Abstract
Background
With newborn screening (NBS) for cystic fibrosis (CF), eradication of Pseudomonas aeruginosa (PA) is possible if PA detection occurs early. A serological response to infection likely precedes culture positivity in CF patients, so PA serological testing is very appealing in this population. However, controversies continue to exist about serology testing, titer cutoffs for enzyme-linked immunosorbent assay (ELISA) antibody tests, and their value in children with CF.
Methods
This longitudinal, prospective study collected respiratory secretions as oropharyngeal swabs or expectorated sputum for culture and also sera over 6 years in 69 patients diagnosed by NBS. Serology assessed PA antibody titers against cell lysate, exotoxin A, and elastase. A novel statistical approach with weighted receiver operating characteristic (ROC) curves was used to determine best antibody titer cutoff values to predict subsequent PA positive cultures.
Results
Using weighted ROC curves, cell lysate was more sensitive than exotoxin A, which was more sensitive than elastase, but all age-specific cutoffs were better than fixed cutoffs previously used. Age-specific serological cutoffs both predict and detect PA respiratory infections with a higher sensitivity and specificity. Serological responses to the PA antigens determined that a response to cell lysate occurs significantly earlier than culture positivity.
Conclusions
Age-specific serological cutoffs rather than fixed values against common PA antigens improve early PA identification in infants and young children diagnosed with NBS. Regular serological assessment with age-specific cutoffs in these children appears to be a worthy diagnostic tool.
Keywords: Pseudomonas aeruginosa, cystic fibrosis, neonatal screening, serology, antibodies
Introduction
With advances in the diagnosis and treatment of patients with cystic fibrosis (CF) during the past decade, particularly widespread early identification through newborn screening (NBS) and improved recognition of the need to ascertain and eradicate Pseudomonas aeruginosa (PA), it has become clear that better methods of detecting PA-associated respiratory infections are sorely needed. Typically, infants diagnosed with CF by NBS are free of PA, whereas about 30% of those diagnosed by traditional methods following signs/symptoms of CF have already acquired the pathogen.1 “The potential to eradicate nonmucoid PA, and even to delay transformation to mucoid species, makes ascertainment of the initial PA infection one of the highest priorities in current clinical management,” as stated by Farrell and Govan.2 The controversy that currently exists is which PA identification method is most favorable in this patient population. Oropharyngeal (OP) swab culture in young CF children is the most widely-used test for PA detection, but studies have shown a variable and limited sensitivity for this screening.3–8 In 2001, Burns et al demonstrated that the identification of PA improved from 72.5% using cultures, obtained by bronchoalveolar lavage (BAL) and OP swab sampling, to 97.5% when combined with serological testing during the first three years of life in a cohort of 40 CF patients.9 The serological analysis performed was an immunoblot that detected antibodies against whole-cell proteins from PA.9
Previous studies have suggested that rising antibody titers to several PA antigens may occur before detection of the organism by culture of respiratory secretions.11–15 In a recent study, da Silva Filho et al10 performed a cross-sectional analysis in 87 CF patients (mean age 9.7 years) comparing three different methods of PA identification: microbiological culture; polymerase chain reaction (PCR) targeting the algD GDP mannose dehydrogenase gene of PA obtained from sputum or OP swab samples; and serum antibodies against three PA antigens, elastase, alkaline protease, and exotoxin A. Microbiological cultures were positive in samples from 42 patients (48.2%), PCR was positive in 53 (60.9%) patients, and serological testing was positive in 38 patients (43.6%).10 The difference among the three methods was not statistically significant, but the combination of PCR and PA serology was significantly superior to single methods, to PCR and microbiological cultures, and to PA serology and microbiological cultures.10 However, the sensitivity and specificity of PA serology and the optimal discriminating or cutoff values for serological values have not been adequately studied, especially in infants and young children with CF. Thus, we designed this analysis to address that gap in knowledge with a working hypothesis that serological cutoffs based on age are more useful in assessing PA infection as compared to a standard cutoff for all ages. Our goal is to identify PA respiratory infections in young children with CF as early as possible using serological values for diagnostic and prognostic purposes.
Methods
Characteristics of study patients
A total of 69 patients that were all diagnosed through NBS at the Madison and Milwaukee CF Centers were followed with characteristics of the study population summarized in Table 1. We included CF patients of the screened group who were assessed successfully with serial serology and had concurrent respiratory secretions cultures. Patients enrolled in this prospective study are described in detail elsewhere.14–15 Our screening project and the PA antibody titer evaluation study were approved by the institutional review boards (IRB’s) of the University of Wisconsin, the Medical College of Wisconsin, and the Children’s Hospital of Wisconsin. The subjects were enrolled with informed consent from their parents. The majority of the subjects had at least one F508del mutation (97%) and pancreatic insufficiency (90%). The population comprised of slightly more males (59%) than females.
Table 1.
Characteristics of study patients diagnosed with CF through NBS (N=69).
| Characteristics | Class | No. (%) |
|---|---|---|
| Center | Madison | 36 (52) |
| Milwaukee | 33 (48) | |
| Sex | Male | 41 (59) |
| Female | 28 (41) | |
| Genotype | ΔF508/ΔF508 | 39 (56) |
| ΔF508/Other | 28 (41) | |
| Other/Other | 2 (3) | |
| Pancreatic status* | PS | 7 (10) |
| PI | 62 (90) | |
| Meconium Ileus | Yes | 13 (19) |
| No | 56 (81) |
Pancreatic status was determined by either 3 day fat absorption studies or clinical/nutritional indicators of intestinal malabsorption.21
Respiratory secretion cultures and serological assay techniques
Samples of respiratory secretions were obtained by vigorous OP swabs during induced coughing or as expectorated sputum. They were cultured and PA identified (when present) every 6 months as part of our evaluation protocol and whenever patients showed increased cough or other signs of pulmonary exacerbations; thus, the average interval between cultures was about 3 months. These specimens were cultured and serological testing was performed every 6 months or at the discretion of the physician as described previously.14–15 Quantitation of anti-PA antibodies was performed on a total of 2134 specimens using enzyme-linked immunosorbent assay (ELISA) to assess IgG, IgA, and IgM antibody titers. A rise in the PA antibody titers, expressed herein primarily as dilutions (e.g., 1:256=8) was indicative of an infection. The antigens used for the ELISA were a cell lysate derived from PA01, purified exotoxin A (from List Biological Laboratories, Inc., Campbell, CA), and elastase toxoid prepared from purified elastase (Nagase and Co., Ltd, Tokyo, Japan). Two-fold dilutions of sera from an adult male without CF (namely co-author PMF, a physician with 30 years of exposure to PA through pediatric pulmonology practice) were included on each plate as a positive control. In addition, sera from CF patients known to have severe PA infections were also used to develop these analytical methods. Full details of the analytical methods are more completely described West et al14.
Statistical methods
Diagnostic values were calculated as illustrated in Table 2. We examined the cultures at the time of the first positive PA culture from birth to 6 years of age or the last cultures from birth to 6 years of age if there was no positive PA culture during this timeframe. These cultures are examined with the concurrent PA serology. The ability of antibody PA titers to discriminate between culture positive and negative was estimated using the receiver operating characteristic (ROC) curve, which is a graphical plot of the sensitivity vs. (1 - specificity) for a binary classifier system as its discrimination threshold varies. The best cutoffs for antibody titers were defined as the point on the ROC curve closest to the upper left corner, with a weighting technique used based on the following considerations. Since in this longitudinal analysis, we could only obtain OP cultures from very young patients and since they have a low sensitivity (44% for age ≤ 1.5 years, 68% for age > 1.5 years) but high specificity (95% for age ≤ 1.5 years and 94% for age > 1.5 years) compared to BAL culture,4 we give a weight of 0.44 for age ≤ 1.5 years and 0.68 for age > 1.5 years for sensitivity of OP culture and 0.95 for age ≤ 1.5 years and 0.94 for age > 1.5 years for specificity of OP culture in our ROC calculation.
Table 2.
Definition of sensitivity, specificity, PPV, and NPV*
| Have first positive culture up to age 6 | ||
|---|---|---|
| Yes | No | |
| Titer | ||
| Positive | a | b |
| Negative | c | d |
Sensitivity=a/(a+c), specificity=d/(b+d), positive predictive value=a/(a+b), negative predictive value=d/(c+d)
Previous studies have demonstrated distinct PA strains when collected simultaneously from the upper and lower airways, suggesting that different strains colonize different anatomic sites in the CF airway.7, 9 Many experts feel that sputum samples are more representative of the lower airway flora as discussed in a review by Gibson et al16. Jung and colleagues7 demonstrated that sputum samples were of equal value as BAL specimens in detecting lower airway PA colonization. Based on this medical literature, we felt that sputum samples were an accurate indicator of lower airway microbiology and the preferred source of airway secretion for management of CF lung disease, so we assigned a weight 1 for sensitivity of sputum and 1 for specificity of sputum culture. This assignment was based on the fact that respiratory culture sampling is difficult for younger patients that do not expectorate sputum with two options available, OP culture or BAL specimens. BAL is a more sensitive measure of PA infection in younger patients and is considered the gold standard. For older patients, sputum cultures are sensitive and specific, as shown in Henig et al,17 so we used this as a standard for our analysis.
To construct a ROC for each age interval, a logistic regression model was used. The culture positivity and negativity was used as the response variable and concurrent antibody titer was used as the predictor. The OUTROC option was used to output the data to make the ROC. There was some slight deviation of culture and serum collections that were scheduled every 6 months, so a window time of ± 3 months was assigned for each collection time.
To determine if the best cutoff values vary over different ages, we examined ROC in each year from birth to year 6, when 80% of the patients had developed positive PA, as defined by positive culture and positive serology as shown by Li et al15. The best cutoff for each antigen for the different ages from year 1 to 6 was determined by a ROC. When calculating the best cutoff, we used the culture status of year 1 and compared this to serological status at year 1 and so forth for years 2 through 6. When examining the diagnostic value of the best cutoffs of serology to detect the first positive PA culture, we compare cultures at the time of the first positive culture or last culture from birth to age 6 years with the concurrent serology. Culture positivity refers to the first positive culture.
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated from a two-way contingency table (Table 2) for serological testing compared to first positive culture up to 6 years of age. Sensitivity is the probability that a patient who is culture positive shows detectable antibodies against PA, and specificity is the probability that a patient who is culture negative shows no detectable antibodies against PA. The PPV is the probability that a patient with positive antibodies against PA is culture positive; the NPV is the probability that a patient with negative antibodies against PA is culture negative; both PPV and NPV take the prevalence of PA into account.
In this study, we calculated the sensitivity, specificity, PPV, and NPV of current antibody titers to identify the first positive culture status. We used age-specific best serological cutoffs as shown in Table 3, e.g. we used 6, 10, 11, 11, 11,11 as cutoffs for year 1, 2, 3, 4, 5, 6, respectively for lysate. We also used the minimum cutoff from age 1 to 6 years as shown in Table 3, e.g., we used 6 for lysate. Finally, we compared using a fixed cutoff value of 8 as used in the West et al14 paper. Calculation of 95% confidence intervals (CI) was performed using a binomial exact method.
Table 3.
The best cutoff values* at each age from weighted ROC analyses using current cell lysate, exotoxin A, and elastase titer to predict concurrent PA status from culture.
| Year 1 [0–1.25 year) | Year 2 [1.25–2.25 years) | Year 3 [2.25–3.25 years) | Year 4 [3.25–4.25 years) | Year 5 [4.25–5.25 years) | Year 6 [5.25–6.25 years) | |
|---|---|---|---|---|---|---|
| Cell lysate | ||||||
| Best cutoff | 6 (1:64) | 10 (1:1,024) | 11 (1:2,048) | 11(1:2,048) | 11(1:2,048) | 11(1:2,048) |
| Sensitivity, % | 73 | 67 | 67 | 67 | 76 | 75 |
| Specificity, % | 50 | 86 | 87 | 72 | 68 | 76 |
| Exotoxin A | ||||||
| Best cutoff | 4 (1:16) | 9 (1:512) | 10 (1:1,024) | 11 (1:2,048) | 11 (1:2,048) | 11(1:2,048) |
| Sensitivity, % | 67 | 75 | 71 | 57 | 62 | 64 |
| Specificity, % | 58 | 77 | 73 | 72 | 72 | 79 |
| Elastase | ||||||
| Best cutoff | 4 (1:16) | 6 (1:64) | 8 (1:256) | 9 (1:512) | 9 (1:512) | 8 (1:256) |
| Sensitivity, % | 27 | 67 | 65 | 57 | 57 | 68 |
| Specificity, % | 84 | 77 | 89 | 85 | 84 | 73 |
| N of culture | 187 | 125 | 112 | 109 | 103 | 105 |
| N of OP | 155 | 93 | 85 | 76 | 72 | 71 |
| PA+, N (%) | 21 (11) | 22 (18) | 28 (25) | 27 (25) | 29 (28) | 30 (29) |
Best cutoff is defined as the value with the highest combination of sensitivity and specificity.
Paired logrank test was used to compare the timing of culture and cell lysate in detecting PA. SAS, version 8.00 (SAS Institute Inc., Cary NC) and S-Plus, version 3.4 (Mathsoft Inc., Seattle WA) software packages were used for statistical analyses.
RESULTS
Diagnostic value of variation in serological cutoffs
Without a gold standard and with inconsistency of OP swab cultures,2 a weighted ROC was used for the first time to determine the best serological cutoff values for ELISA antibody titers for cell lysate, exotoxin A, and elastase to predict PA positivity in cultures. The area under the curve (AUC) was calculated as shown in Fig 1, but there is no significant difference among three antigens. Using these weighted ROC results, Table 3 illustrates that variable but best serological cutoff values associated with concurrent PA culture-positivity increase the sensitivity and specificity for each antigen. The sensitivity and specificity of the best serological cutoff levels were slightly lower during Year 1 for each antigen but improved for subsequent years. ROC curves were constructed for serological cutoffs for the three PA antigens for specific ages. Age-adjusted serological cutoff values were different for children in year 1 and 2 with no large difference for the other age groups. There were more OP cultures in year 1 (83%) than year 2 (74%) with OP cultures being used in weighting technique, so weighting technique may affect this difference. The proportion of OP cultures and sputum cultures for all ages are provided in the Tables. The total number of OP and sputum cultures is provided in the tables.
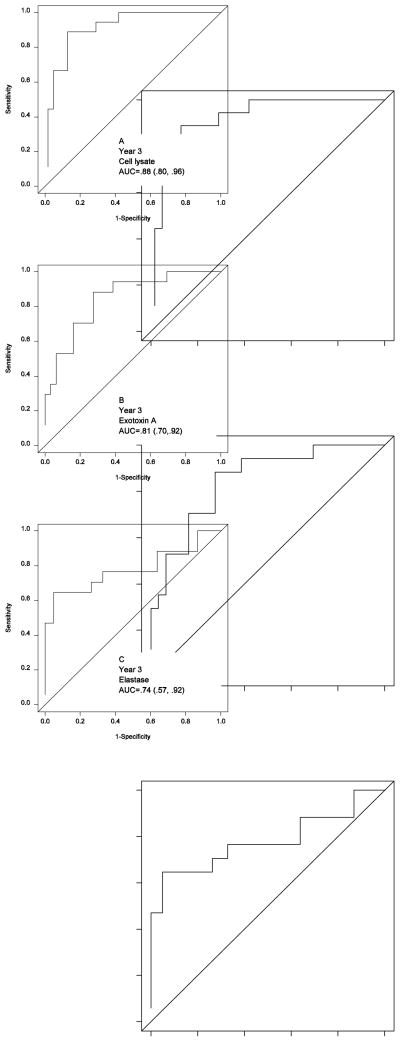
Figure 1.
ROC curves for cell lysate (A), exotoxin A (B), and elastase (C) antibody titers to discriminate PA infection using culture as the standard at the age of year 3. Since OP cultures obtained in early childhood of CF patients have varied sensitivity and specificity compared to bronchoalveolar lavage culture4, we give varied weight if OP culture is used in our ROC curve calculations.
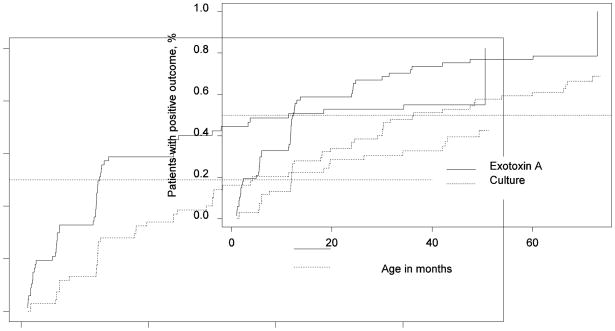
Fig 1 is representative of the ROC curves from the cohort with age-specific serological cutoffs for Year 3. There were slight differences in the area under of the curves among the three antigenic antibody responses, suggesting a trend. Cell lysate was more sensitive than exotoxin A, which was more sensitive than elastase; however, these differences did not meet statistical significance using 95% CI. Fig 2 is a timing graph comparing respiratory culture and exotoxin A antibody results using age-specific cutoffs from the analysis of this data. Fig 2 further reiterates the fact that a serological response occurs well before a positive culture, the median time for identification of exotoxin A antibodies was 1.02 years compared to the median time for culture being 3.2 years (p=004), paired logrank test.
Figure 2.
Timing graph comparing culture and concurrent exotoxin A titer using age-specific serological cutoffs. The median time of positive culture is 3.02 years with 95% CI of 2.04–5.52, the median time of positive exotoxin A titer is 1.02 years with 95% CI of 0.98–2.01years (P=.004 by paired logrank test).
Prognostic value of serological results
Table 3 illustrates the variation in the best cutoff serological values over time from weighted ROC using current cell lysate, exotoxin A, and elastase titer to identify PA status from culture. Table 4 demonstrates the assessment of minimum serological cutoff values used for the three PA antigens to predict the first PA isolate on culture from diagnosis to age 6 years. The PPV and NPV values for cell lysate and exotoxin values were similar and were slightly higher than elastase. Focusing on the sensitivity of these three antigens, cell lysate had the highest followed by exotoxin A and then elastase. Specificity of the antigens was found to be indirectly correlated with sensitivity. Table 4 also illustrates the antibody titer values using age-specific cutoffs as shown in Table 3 for cell lysate, exotoxin A, and elastase. In addition, Table 4 uses a single, specific cutoff of 8 (1:256) as reported in West et al12 to predict first PA positive culture from diagnosis to age 6 years when patients developed their first PA positive culture. Using age-specific serological cutoffs, our analysis found a decrease in sensitivity for cell lysate and exotoxin and an increased for elastase with an associated increase in specificity, PPV, and NPV. With variable antibody responses to the three PA antigens during the study at different time periods, serological analysis with multiple, individual PA antigens appear to be the optimal way to optimize sensitivity and specificity.
Table 4.
Using the current antibody titers with different cutoff values to predict first PA+ in young children with CF from diagnosis to age 6 years when 44/69 (64%) of the patients have their first PA positive culture.
| Cell lysate | Exotoxin A | Elastase | |
|---|---|---|---|
| Using minimum cutoffs (6 (1:64) for lysate, 4 (1:16) for exotoxin A, and 4 (1:16) for elastase) | |||
| Sensitivity, % (95% CI) | 91 (70, 99) | 84 (62, 97) | 43 (22, 66) |
| Specificity, % (95% CI) | 9 (1, 32) | 24 (9, 49) | 38 (19,64) |
| Positive predictive value, % (95% CI) | 51 (34, 68) | 53 (35, 71) | 41 (22, 66) |
| Negative predictive value, % (95% CI) | 52 (7, 93) | 60 (24,91) | 39 (19, 64) |
| Using age-specific cutoffs as shown in Table 3 for cell lysate, exotoxin A, and elastase | |||
| Sensitivity, % (95% CI) | 52 (27, 73) | 49 (27, 73) | 36 (15, 59) |
| Specificity, % (95% CI) | 90 (68, 99) | 90 (68, 99) | 81 (56, 94) |
| Positive predictive value, % (95% CI) | 84 (52, 98) | 84 (52, 98) | 65 (31, 89) |
| Negative predictive value, % (95% CI) | 65 (44, 81) | 64 (44, 81) | 55 (36, 74) |
| Using a cutoff of 8 (1:256) as in West et al 12 paper | |||
| Sensitivity, % (95% CI) | 73 (48, 89) | 63 (38, 82) | 20 (6, 44) |
| Specificity, % (95% CI) | 43 (23, 68) | 57 (32, 77) | 81 (56, 94) |
| Positive predictive value, % (95% CI) | 56 (37, 77) | 60 (36, 79) | 51 (16, 84) |
| Negative predictive value, % (95% CI) | 61 (32, 84) | 60 (34, 80) | 50 (32, 68) |
Finally, with regard to predicting the occurrence of PA positivity, we examined serologic titers before the cultures were positive. More specifically, assessed 44 culture positive and 25 culture negative patients with regard to exotoxin A titers, and analyzed the serological data 6 months before the cultures showed PA for the first time. Using the age-specific cutoff values, we found that those with high titers and PA positivity (44% concordance) were significantly different than the culture negative patients with low titers (89% concordance; P=.018 by chi square test). Thus, PA serology has some predictive capability 6 months ahead of the culture results. This implies potential prognostic value, as well as diagnostic value.
DISCUSSION
The routine use of PA serology in young patients CF remains limited and somewhat controversial in part due to the lack of the availability of a standardized commercial ELISA test, which has constrained clinical research on this topic. The issues related to PA serological testing in young children with CF were reviewed by Farrell and Govan8 in a discussion of two reports addressing PA serology in children with CF. These contrasting studies by Kappler et al18 and Tramper-Stranders et al19 used the same commercially available ELISA test that measured alkaline protease, elastase, and exotoxin A; however, different serological cutoff values were used to define a positive antibody titer. Kappler et al18 used the manufacturer’s recommended cutoff value defining a positive titer as > 1:500; whereas, Tramper-Stranders et al19 used ROC determined cutoff values. With limited studies addressing this very important issue in CF, another recent paper by Ratjen et al20 found that the sensitivity values were highest for alkaline protease at 85%, followed by elastase, and then exotoxin A; however, combining the three ELISA’s improved sensitivity further.
A very relevant problem in younger CF patients is the assessment of these patients by OP swabs rather than sputum or BAL, with the latter being considered the gold standard by most experts. In addition to commonly assessing this patient population with less than optimal culture techniques, there is a continuum of PA colonization and infection in CF that is difficult to differentiate, especially in the initial periods of PA isolation by culture.15–16 Serological antibody titers predict isolation of PA on culture, as much as 12 months before bacteriologic isolation as previously described.14–15 Our findings reiterate that once again, with a significant rise in cell lysate titers well before identification of PA on culture (Fig 2).
Kappler et al18, studying a mixed population of CF patients with regard to age, used the threshold recommended by the manufacturer and demonstrated that the combination of three antibody titers with a >1:500 serological cutoff yielded the best results with a sensitivity of 86%, specificity of 96%, and a positive predictive value of 97%; they also found even higher values in CF patients for whom sputum cultures were available (sensitivity 95%, specificity 100%, and positive predictive value 100%). Based on their findings, the authors concluded a rising antibody titer indicates probable PA infection with recommendations of PA eradication treatment even in the absence of a positive microbiological culture.18
On the other hand, with the use of ROC curves to create serological cutoff values, Tramper-Stranders et al16 found a sensitivity of 96% and specificity of 79%. The sensitivity of individual antibody tests was 87% for elastase, 79% for exotoxin A, and 76% for alkaline protease.19 In contrast to our results, the first positive microbiological culture for PA was preceded by positive serology in only 5 of 13 patients (38%) in their prospective study. Therefore, their conclusion was that serological testing is sensitive for identification of chronic PA but fails to detect early colonization in young CF patients thus reliance upon microbiological cultures continues.19
Our study further addresses the very important question of the potential value of serological testing for PA in CF patients in early childhood and adds information to the recent studies demonstrated conflicting findings. Due to OP swab culture variations and imperfect sensitivity, weighted ROC curves were used for the first time in our study. Tramper-Stranders et al19 adjusted serological cutoff values extrapolated from ROC curves but did not use age-specific cutoff values. Ratjen et al20 also used ROC curves but not in a weighted or age-specific fashion. In our study, adjusting cutoff values extrapolated from weighted ROC curves along with age-specific cutoffs clearly revealed that serological tests against common PA antigens was better at identifying concurrent and future PA respiratory infections than a single cutoff value across all ages. These results suggest that the regular determination of multiple serum antibodies against PA in infants and young patients with CF is reliable when age-specific serological cutoffs are used.
However, there are some limitations in this investigation such as relatively small study population (N=69), our reliance on OP cultures at approximately 3 month intervals, and the imperfect specificity of the antigens used to detect immunologic responses to PA. These age-specific serological cutoff values also allowed for the detection of rising serological titers in this population before the isolation of PA on OP swab cultures with cell lysate being the most significant. West et al14 demonstrated in their publication that exotoxin A and cell lysate assays were similar with detection by cell lysate occurring earlier at a mean of 11.9 months before the first isolation of PA compared to a mean of 5.6 months for exotoxin A; elastase antibodies always appeared much later at a mean of 41.1 months after the first isolate of PA and was associated with disease progression, including cough and worsening chest x-ray scores. Subsequently, Li et al15 found that 92% of patients with CF had mucoid PA strains but that a median period of 10.9 years exists from initial PA acquisition as a “window of opportunity” for eradication. With our combined findings, we conclude that PA serology would thus be useful clinically for diagnosing PA early on before PA isolation by culture. With serological diagnostic capability, PA eradication therapy could be optimized by treatment before culture isolation. Another potential use of PA serology could include monitoring of PA infection for those patients already colonized with PA as an objective measurement to determine when treatment is indicated. Further comprehensive research, however, will be needed to improve microbiological and serological techniques to optimize patient care in the young CF patient. The development of a commercially available panel of PA antigens with clinically significant virulence factors needs to occur. We believe that further research and development of PA serology will facilitate achievement of the important goal of identifying PA respiratory infections in young children with CF as early as possible.
Acknowledgments
We thank the other investigators who have participated in the Wisconsin Cystic Fibrosis Neonatal Screening Project, its longtime coordinator, Anita Laxova, and Jeffrey P. Davis, MD, of the University of Wisconsin for advice on experimental design and interpretation of early results.
This study was approved by the institutional review boards of all participating centers, and informed consent was received from the parents of the participating patients.
Financial support was provided by the National Institutes of Health (grant NIDDK 5 R01 DK34108-17) with the work primarily done at the University of Wisconsin School of Medicine and Public Health.
Footnotes
The authors report that there is no potential conflict of interest.
References
- 1.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122:1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 2.Farrell PM, Govan JR. Pseudomonas serology: confusion, controversy, and challenges. Thorax. 2006;61:645–647. doi: 10.1136/thx.2006.062612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tramper-Stranders GA, van der Ent CK, Wolfs TF. Detection of Pseudomonas aeruginosa in patients with cystic fibrosis. J Cyst Fibros. 2005;4:37–43. doi: 10.1016/j.jcf.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey BW, Wentz KR, Smith AL, Richardson M, Williams-Warren J, Hedges DL, Gibson R, Redding GJ, Lent K, Harris K. Predictive value of oropharyngeal cultures for identifying lower airway bacteria in cystic fibrosis patients. Am Rev Respir Dis. 1991;144:331–337. doi: 10.1164/ajrccm/144.2.331. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DS, Grimwood K, Carlin JB, Carzino R, Olinsky A, Phelan PD. Bronchoalveolar lavage or oropharyngeal cultures to identify lower respiratory pathogens in infants with cystic fibrosis. Pediatr Pulmonol. 1996;21:267–275. doi: 10.1002/(SICI)1099-0496(199605)21:5<267::AID-PPUL1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld M, Emerson J, Accurso F, Armstrong D, Castile R, Grimwood K, Hiatt P, McCoy K, McNamara S, Ramsey B, Wagener J. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol. 1999;28:321–328. doi: 10.1002/(sici)1099-0496(199911)28:5<321::aid-ppul3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Jung A, Kleinau I, Schönian G, Bauernfeind A, Chen C, Griese M, Döring G, Göbel U, Wahn U, Paul K. Sequential genotyping of Pseudomonas aeruginosa from upper and lower airways of cystic fibrosis patients. Eur Respir J. 2002;20:1457–1463. doi: 10.1183/09031936.02.00268002. [DOI] [PubMed] [Google Scholar]
- 8.Kabra SK, Alok A, Kapil A, Aggarwal G, Kabra M, Lodha R, Pandey RM, Sridevi K, Mathews J. Can throat swab after physiotherapy replace sputum for identification of microbial pathogens in children with cystic fibrosis? Indian J Pediatr. 2004;71:21–23. doi: 10.1007/BF02725650. [DOI] [PubMed] [Google Scholar]
- 9.Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, Rosenfeld M, Hiatt P, McCoy K, Castile R, Smith AL, Ramsey BW. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis. 2001;183:444–452. doi: 10.1086/318075. [DOI] [PubMed] [Google Scholar]
- 10.da Silva Filho LV, Tateno AF, Martins KM, Azzuz Chernishev AC, Garcia Dde O, Haug M, Meisner C, Rodrigues JC, Döring G. The combination of PCR and serology increases the diagnosis of Pseudomonas aeruginosa colonization/infection in cystic fibrosis. Pediatr Pulmonol. 2007;42:938–944. doi: 10.1002/ppul.20686. [DOI] [PubMed] [Google Scholar]
- 11.Pressler T, Pedersen SS, Espersen F, Høiby N, Koch C. IgG subclass antibodies to Pseudomonas aeruginosa in sera from patients with chronic Ps. aeruginosa infection investigated by ELISA. Clin Exp Immunol. 1990;81:428–434. doi: 10.1111/j.1365-2249.1990.tb05351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brett MM, Ghoneim AT, Littlewood JM. Serum antibodies to Pseudomonas aeruginosa in cystic fibrosis. Arch Dis Child. 1986;61:1114–1120. doi: 10.1136/adc.61.11.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brett MM, Ghoneim AT, Littlewood JM. Serum IgG antibodies in patients with cystic fibrosis with early Pseudomonas aeruginosa infection. Arch Dis Child. 1987;62:357–361. doi: 10.1136/adc.62.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West SE, Zeng L, Lee BL, Kosorok MR, Laxova A, Rock MJ, Splaingard MJ, Farrell PM. Respiratory infections with Pseudomonas aeruginosa in children with cystic fibrosis: early detection by serology and assessment of risk factors. JAMA. 2002;287:2958–2967. doi: 10.1001/jama.287.22.2958. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Kosorok MR, Farrell PM, Laxova A, West SE, Green CG, Collins J, Rock MJ, Splaingard ML. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293:581–588. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 16.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 17.Henig NR, Tonelli MR, Pier MV, Burns JL, Aitken ML. Sputum induction as a research tool for sampling the airways of subjects with cystic fibrosis. Thorax. 2001;56:306–311. doi: 10.1136/thorax.56.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kappler M, Kraxner A, Reinhardt D, Ganster B, Griese M, Lang T. Diagnostic and prognostic value of serum antibodies against Pseudomonas aeruginosa in cystic fibrosis. Thorax. 2006;61:684–688. doi: 10.1136/thx.2005.049536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tramper-Stranders GA, van der Ent CK, Slieker MG, Terheggen-Lagro SW, Teding van Berkhout F, Kimpen JL, Wolfs TF. Diagnostic value of serological tests against Pseudomonas aeruginosa in a large cystic fibrosis population. Thorax. 2006;61:689–693. doi: 10.1136/thx.2005.054726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratjen F, Walter H, Haug M, Meisner C, Grasemann H, Döring G. Diagnostic value of serum antibodies in early Pseudomonas aeruginosa infection in cystic fibrosis patients. Pediatr Pulmonol. 2007;42:249–255. doi: 10.1002/ppul.20562. [DOI] [PubMed] [Google Scholar]
- 21.Farrell PM, Kosorok MR, Laxova A, Shen G, Koscik RE, Bruns T, Splaingard M, Mischler EH. Nutritional benefits of newborn screening for cystic fibrosis. NEJM. 1997;337:963–969. doi: 10.1056/NEJM199710023371403. [DOI] [PubMed] [Google Scholar]