acclimated to room temperature (21 °C), and tested within 3 days of receipt. L-glutamic acid and scopolamine hydrobromide were purchased from Tocris Biosciences (St. Louis, MO) and caffeine was purchased from Sigma-Aldrich (St. Louis, MO). Cocaine hydrochloride was provided by the National Institute on Drug Abuse. Stock solutions of glutamate and cocaine were prepared daily in tap water containing AmQuel® water conditioner (sodium hydroxymethanesulfonate) (1 ml Amquel per 1 gallon of water). All treatment solutions were diluted with tap water containing AmQuel® water conditioner.
Behavioral experiments
Individual planarians were placed randomly into a plastic petri dish (5.5 cm diameter) containing a specific drug or drug combination and observed by a well-trained experimenter who was blinded to the treatment. Planarian activity counts were defined as the number of C-like hyperkinesias during the first 1 min of drug exposure as previously described and shown (Rawls et al., 2009). Each experiment used independent groups of planarians that were not reused for additional experiments. In all cases except for the caffeine experiments, planarians were only exposed to a specific treatment for 1 min. Experiments were conducted between 1 PM and 5 PM to control for potential circadian effects on planarian activity. The planarian C-like hyperactivity was not due to effects of the drugs on pH of the medium, a possibility that was addressed in a recent study demonstrating that topiramate antagonizes C-like hyperactivity induced by NMDA (Rawls et al., 2009). What we found was that the pH of the test water, in the absence of drug, was 7.0, and that the pH of a 3 mM NMDA solution was approximately 6.6. In the case in which planarians were exposed only to acetate buffer (pH = 6.6), they did not display C-like hyperactivity (Rawls et al., 2009). In fact, only when the pH of the acetate buffer was lowered to less than 5.0 did planarians display hyperactivity. The concentrations of cocaine and glutamate used in the current study have a pH greater than 5. More evidence that the hyperactivity observed here was due to a specific drug action, rather than a lowering of the pH, was that planarians exposed to GABA, even at concentrations as high as 25 mM, did not display significant hyperactivity (Fig. 1).
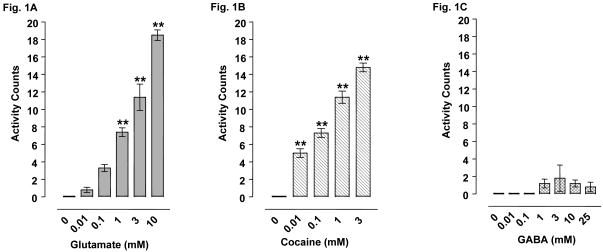
Fig. 1.
Effect of acute (A) glutamate (0.01-10 mM), (B) cocaine (0.01-3 mM), or (C) GABA (0.01-25 mM) administration on planarian activity. Data are expressed as mean activity counts ± S.E.M. during 1-min of drug exposure. **p < 0.01 compared to water control. N = 8 planarians per group.
Does acute glutamate, cocaine or GABA exposure increase planarian activity counts?
Individual planarians were exposed to glutamate (0, 0.01, 0.1, 1, 3, 10 mM), cocaine (0, 0.01, 0.1, 1, 3, 10 mM) or GABA (0, 0.01, 0.1, 1, 3, 10, 25 mM) and activity counts were quantified for 1 min.
Does repeated cocaine or glutamate exposure produce planarian behavioral sensitization (pBS)?
Individual planarians were initially exposed to cocaine (0.1, 1, 3 mM) for 1 min on day 1; re-exposed for 1 min to the same cocaine concentration (0.1, 1, 3 mM) 60 min later on day 1; and then underwent 2 days of abstinence before being reintroduced to the same cocaine concentration (0.1, 1, 3 mM) for 1 min on day 4. Activity counts were observed and quantified during each 1-min cocaine exposure on days 1 and 4. Control planarians were included for each respective cocaine concentration. Activity counts in control planarians were obtained during 1 min of exposure to drug-free water on day 1; during 1 min of exposure to drug-free water 60 min later on day 1; and during 1 min of exposure to cocaine (0.1, 1, 3 mM) on day 4. An identical paradigm was used for experiments in which planarians were repeatedly exposed to glutamate (0.1, 1, 3 mM).
Do planarians exposed only once to cocaine or glutamate display behavioral sensitization after 2 or 6 days of drug abstinence?
Individual planarians were exposed once to either 0.1 mM cocaine or drug-free water for 1 min on day 1 and then treated for 1 min with 0.1 mM cocaine 2 days later. In separate experiments, individual planarians exposed once to either 0.1 mM cocaine or drug-free water for 1 min on day 1 were treated for 1 min with 0.1 mM cocaine 6 days later. The identical paradigm was used for the 0.1 mM glutamate experiments.
Do cocaine and glutamate display cross-sensitization in planarians?
Individual planarians were exposed to cocaine (0.1 mM), glutamate (0.1 mM) or drug-free water for 1 min on day 1. Following 2 days of drug abstinence, planarians from each group were exposed to cocaine (0.1 mM) or glutamate (0.1 mM) for 1 min on day 4.
Does repeated scopolamine exposure cause planarian behavioral sensitization (pBS)?
Individual planarians were initially exposed to scopolamine (0.1, 1, 3 mM) for 1 min on day 1; re-exposed for 1 min to the same scopolamine concentration (0.1, 1, 3 mM) 60 min later on day 1; and then underwent 2 days of abstinence before being reintroduced to the same scopolamine concentration (0.1, 1, 3 mM) for 1 min on day 4. Activity counts were observed and quantified during each 1-min scopolamine exposure on days 1 and 4. Control planarians were included for each respective scopolamine concentration. Activity counts in control planarians were obtained during 1 min of exposure to drug-free water on day 1; during 1 min of exposure to drug-free water 60 min later on day 1; and during 1 min of exposure to scopolamine (0.1, 1, 3 mM) on day 4.
Does repeated caffeine exposure cause planarian behavioral sensitization (pBS)?
Pilot experiments indicated that caffeine concentrations of less than 0.1, 1 and 3 mM did not produce reproducible and quantifiable C-like hyperkinesias during 1 min of exposure. However, when the observation interval was extended to 5 min, caffeine concentrations of 1 and 3 mM did produce quantifiable C-like hyperkinesias. Thus, we selected concentrations of 1 and 3 mM and chose a drug-exposure interval of 5 min, rather than 1 min, for the caffeine sensitization experiments. In these experiments, individual planarians were initially exposed to caffeine (1, 3 mM) for 5 min on day 1; re-exposed for 5 min to the same caffeine concentration (1, 3 mM) 60 min later on day 1; and then underwent 2 days of abstinence before being reintroduced to the same caffeine concentration (1, 3 mM) for 5 min on day 4. Activity counts were observed and quantified during each 5-min caffeine exposure on days 1 and 4. Control planarians were included for each respective caffeine concentration. Activity counts in control planarians were obtained during 1 min of exposure to drug-free water on day 1; during 1 min of exposure to drug-free water 60 min later on day 1; and during 1 min of exposure to caffeine (1, 3 mM) on day 4.
Data analysis
For acute experiments, comparisons of group means (± S.E.M.) were evaluated by one-way ANOVA followed (if p < 0.05) by a Dunnett's post-hoc analysis. For chronic experiments, comparisons of group means (± S.E.M.) were analyzed using two-way ANOVA with pretreatment and treatment factors (or pretreatment and abstinence period factors) followed by a Bonferroni test for multiple comparisons. Within-subject comparisons of group means (± S.E.M.) were evaluated using one-way ANOVA followed (if p < 0.05) by a Dunnett's test. Values of p < 0.05 were considered statistically significant.
Results
Acute cocaine or glutamate administration increased activity
Planarians exposed to water did not display C-like hyperkinesias (i.e., activity counts) (Fig. 1A-C). Glutamate exposure produced a concentration-related increase in C-like hyperkinesias [F(5, 38) = 79.2, p < 0.0001] (Fig. 1A). Dunnett's post-hoc analysis showed that activity counts were significantly greater in planarians exposed to glutamate (1, 3, 10 mM) than in planarians exposed to water (p < 0.01). Cocaine-treated planarians also displayed a concentration-dependent increase in activity counts [F(4, 31) = 87.41, p < 0.0001] (Fig. 1B). Dunnett's post-hoc analysis indicated that cocaine (0.01, 0.1, 1, 3 mM) produced significant C-like hyperkinesias compared to water (p < 0.01) (Fig. 1B). Planarians exposed to GABA (0.01, 0.1, 1, 3, 10, 25 mM) did not display enhanced activity counts compared to planarians exposed to water [F(6, 42) = 1.054, p > 0.05] (Fig. 1C).
Cocaine produced planarian behavioral sensitization (pBS)
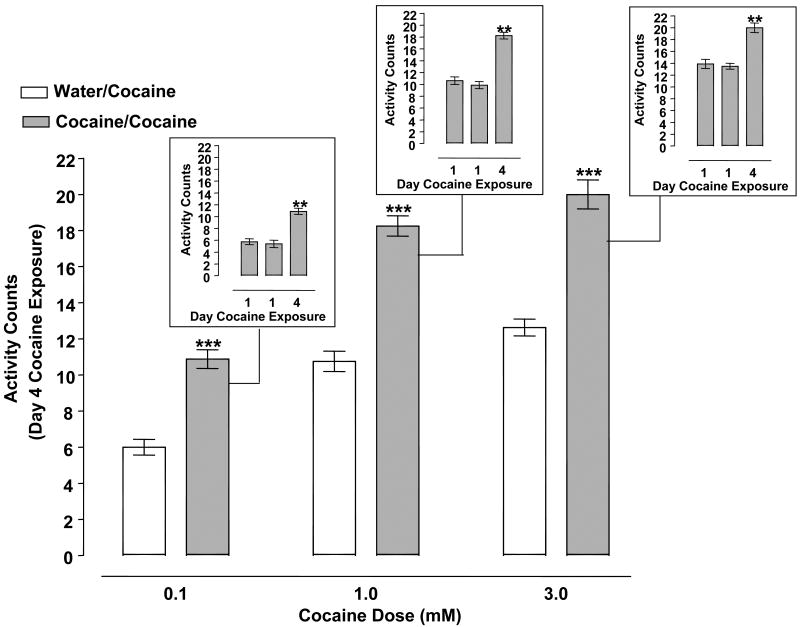
Activity counts of planarians pretreated with water or cocaine (0.1, 1, 3 mM) twice on day 1 and then treated with cocaine (0.1, 1, 3 mM) on day 4 are displayed in Fig. 2. Two-way ANOVA (pretreatment, treatment) showed a significant pretreatment effect [F(1, 42) = 179.4, p < 0.0001] and treatment effect [F(2, 42) = 93.88, p < 0.0001] whereas the interaction effect did not quite attain statistical significance [F(2, 42) = 3.03, p = 0.0592]. Post-hoc analysis revealed that 0.1, 1 and 3 mM cocaine exposure on day 4 produced significantly greater activity counts in planarians pretreated with cocaine twice on day 1 than in planarians previously naïve to cocaine (p < 0.001 water/cocaine versus cocaine/cocaine). One-way ANOVA comparing within-group means for the cocaine-exposed planarians showed a significant main effect for the 0.1 mM cocaine experiments [F (2, 21) = 31.33, p < 0.0001]; 1 mM cocaine experiments [F (2, 21) = 59.40, p < 0.0001]; and 3 mM cocaine experiments [F (2, 21) = 26.92, p < 0.0001] (Fig. 2 insets). Dunnett's post-hoc analysis indicated that planarians displayed enhanced activity counts following cocaine challenge (0.1, 1 or 3 mM) on day 4 compared to initial cocaine exposure on day 1 (p < 0.001 day 1 versus day 4).
Fig. 2.
Cocaine (0.1 - 3 mM) produced behavioral sensitization. Planarians pretreated twice with cocaine (0.1, 1, 3 mM) on day 1 (60 min apart) underwent 2 days of withdrawal before being challenged with cocaine (0.1, 1, 3 mM) on day 4 (cocaine/cocaine groups). Control planarians for each respective cocaine concentration were pretreated twice with water on day 1 and then exposed to cocaine (0.1, 1, 3 mM) on day 4 (water/cocaine groups). Data are expressed as mean activity counts ± S.E.M. during 1 min of cocaine exposure on day 4. ***p < 0.001 versus respective water/cocaine group. Insets) Data are expressed as mean activity counts ± S.E.M. in planarians exposed to cocaine (0.1, 1, 3 mM) for 1 min on days 1 (twice) and 4. **p < 0.01 versus day 1 exposure. N = 8 planarians per group.
Glutamate produced planarian behavioral sensitization (pBS)
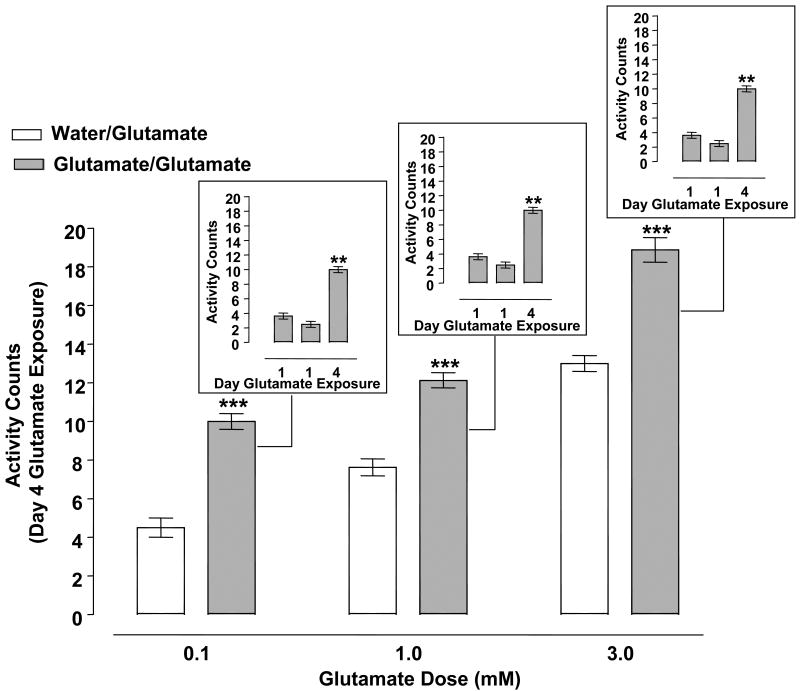
Activity counts of planarians pretreated with water or glutamate (0.1, 1, 3 mM) twice on day 1 and then treated with glutamate (0.1, 1, 3 mM) on day 4 are displayed in Fig. 3. Two-way ANOVA (pretreatment, treatment) showed a significant pretreatment effect [F(1, 42) = 147.6, p < 0.0001] and treatment effect [F(2, 42) = 139.5, p < 0.0001] but not a significant interaction effect [F(2, 42) = 0.8876, p = 0.4192]. Post-hoc analysis revealed that 0.1, 1 and 3 mM glutamate exposure on day 4 produced significantly greater activity counts in planarians pretreated with glutamate twice on day 1 than in planarians previously naïve to glutamate (p < 0.001 water/glutamate versus glutamate/glutamate). One-way ANOVA comparing within-group means for the glutamate-exposed planarians showed a significant main effect for the 0.1 mM glutamate experiments [F (2, 21) = 97.27, p < 0.0001]; 1 mM glutamate experiments [F (2, 21) = 42.97, p < 0.0001]; and 3 mM glutamate experiments [F (2, 21) = 59.27, p < 0.0001] (Fig. 3 insets). Dunnett's post-hoc analysis indicated that planarians displayed enhanced activity counts following glutamate challenge (0.1, 1, 3 mM) on day 4 compared to initial glutamate exposure on day 1 (p < 0.001 day 1 versus day 4).
Fig. 3.
Glutamate (0.1 - 3 mM) produced behavioral sensitization. Planarians pretreated twice with glutamate (0.1, 1, 3 mM) on day 1 (60 min apart) underwent 2 days of withdrawal before being challenged with glutamate (0.1, 1, 3 mM) on day 4 (glutamate/glutamate groups). Control planarians for each respective glutamate concentration were pretreated twice with water on day 1 and then exposed to glutamate (0.1, 1, 3 mM) on day 4 (water/glutamate groups). Data are expressed as mean activity counts ± S.E.M. during 1 min of glutamate exposure on day 4. ***p < 0.001 versus respective water/glutamate group. Insets) Data are expressed as mean activity counts ± S.E.M. in planarians exposed to glutamate (0.1, 1, 3 mM) for 1 min on days 1 (twice) and 4. **p < 0.01 versus day 1 exposure.
A single cocaine or glutamate exposure produced planarian behavioral sensitization (pBS) following abstinence intervals of 2 and 6 days
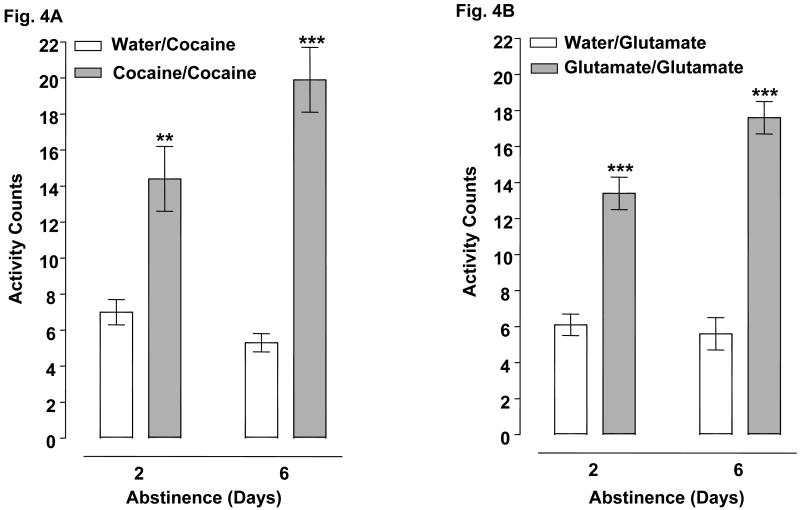
Fig. 4 presents results from experiments in which planarians were exposed once (day 1) to either 0.1 mM cocaine or 0.1 mM glutamate and then administered a challenge dose of 0.1 mM cocaine or 0.1 mM glutamate 2 or 6 days later. For the cocaine experiments, two-way ANOVA (pretreatment, abstinence interval) showed significant pretreatment [F (1, 28) = 68.40, p < 0.0001] and interaction [F (1, 28) = 7.428, p = 0.0109] effects but not a significant effect of abstinence interval by itself [F (1, 28) = 1.987, p = 0.1696] (Fig. 4A). Bonferroni analysis showed that planarians exposed once to 0.1 mM cocaine on day 1 and then reintroduced to 0.1 mM cocaine following 2 or 6 days of abstinence displayed significantly greater activity counts than did planarians that were previously naïve to cocaine (p < 0.01 or p < 0.001 for water/cocaine versus cocaine/cocaine following a 2- or 6-day abstinence) (Fig. 4A). For the 0.1 mM glutamate experiments, two-way ANOVA (pretreatment, abstinence interval) showed significant pretreatment [F (1, 28) = 123.2, p < 0.0001], abstinence interval [F (1, 28) = 0.0393, p < 0.0001] and interaction [F (1, 28) = 7.499, p = 0.0106] effects (Fig. 4B). Bonferroni analysis showed that planarians exposed once to 0.1 mM glutamate on day 1 and then reintroduced to 0.1 mM glutamate following 2 or 6 days of abstinence displayed significantly greater activity counts than did planarians that were previously naïve to glutamate (p < 0.001 for water/glutamate versus glutamate/glutamate following 2 and 6 days of abstinence) (Fig. 4B). The effect of 0.1 mM glutamate pretreatment was sensitive to the period of glutamate abstinence as a subsequent 0.1 mM glutamate challenge produced significantly greater activity counts following 6 days of abstinence (3.6-fold sensitization) than after 2 days of abstinence (2.5-fold sensitization).
Fig. 4.
A single cocaine or glutamate exposure produced behavioral sensitization after 2 and 6 days of drug abstinence. 4A) Planarians treated with 0.1 mM cocaine or water once on day 1 were exposed to 0.1 mM cocaine 2 or 6 days later. Data are expressed as mean activity counts ± S.E.M. during 1 min of cocaine exposure following 2 or 6 days of abstinence. **p < 0.01 or ***p < 0.001 compared to respective water/cocaine group. N = 8 planarians per group. 4B) Planarians treated with 0.1 mM glutamate or water once on day 1 were exposed to 0.1 mM glutamate 2 or 6 days later. Data are expressed as mean activity counts ± S.E.M. during 1 min of glutamate exposure following 2 or 6 days of abstinence. ***p < 0.001 compared to respective water/glutamate group. N = 8 planarians per group.
Cocaine and glutamate displayed cross-sensitization
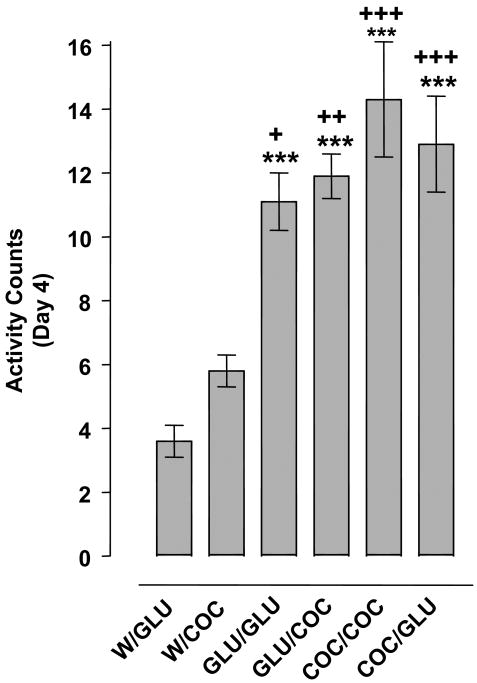
Fig. 5 presents results from experiments in which planarians with prior 0.1 mM glutamate exposure (twice on day 1) were challenged on day 4 with 0.1 mM cocaine, and vice versa. One-way ANOVA indicated a significant drug effect [F (5, 42) = 14.86, p < 0.0001]. Cocaine (0.1 mM) once again produced sensitization, evident from the observation that planarians exposed to cocaine (0.1 mM) displayed greater hyperactivity in response to their challenge administration of 0.1 mM cocaine (day 4) than planarians exposed to cocaine (0.1 mM) for the first time (p < 0.001). Glutamate also produced sensitization as day 4 glutamate administration (0.1 mM) produced greater hyperactivity in planarians previously exposed to glutamate (0.1 mM) than in planarians previously naïve to glutamate (p < 0.001). Cocaine produced cross-sensitization to glutamate, such that glutamate administration (0.1 mM) on day 4 produced greater hyperactivity in planarians with prior cocaine (0.1 mM) experience than in cocaine-naïve planarians (p < 0.001). In planarians exposed to 0.1 mM cocaine or glutamate on day 1, glutamate administration (0.1 mM) on day 4 produced hyperactivity that was not significantly different (p > 0.05). Glutamate also produced cross-sensitization to cocaine, as cocaine exposure (0.1 mM) on day 4 produced hyperactivity that was significantly greater in planarians exposed to glutamate (0.1 mM) on day 1 than in glutamate-naïve planarians (p < 0.01). In planarians exposed to either cocaine (0.1 mM) or glutamate (0.1 mM) on day 1, hyperactivity produced by cocaine (0.1 mM) on day 4 was not significantly different (p > 0.05).
Fig. 5.
Cocaine and glutamate display cross-sensitization. Planarians exposed to water (W), cocaine (COC) (0.1 mM) or glutamate (GLU) twice on day 1 (60 min apart) were treated with COC (0.1 mM) or GLU (0.1 mM) on day 4. Data are expressed as mean activity counts ± S.E.M. during 1 min of drug exposure on day 4. ***p < 0.001 compared to the W/GLU group; +p < 0.05, ++p < 0.01, and +++p < 0.001 compared to the W/COC group. N = 8 planarians per group.
Scopolamine or caffeine exposure did not cause planarian behavioral sensitization (pBS)
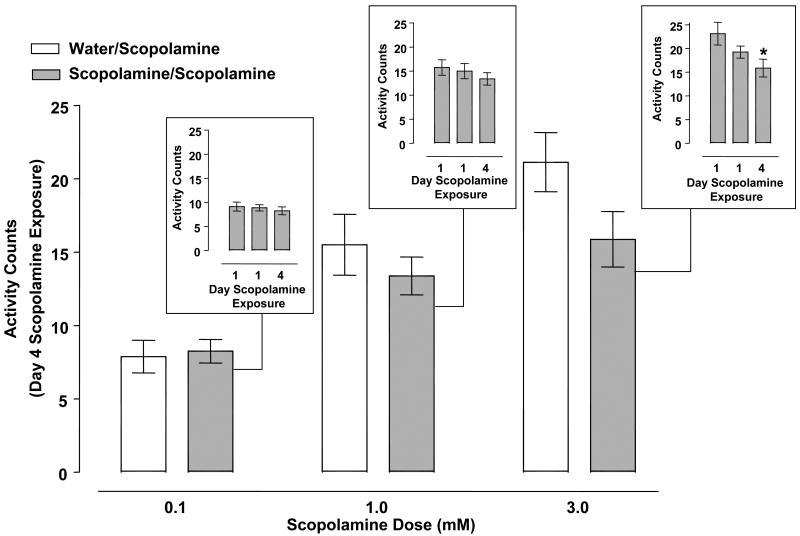
Fig. 6 displays the activity counts of planarians pretreated with water or scopolamine (0.1, 1, 3 mM) twice on day 1 and then treated with scopolamine (0.1, 1, 3 mM) on day 4. Scopolamine concentrations of 0.1, 1 and 3 mM induced C-like hyperkinesias following acute administration on day 1 (Fig. 6 insets). Two-way ANOVA (pretreatment, treatment) of the behavioral data showed a significant treatment effect [F(2, 42) = 18.83, p < 0.0001] but not significant pretreatment [F(1, 42) = 2.777, p = 0.1030] and interaction [F(2, 42) = 1.351, p = 0.2701] effects. A one-way ANOVA comparing within-group means for scopolamine-exposed planarians showed a significant main effect for the 3 mM scopolamine group [F (2, 21) = 3.624, p < 0.05] (Fig. 6 insets). Dunnett's post-hoc analysis indicated that scopolamine challenge (3 mM) on day 4 produced fewer activity counts than the same concentration of scopolamine (3 mM) produced upon initial exposure on day 1 (p < 0.05 day 1 versus day 4).
Fig. 6.
Scopolamine (0.1 - 3 mM) did not produce behavioral sensitization. Planarians pretreated twice with scopolamine (0.1, 1, 3 mM) on day 1 (60 min apart) underwent 2 days of withdrawal before being challenged with scopolamine (0.1, 1, 3 mM) on day 4 (scopolamine/scopolamine groups). Control planarians for each respective scopolamine concentration were pretreated twice with water on day 1 and then exposed to scopolamine (0.1, 1, 3 mM) on day 4 (water/scopolamine groups). Data are expressed as mean activity counts ± S.E.M. during 1 min of scopolamine exposure on day 4. Insets) Data are expressed as mean activity counts ± S.E.M. in planarians exposed to scopolamine (0.1, 1, 3 mM) for 1 min on days 1 (twice) and 4. *p < 0.05 versus day 1 exposure. N = 8 planarians per group.
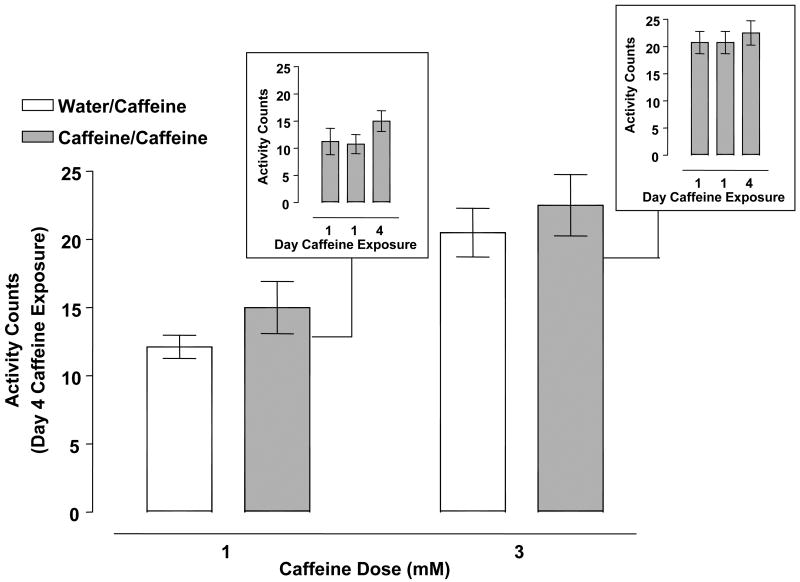
Activity counts of planarians pretreated with water or caffeine (1, 3 mM) twice on day 1 and then treated with caffeine (1, 3 mM) on day 4 are displayed in Fig. 7. The data displayed in Fig. 7 represent activity counts quantified over a 5-min interval. Caffeine concentrations of 1 and 3 mM produced C-like hyperkinesias following acute exposure (Fig. 7 insets). Two-way ANOVA (pretreatment, treatment) showed a significant treatment effect [F(1, 28) = 27.67, p < 0.0001] but not significant pretreatment [F(1, 28) = 1.578, p = 0.2194] and interaction [F(1, 28) = 0.000, p = 1.000] effects. One-way ANOVA comparing within-group means for the caffeine-exposed planarians confirmed the lack of a sensitized response to caffeine concentrations of 1 mM [F (2, 21) = 1.288, p = 0.2967] and 3 mM [F (2, 21) = 0.2284, p = 0.7978] (Fig. 7 insets).
Fig. 7.
Caffeine (1, 3 mM) did not produce behavioral sensitization. Planarians pretreated twice with caffeine (0.1, 1, 3 mM) on day 1 (60 min apart) underwent 2 days of withdrawal before being challenged with caffeine (1, 3 mM) on day 4 (caffeine/caffeine groups). Control planarians for each respective caffeine concentration were pretreated twice with water on day 1 and then exposed to caffeine (1, 3 mM) on day 4 (water/caffeine groups). Data are expressed as mean activity counts ± S.E.M. during 5 min of caffeine exposure on day 4. Insets) Data are expressed as mean activity counts ± S.E.M. in planarians exposed to caffeine (1, 3 mM) for 5 min on days 1 (twice) and 4. N = 8 planarians per group.
Discussion
We report for the first time that planarians display behavioral sensitization to cocaine, one of the world's most addictive drugs. Planarian behavioral sensitization (pBS) to cocaine challenge was detectable following a single cocaine exposure and was present after cocaine abstinence periods of two and six days. These aspects of pBS were similar to the behavioral sensitization produced by cocaine in mammals and drosophila (King et al., 1992; McClung and Hirsh, 1998; Post, 1980; Vanderschuren and Kalivas, 2000). Glutamate, a major excitatory neurotransmitter in the mammalian brain that possesses a different mechanism of action than cocaine, produced sensitization similar to cocaine, indicating that pBS was not cocaine-specific (Nicholls and Sihra, 1986). Pharmacological selectivity was observed for the phenomenon, however, as neither scopolamine or caffeine produced pBS despite increasing planarian activity following acute exposure. Another key finding was that planarians displayed a robust cross-sensitization to cocaine and glutamate, evident from the observation that cocaine produced behavioral sensitization in planarians previously exposed to glutamate and vice versa.
Although cocaine produces behavioral and neurochemical sensitization in mammals, including humans, the nature of the sensitization varies widely with a host of factors unrelated to the drug itself. These factors include cocaine exposure patterns (intermittent versus continuous); dosing schedules; pharmacokinetic factors; route of cocaine administration; testing environment (e.g., context-paired versus context-unpaired); anticipation of cocaine administration; the use of multiple, and often, diverse biological measures to quantify sensitization; and poly-drug abuse by addicts, a common practice which affects and complicates sensitization phenomena produced by the co-administered drugs (Lau et al. 1991; Yeh and Haertzen 1991; Nestby et al., 1997; Vanderschuren and Kalivas, 2000; Narendran and Martinez, 2008; Boileau et al., 2006). The inability to control these confounds is a principal reason that sensitization is often difficult to quantify, and sometimes even to demonstrate, in mammals. Early human sensitization studies were limited to uncontrolled observations and open trials in cocaine and amphetamine users who reported an increased tendency to develop psychosis over time despite using lower doses of the drugs (Satel et al., 1991; Brady et al., 1991). More recent clinical investigations have used multiple parameters (e.g. increased speech, increased eye blink, enhanced mood, enhanced energy, vital signs, pupil diameter, subjective effects, hormone levels, electroencephalographic recordings, dopamine transmission) to quantify sensitization (Rothman et al., 1994; Sax and Strakowski, 2001). Results from clinical trials have been predictably inconsistent as repeated psychostimulant exposure has been shown to cause tolerance (Narendran and Martinez, 2008) as well as persistent behavioral and neurochemical sensitization (Boileau et al., 2006).
It follows that a promising application of the present finding - identification of a sensitive and reproducible measure of behavioral sensitization and cross-sensitization in planarians - is to study aspects of drug-induced behavioral sensitization that are methodologically challenging to investigate in higher-order species. Planarians possess key substrates which have been identified as primary mediators of mammalian behavioral sensitization; display hyperactivity following acute psychostimulant exposure; and lack a blood-brain barrier (Rowlands and Pagán, 2008; Pagán et al. 2008; Palladini et al., 1996; Margotta et al., 1997). Studying behavioral sensitization in planarians offers the ability to: (1) measure behavioral sensitization with a simple biological parameter that is sensitive, reproducible, quantifiable, cost-effective, and time-effective; (2) minimize interpretive complications related to pharmacokinetic factors such as drug absorption, distribution, metabolism, elimination, and blood-brain-barrier passage; (3) precisely identify the drug concentration, as opposed to just dose, which is less amenable to rigorous quantitative analysis; (4) continuously expose the animal to an abused drug - by soaking it in a petri dish - a feat accomplished easily with planarians because of their aquatic nature, thus offering a distinct advantage over mammalian models that rely on multiple injections or mini-pumps to achieve constant drug concentrations; (5) reduce the impact of higher-order confounding influences, such as the effects of handling, familiarity of the testing environment, strain, stress from procedures, and anticipation of drug administration; (6) test cocaine, and most other abused drugs, in a variety of dosing schedules and drug exposure patterns due to the exceptional adaptability of the planarian model; (7) adapt the behavioral sensitization model to translational use, such as the preclinical testing and in vivo screening of very small amounts of novel synthetic compounds, which are available in limited quantity, for their potential as abuse-deterrent and CNS-active therapeutics; (8) study the effects of poly-drug exposure, particularly as related to screening compounds for their efficacy against adverse effects produced by simultaneous exposure to multiple abused drugs; and (9) reduce the use of mammals in research.
At present, drosophila is the major invertebrate used to investigate behavioral sensitization. Drosophila studies have been invaluable in the identification and characterization of genetic- and circadian-related mechanisms of cocaine-induced sensitization (Heberlein et al., 2009; Quinn et al., 1974; Griffith et al., 1993; McClung and Hirsh, 1998; Andretic et al., 1999; Dimitrijevic et al., 2004). Our results now suggest that planarians are capable of providing equally important insight into additional aspects of drug-induced sensitization that have not been addressed in existing models. Unlike drosophila, which has a relatively short life span of 2-3 weeks, planarians are practically immortal because they undergo continuous cellular proliferation as adults (Saló, 2006). This makes planarians easily adaptable to investigate one of sensitization's hallmark features, endurance, a phenomenon which can persist for up to a year following cessation of drug exposure in mammals. Drug administration is also much more convenient in planarians than in drosophila (McClung and Hirsh, 1998; Hatsukami et al., 1990). Due to their natural aquatic habitat, planarians can be simply soaked in a known concentration of drug whereas drosophila requires drug volatilization for effective delivery. This is a likely reason that drosophila sensitization studies have focused almost exclusively on cocaine. The ease with which drugs can be administered to planarians now offers the attractive opportunity to investigate behavioral sensitization produced by broad classes of abused drugs and different drug combinations.
It should be noted that our initial experiments showed that planarians exposed once to cocaine, and then challenged with cocaine 60 min later, did not display detectable behavioral sensitization. We conjectured the lack of pBS was due to one of two possibilities: a single cocaine exposure was insufficient to induce behavioral sensitization or the time interval between initial cocaine exposure and cocaine reintroduction was too short. We favored the latter explanation because a single cocaine administration produces behavioral sensitization in rodents and drosophila (Peris and Zahniser, 1987; Kalivas and Alesdatter, 1993; McClung and Hirsh, 1998; Post, 1980; Post et al., 1988). However, detectable sensitization in both species requires a cocaine withdrawal period of minimum length, as sensitization in drosophila is first observed following 6 h of cocaine withdrawal but is not detectable following only 3 h of abstinence (McClung and Hirsh, 1998). In the case in which planarians were exposed to cocaine once and then reintroduced to cocaine following more extended withdrawal intervals of two or six days, pBS was in fact detectable, a finding that is consistent with the persistent sensitization produced by cocaine in drosophila (up to 24 h) and mammals (months) (McClung and Hirsh, 1998; Vanderschuren and Kalivas, 2000; Robinson and Camp, 1987).
Three mechanisms may explain cocaine-induced pBS. One explanation is new gene expression induced by initial cocaine exposure. Behavioral sensitization in mammals and drosophila requires the new expression of genes, such as immediate early genes and genes encoding for tyrosine hydroxylase, dopamine D1 and D2 receptors, dynorphin, tyramine, and LIM-only proteins, following initial cocaine exposure (Vrana et al., 1993; Steiner and Gerfen, 1993; Bhat and Baraban, 1993; Unterwald et al., 1994; Andretic et al., 1999; Wolf, 1999; Heberlein et al., 2009). A second explanation is initial cocaine exposure activates signaling components downstream of dopamine receptors, such as the cyclic AMP cascade or extracellular signal-regulated kinase (ERK) pathway, or activates components of the glutamate system, such as NMDA and AMPA receptors (Vanderschuren and Kalivas, 2000). A third consideration is accumulation of cocaine or an active cocaine metabolite over time. We believe this possibility is unlikely because there are not any cocaine metabolites in vertebrates that are known to display greater activity than cocaine itself. Furthermore, if a metabolite were responsible for cocaine-induced sensitization in planarians, it would require a relatively slow metabolism of cocaine since our data indicated that pBS was only detectable following a minimum time interval between initial cocaine exposure and cocaine challenge.
Akin to cocaine, glutamate, produced hyperactivity following acute exposure, sensitization of hyperactivity following repeated and intermittent exposure, and detectable pBS following a single exposure. Given that cocaine and glutamate both produced pBS, we investigated the presence of cross-sensitization between the two substances. Cross-sensitization is a phenomenon in which the response of a new drug is enhanced by prior experience with a different drug (Lett, 1989). We found that prior exposure to glutamate enhanced hyperactivity produced by cocaine and vice versa. The identification of cross-sensitization between cocaine and glutamate in planarians implies a common underlying mechanism for the substances and is consistent with vertebrate studies showing that prior exposure to glutamate, or to agents that increase extracellular glutamate, enhance psychostimulant-induced behavioral responses (Vanderschuren and Kalivas, 2000; Dean et al., 1988; Mitchell et al., 1988; Aked et al., 2005).
One final point is that the acute and sensitized behavioral responses observed here displayed pharmacological selectivity. Glutamate and three different drugs – cocaine, glutamate, and caffeine –produced an increase in planarian hyperactivity following acute exposure, findings that are consistent with those shown in mammals (Hooks et al., 1991; Kaplan et al., 1992). In contrast, GABA, the principal inhibitory neurotransmitter in the mammalian brain that is also synthesized and utilized by planarians (Eriksson and Panula, 1994; Raffa et al., 2007, did not increase planarian hyperactivity. This finding was not entirely unexpected considering the well documented role of GABA systems, particularly those specific to the GABAB receptor, in suppressing several of cocaine's characteristic effects, including acute hyperactivity, sensitization of hyperactivity, self-administration, and mesolimbic dopamine release, in rodents (Kalivas et al., 1990; Fadda et al., 2003; Frankowska et al., 2009). Regarding sensitized responses, cocaine and glutamate both produced significant pBS whereas scopolamine and caffeine did not. In fact, some degree of tolerance to hyperactivity was actually observed for the highest concentration of scopolamine, a finding that has been demonstrated in rodents (Hooks et al., 1991). Planarians with prior caffeine experience did display enhanced hyperactivity to a challenge concentration of caffeine, but the effect did not reach statistical significance. The inability of caffeine to produce significant pBS at the concentrations used here is different from rodents and may be related to the caffeine concentrations tested or to variations in adenosine receptor profiles between the two species, particularly since A1 and A2 receptors may not be equally important to caffeine-induced behavioral sensitization in rodents (Kaplan et al., 1992; Hsu et al., 2009).
In conclusion, the present demonstration of drug-induced behavioral sensitization and cross-sensitization in planarians suggests fundamental pathways involved in cocaine-induced behavioral sensitization are conserved in these simple flatworms (Vezina, 2004). We propose planarians as a sensitive, translational in vivo system with broad applicability to investigate biological processes involved in behavioral sensitization produced by different classes of abused drugs and to screen small amounts of novel compounds in limited availability for their abuse-deterrent potential.
Acknowledgments
Funding Sources: National Institutes on Drug Abuse grants DA022694 (SMR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aked J, Coizet V, Clark D, Overton PG. Local injection of a glutamate uptake inhibitor into the ventral tegmental area produces sensitization to the behavioural effects of d-amphetamine. Neuroscience. 2005;134:361–367. doi: 10.1016/j.neuroscience.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285:1066–1068. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- Bell K, Kalivas PW. Context-specific cross-sensitization between systemic cocaine and intra-accumbens AMPA infusion in the rat. Psychopharmacology. 1996;127:377–383. doi: 10.1007/s002130050101. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Baraban JM. Activation of transcription factor genes in striatum by cocaine: role of both serotonin and dopamine systems. J Pharmacol Exp Ther. 1993;267:496–505. [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Brady KT, Lydiard RB, Malcolm R, Ballenger JC. Cocaine-induced psychosis. J Clin Psychiatry. 1991;52:509–512. [PubMed] [Google Scholar]
- Cretì P, Capasso A, Grasso M, Parisi E. Identification of a 5-HT1A receptor positively coupled to planarian adenylate cyclase. Cell Biol Int Rep. 1992;16:427–432. doi: 10.1016/s0309-1651(06)80062-7. [DOI] [PubMed] [Google Scholar]
- Dean P, Mitchell IJ, Redgrave P. Contralateral head movements produced by microinjection of glutamate into superior colliculus of rats: evidence for mediation by multiple output pathways. Neuroscience. 1988;24:491–500. doi: 10.1016/0306-4522(88)90344-2. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic N, Dzitoyeva S, Manev H. An automated assay of the behavioral effects of cocaine injections in adult Drosophila. J Neurosci Methods. 2004;137:181–184. doi: 10.1016/j.jneumeth.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Eriksson KS, Panula P. gamma-Aminobutyric acid in the nervous system of a planarian. J Comp Neurol. 1994;345:528–536. doi: 10.1002/cne.903450405. [DOI] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse. 2003;50:1–6. doi: 10.1002/syn.10238. [DOI] [PubMed] [Google Scholar]
- Frankowska M, Nowak E, Filip M. Effects of GABAB receptor agonists on cocaine hyperlocomotor and sensitizing effects in rats. Pharmacol Rep. 2009;61:1042–1049. doi: 10.1016/s1734-1140(09)70166-5. [DOI] [PubMed] [Google Scholar]
- Gambarana C, Ghiglieri O, Montis MG, Tagliamonte A. Under continuous dizocilpine infusion an N-methyl-D-aspartate receptor independent form of cocaine sensitization develops in rats. Behav Pharmacol. 1998;9:61–68. [PubMed] [Google Scholar]
- Griffith LC, Verselis LM, Aitken KM, Kyriacou CP, Danho W, Greenspan RJ. Inhibition of calcium/calmodulin-dependent protein kinase in Drosophila disrupts behavioral plasticity. Neuron. 1993;10:501–509. doi: 10.1016/0896-6273(93)90337-q. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Keenan R, Carroll M, Colon E, Geiske D, Wilson B, Huber M. A method for delivery of precise doses of smoked cocaine-base to humans. Pharmacol Biochem Behav. 1990;36:1–7. doi: 10.1016/0091-3057(90)90116-y. [DOI] [PubMed] [Google Scholar]
- Heberlein U, Tsai LT, Kapfhamer D, Lasek AW. Drosophila, a genetic model system to study cocaine-related behaviors: a review with focus on LIM-only proteins. Neuropharmacology. 2009;56 1:97–106. doi: 10.1016/j.neuropharm.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav. 1991;38:467–470. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Hsu CW, Chen CY, Wang CS, Chiu TH. Caffeine and a selective adenosine A2A receptor antagonist induce reward and sensitization behavior associated with increased phospho-Thr75-DARPP-32 in mice. Psychopharmacology. 2009;204:313–325. doi: 10.1007/s00213-009-1461-3. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Eberhardt H. Modulation of A10 dopamine neurons by gamma-aminobutyric acid agonists. J Pharmacol Exp Ther. 1990;253:858–866. [PubMed] [Google Scholar]
- Kalivas PW, Alesdatter JE. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;267:486–495. [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem. 1998;70:1497–1502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Greenblatt DJ, Kent MA, Cotreau MM, Arcelin G, Shader RI. Caffeine-induced behavioral stimulation is dose-dependent and associated with A1 adenosine receptor occupancy. Neuropsychopharmacology. 1992;6:145–153. [PubMed] [Google Scholar]
- Karler R, Calder LD, Chaudhry IA, Turkanis SA. Blockade of “reverse tolerance” to cocaine and amphetamine by MK-801. Life Sci. 1989;45:599–606. doi: 10.1016/0024-3205(89)90045-3. [DOI] [PubMed] [Google Scholar]
- Karler R, Calder LD, Bedingfield JB. Cocaine behavioral sensitization and the excitatory amino acids. Psychopharmacology. 1994;115:305–310. doi: 10.1007/BF02245070. [DOI] [PubMed] [Google Scholar]
- King GR, Joyner C, Lee T, Kuhn C, Ellinwood EH., Jr Intermittent and continuous cocaine administration: residual behavioral states during withdrawal. Pharmacol Biochem. 1992;43:243–248. doi: 10.1016/0091-3057(92)90664-2. [DOI] [PubMed] [Google Scholar]
- Lau CE, Imam A, Ma F, Falk JL. Acute effects of cocaine on spontaneous and discriminative motor functions: relation to route of administration and pharmacokinetics. J Pharmacol Exp Ther. 1991;257:444–456. [PubMed] [Google Scholar]
- Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 1989;98:357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- Li Y, Vartanian AJ, White FJ, Xue CJ, Wolf ME. Effects of the AMPA receptor antagonist NBQX on the development and expression of behavioral sensitization to cocaine and amphetamine. Psychopharmacology. 1997;134:266–276. doi: 10.1007/s002130050449. [DOI] [PubMed] [Google Scholar]
- Margotta V, Caronti B, Meco G, Merante A, Ruggieri S, Venturini G, Palladini G. Effects of cocaine treatment on the nervous system of planaria (Dugesia gonocephala s. l.). Histochemical and ultrastructural observations. Eur J Histochem. 1997;41:223–230. [PubMed] [Google Scholar]
- McClung C, Hirsh J. Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr Biol. 1998;8:109–112. doi: 10.1016/s0960-9822(98)70041-7. [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Redgrave P, Dean P. Plasticity of behavioural response to repeated injection of glutamate in cuneiform area of rat. Brain Res. 1988;460:394–397. doi: 10.1016/0006-8993(88)90389-7. [DOI] [PubMed] [Google Scholar]
- Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62:851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- Nestby P, Schotte A, Janssen PF, Tjon GH, Vanderschuren LJ, De Vries TJ, Mulder AH, Leysen JE, Schoffelmeer AN. Striatal dopamine receptors in rats displaying long-term behavioural sensitization to morphine. Synapse. 1997;27:262–265. doi: 10.1002/(SICI)1098-2396(199711)27:3<262::AID-SYN10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Sihra TS. Synaptosomes possess an exocytotic pool of glutamate. Nature. 1986;321:772–773. doi: 10.1038/321772a0. [DOI] [PubMed] [Google Scholar]
- Pagán OR, Rowlands AL, Azam M, Urban KR, Bidja AH, Roy DM, Feeney RB, Afshari LK. Reversal of cocaine-induced planarian behavior by parthenolide and related sesquiterpene lactones. Pharmacol Biochem Behav. 2008;89:160–70. doi: 10.1016/j.pbb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Palladini G, Ruggeri S, Stocchi F, De Pandis MF, Venturini G, Margotta V. A pharmacological study of cocaine activity in planaria. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;115:41–45. doi: 10.1016/s0742-8413(96)00053-9. [DOI] [PubMed] [Google Scholar]
- Peris J, Zahniser NR. One injection of cocaine produces a long-lasting increase in [3H]-dopamine release. Pharmacol Biochem Behav. 1987;27:533–535. doi: 10.1016/0091-3057(87)90361-3. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM. Intermittent versus continuous stimulation: effect of time interval on the development of sensitization or tolerance. Life Sci. 1980;26:1275–1282. doi: 10.1016/0024-3205(80)90085-5. [DOI] [PubMed] [Google Scholar]
- Post RM, Weiss SR, Pert A. Cocaine-induced behavioral sensitization and kindling: implications for the emergence of psychopathology and seizures. Ann N Y Acad Sci. 1988;537:292–308. doi: 10.1111/j.1749-6632.1988.tb42114.x. [DOI] [PubMed] [Google Scholar]
- Quinn WG, Harris WA, Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa RB, Valdez JM. Cocaine withdrawal in Planaria. Eur J Pharmacol. 2001;430:143–145. doi: 10.1016/s0014-2999(01)01358-9. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Stagliano GW, Ross G, Powell JA, Phillips AG, Ding Z, Rawls SM. The kappa-opioid receptor antagonist nor-BNI inhibits cocaine and amphetamine, but not cannabinoid (WIN 52212-2), abstinence-induced withdrawal in planarians: an instance of ‘pharmacologic congruence’. Brain Res. 2008;1193:51–56. doi: 10.1016/j.brainres.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Gomez T, Stagliano GW, Raffa RB. Measurement of glutamate and aspartate in Planaria. J Pharmacol Toxicol Methods. 2006;53:291–295. doi: 10.1016/j.vascn.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Gomez T, Raffa RB. An NMDA antagonist (LY 235959) attenuates abstinence-induced withdrawal of planarians following acute exposure to a cannabinoid agonist (WIN 55212-2) Pharmacol Biochem Behav. 2007;86:499–504. doi: 10.1016/j.pbb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Cavallo F, Capasso A, Ding Z, Raffa RB. The beta-lactam antibiotic ceftriaxone inhibits physical dependence and abstinence-induced withdrawal from cocaine, amphetamine, methamphetamine, and clorazepate in planarians. Eur J Pharmacol. 2008;584:278–284. doi: 10.1016/j.ejphar.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Reid MS, Berger SP. Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. Neuroreport. 1996;7:1325–1329. doi: 10.1097/00001756-199605170-00022. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DM. Long-lasting effects of escalating doses of d-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior and spontaneous nocturnal locomotion. Pharmacol Biochem Behav. 1987;26:821–827. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Gorelick DA, Baumann MH, Guo XY, Herning RI, Pickworth WB, Gendron TM, Koeppl B, Thomson LE, 3rd, Henningfield JE. Lack of evidence for context-dependent cocaine-induced sensitization in humans: preliminary studies. Pharmacol Biochem Behav. 1994;49:583–588. doi: 10.1016/0091-3057(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Rowlands AL, Pagán OR. Parthenolide prevents the expression of cocaine-induced withdrawal behavior in planarians. Eur J Pharmacol. 2008;583:170–172. doi: 10.1016/j.ejphar.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Saló E. The power of regeneration and the stem-cell kingdom: freshwater planarians (Platyhelminthes) Bioessays. 2006;28:546–559. doi: 10.1002/bies.20416. [DOI] [PubMed] [Google Scholar]
- Satel SL, Price LH, Palumbo JM, McDougle CJ, Krystal JH, Gawin F, Charney DS, Heninger GR, Kleber HD. Clinical phenomenology and neurobiology of cocaine abstinence: a prospective inpatient study. Am J Psychiatry. 1991;148:1712–1716. doi: 10.1176/ajp.148.12.1712. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM. Behavioral sensitization in humans. J Addict Dis. 2001;20:55–65. doi: 10.1300/J069v20n03_06. Review. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993;13:5066–5081. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda S, Stagliano GW, Borenstein MR, Raffa RB. A reverse-phase HPLC and fluorescence detection method for measurement of 5-hydroxytryptamine (serotonin) in Planaria. J Pharmacol Toxicol Methods. 2005;51:73–76. doi: 10.1016/j.vascn.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Ho A, Rubenfeld JM, Kreek MJ. Time course of the development of behavioral sensitization and dopamine receptor up-regulation during binge cocaine administration. J Pharmacol Exp Ther. 1994;270:1387–1396. [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. Review. [DOI] [PubMed] [Google Scholar]
- Venturini G, Stocchi F, Margotta V, Ruggieri S, Bravi D, Bellantuono P, Palladini G. A pharmacological study of dopaminergic receptors in planaria. Neuropharmacology. 1989;28:1377–1382. doi: 10.1016/0028-3908(89)90013-0. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. Review. [DOI] [PubMed] [Google Scholar]
- Vrana SL, Vrana KE, Koves TR, Smith JE, Dworkin SI. Chronic cocaine administration increases CNS tyrosine hydroxylase enzyme activity and mRNA levels and tryptophan hydroxylase enzyme activity levels. J Neurochem. 1993;61:2262–2268. doi: 10.1111/j.1471-4159.1993.tb07468.x. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Bonci A. Dose-dependent changes in the synaptic strength on dopamine neurons and locomotor activity after cocaine exposure. Synapse. 2008;62:790–795. doi: 10.1002/syn.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Jeziorski M. Coadministration of MK-801 with amphetamine, cocaine or morphine prevents rather than transiently masks the development of behavioral sensitization. Brain Res. 1993;613:291–294. doi: 10.1016/0006-8993(93)90913-8. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Cocaine addiction: clues from Drosophila on drugs. Curr Biol. 1999;9:R770–772. doi: 10.1016/S0960-9822(00)80009-3. [DOI] [PubMed] [Google Scholar]
- Yeh SY, Haertzen CA. Cocaine-induced locomotor activity in rats. Pharmacol Biochem Behav. 1991;39:723–727. doi: 10.1016/0091-3057(91)90154-t. [DOI] [PubMed] [Google Scholar]