Abstract
A novel excite-coupled Trapping Ring Electrode Cell (eTREC) was designed and developed. eTREC technology provides greater linearity in the excitation electric field along with minimized variation in radial trapping field during detection. The variation in the radial trapping electric field is reduced through postexcitation modulation of the trapping potentials applied to the Trapping Ring Electrode Cell (TREC). Linearization of the electric field generated during radio frequency (RF) excitation is accomplished by coupling the RF excitation to a novel electrode arrangement superimposed onto the trapping rings of a TREC. The coupling of RF excitation to the trap plates effectively reduces z-axis ejection and allows for a more uniform postexcitation radius for the entire ion population. Using this technology, sensitivity was increased by >50%, resolution of 13C2 and 34S fine structure peaks was achieved with the peptide MMMMG (∼330 000 RP) on a 3 T system, and the limit of detection was significantly reduced.
Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FTICR-MS)1 is a high-performance technique capable of simultaneous high resolution, high mass measurement accuracy, wide dynamic range, and high sensitivity measurements.2–5 FT-ICR-MS continues to be used in many fields to solve difficult problems. Analysis of complex samples acquired from the fields of proteomics,6,7 metabolomics,8,9 petroleomics,10,11 and others require instrumentation of the highest performance to extract the maximum amount of information possible. Few instruments meet the performance demands imposed by the aforementioned fields of interest; however, FT-ICR-MS has been routinely applied in these areas due to its extraordinary performance criteria.
In spite of its outstanding attributes, FT-ICR-MS technology requires further development to realize its fullest potential. Performance limitations such as radial electric field variation in the cell and mass discrimination during ion transmission are still areas of FT-ICR-MS technology which require improvement. The most recent efforts from our laboratory to improve FT-ICR-MS performance have been focused on ion transmission12 and ion detection.13 The data presented in this manuscript has a focus on ion detection. The heart of FT-ICR-MS is the cell which is used for confinement and m/z analysis. Fine details of cell operation and the many incarnations of the ICR cell3,13–21 are the focus of review articles.22,23 Ion confinement in all cells is achieved through the application of an axial electric field and a uniform magnetic field. Ions that reach the cell and are trapped have a distribution or spread in trapped ion kinetic energy. A spread in trapped ion kinetic energy leads to a spread in the extent of z-axis oscillation within the cell. The spread in trapped ion kinetic energy is present before and after ion excitation and during the detection process. Radial electric field varies along the z-axis at the postexcitation cyclotron radius (rc) even in cells developed to have quadrupolar or harmonic trapping potentials (ideal electric field environment). Therefore, ions of the same m/z, but different z-axis kinetic energy, experience a distribution in radial electric field during ion detection. Radial electric field magnitude defines magnetron frequency. Ions of the same m/z, but different z-axis kinetic energy, will have a distribution in magnetron frequencies which arises from the variation in radial field.24 The detected or observed ion cyclotron frequency (ωo) is related to the “unperturbed” cyclotron frequency (ω+) and magnetron frequency (ω−) through the following expression:23
| (1) |
Contribution of magnetron frequency to the observed frequency is orders of magnitude smaller than the cyclotron frequency for the m/z regime most commonly analyzed; however, relatively small frequency differences result in rapid ion cloud dephasing or distorted peak shape. The excitation event defines the initial phase coherence of cyclotron motion. Therefore, ions of the same m/z begin in phase, but due to slight differences in magnetron frequency, ions lose phase coherence, and consequently, signal loss is observed.25 There are other mechanisms for signal loss in ICR experiments such as collisional damping; however, these contributions are normally minimized by operation at low background pressure.
Our studies of electron promoted ion coherence (EPIC)24–26 showed that radial electric fields are very important for control of ion cloud dephasing and, thus, can strongly affect instrument performance. The effect of radial electric field variation on cell performance was also observed by Kim et al. upon inversion of the sidekick potential of an Infinity cell during detection.27 Further investigation into the radial field effects on trapped ion kinetic energy in an Infinity cell have indicated that the extent of oscillation has a profound impact on observed frequency. Specifically, ion populations of the same m/z with different pre-excitation z-axis kinetic energy24 are observed at different cyclotron frequencies. The difference in observed frequency between “hot” and “cool” ion populations of the same m/z was eliminated through the application of EPIC, which modulates the radial fields during ion detection. Development of a cell with the capability to minimize radial electric field variation at the cyclotron radius (rc) has provided further support of the hypothesis that radial field variation along the z-axis at the postexcitation cyclotron radius dramatically influences instrument performance.13 Using Trapping Ring Electrode Cell (TREC), we observed a 10-fold gain in signal intensity, decreased ion frequency drift, and resolving power near the theoretical limit. Although our experimentation with TREC supported the radial field variation hypothesis, no method for correcting nonideal excitation fields was incorporated in this design.
The coupling of radio frequency (RF) excitation to the trapping electrode surfaces of the ICR cell has been demonstrated by multiple researchers and has been integrated into commercial instruments.14,17,28,29 The motive for incorporating excitation coupling is to correct for nonideality in the electric field present during the excitation event. The finite geometry of the closed cell design causes deviation in excite potentials at the extremes of the cell (near the end-cap electrodes, or near the edge of the excite/detect electrodes). This deviation results in a component of the electric field directed axially and, thus, provides an undesirable excitation of the z-axis oscillation of the ion cloud.30 Excitation of the z-axis motion can cause ejection from the cell and/or provide a poorly defined cyclotron radius (increase in z–x spread). This phenomenon has long been recognized and surmounted in a variety of ways.14,17,29,31 The Infinity cell17 is a primary example of how z-axis ejection can be surmounted through shimming the applied RF potential; however, the Infinity cell lacks the ability to optimize the trapping field during detection.
An ideal dipolar excitation field is produced in the limit of infinite cell length. In the plane which bisects the excitation electrodes axially, the shape of this field is linear as a function of increasing radius without regard to z-displacement. An important consequence of finite excitation electrodes in all cell designs is that they effectively short the RF potential applied to ground at their boundaries. The result is that the excitation field changes greatly as a function of z-displacement in uncoupled cell designs. In the limit of infinite cell radius the trapping radial electric field variation along the z-axis at any cyclotron radius would be zero. Another important consequence of finite trapping electrodes is nonzero radial electric field at all radii other than r = 0. Virtually all cell designs to date have been crafted such that excitation field or detection field have been optimized, or a compromise has been made between the two. The ability to correct for nonideal electric fields present during excitation and minimize radial field variation during detection has not been demonstrated with any cell technology. As we and others have shown, radial fields hinder the performance achievable by causing ion dephasing to occur more rapidly. Simulations indicate eTREC technology permits ion excitation to uniform, unstratified cyclotron orbit without z-axis ejection. In addition, the ability to moderate ion dephasing by minimizing radial field variation at any cyclotron radius is maintained. Many benefits result from the eTREC technique, such as improved S/N, limit of detection (LOD), resolution, and sensitivity.
Here, we present a novel method of excitation coupling to a modified closed cylindrical cell. Through the use of printed circuit boards for end-cap electrodes, one can produce trapping electrodes with specified geometries to a high degree of precision for a relatively low cost. In addition, minimization of design complexity can also be achieved with printed circuit board-based design. A capacitive RF voltage divider provides the basis for the RF voltage gradient in this excite-coupled Trapping Ring Electrode Cell (eTREC).
Experimental Section
eTREC Design
The conceptual illustration and schematic for the eTREC design was rendered using SolidWorks 2004 (SolidWorks Corp., Suresnes, France). The trap plates were designed in a circuit board layout program EAGLE ver. 4.13 (CadSoft Computer, Pembroke Pines, FL). The printing and machining of the boards was performed by Advanced Circuits (Advanced Circuits, Aurora, CO). The material chosen was FR-4. Out-gassing of the FR-4 circuit board was tested through the addition of a 4 in. × 8 in. rectangular multilayered sheet of this material to the UHV cross and monitoring the achievable base pressure of the high vacuum region. No discernible change in background pressure was observed and others have used this material operating under UHV with minimal outgassing.32 The eTREC plates consist of 10 rings per plate (20 independently controlled DC ring potentials total) spaced by a 0.007 in. gap with a width of 0.066 in. Tolerances on these dimensions are ±0.002 in. Nine of the 10 rings are segmented radially (with the exception of the innermost ring to prevent charging of nonconductor surfaces) again to form four quadrants (90° extent) to which two opposing segments have excitation coupled and adjacent segments have only DC applied. The excitation coupling was performed through a capacitive voltage divider using 680 pF surface mount capacitors (Digikey, Thief River Falls, MN). The RF voltage applied to each ring arc segment beginning from largest radius to smallest radius has about a 10% Vp-p drop between each arc. This RF voltage drop occurs at each ring arc segment until the center ring is reached which it is then terminated to ground allowing little to no RF onto this ring, effectively making it a DC only ring. The center ring is through hole plated to prevent charging of exposed fiberglass-epoxy composite. RF excitation was prevented from coupling to DC only quadrants via 1 MΩ resistors (Digikey, Thief River Falls, MN) while allowing DC potentials to be coupled to entire rings. Time constants for the switching of DC potentials with all components in place were ∼50–100 μs. All components were soldered to the face of the plate external to the trap. Aluminum 2–56 socket cap screws were used to assemble the cell (Fastener Express, Laguna Hills, CA). All 20 rings were wired separately, affording independent control over the DC potentials on each ring. Kapton coated wire (MDC Vacuum Products Corp., Hayward, CA) was used for electrical connections to the trapping ring electrodes. Tin-coated copper ring-tongue solderless connectors (McMaster-Carr, Los Angeles, CA) were used to connect the Kapton wire to the terminals on the cell. The cell had an inner diameter of 1.875 in. and a length of 2 in. between trapping electrodes.
eTREC Operation
Generation of independently variable DC voltages for each of the ring electrodes was accomplished using a program developed in-house within LabVIEW 8.0. The process occurs through two computers working in concert, a MIDAS33 data station and a computer housing the National Instruments hardware. eTREC operation is identical to TREC with two exceptions: the first exception is a solid-state relay wired in series with the cylindrical excitation electrodes and the respective trap plate quadrant allows for switching between excite-coupled and uncoupled states using TTL. This arrangement allows for common, TREC, excite-coupled common (ecommon), and eTREC conditions to be accessible to the user without breaking the vacuum or rewiring. Use of similar wiring schemes has been established by Easterling et al.34 for switching between coupled and uncoupled cell states. The second exception is the original LabVIEW program used for TREC was modified to include 10 additional DC potentials to supply the added ring electrodes in the eTREC design.
SIMION Modeling
Modeling of eTREC was performed using SIMION 3D version 7.0 (D. A. Dahl, Idaho National Engineering and Environmental Laboratory). The model was designed to scale with the actual TREC design using 0.25 mm/grid unit and refined to a convergence level of 10−5. Equipotential contour plots illustrate the approximate potentials within the cell during experiments with common, TREC, ecommon, and eTREC voltage conditions. RF excitation electric field equipotential contours were generated by placing DC potentials of opposite polarity and magnitude reflected about the z–y plane of the cell. The axial and radial field component was recorded and averaged over the entire volume of the cell by acquiring data incrementing cyclotron radius (r = 0, 5, 10, 15, 20 mm) and translating along the z-axis (z= 0, 2, 4, 6, …, 50 mm). No TREC (or eTREC) voltage profile was applied during these experiments because voltages during TREC/eTREC experiments are modulated postexcitation.
The extent to which z-axis ejection occurred in each cell was also studied with SIMION modeling. A closed cylindrical cell with identical dimensions and aspect ratio was constructed. The user program from the ICR simulation included with SIMION was adapted and used for excitation in both the eTREC and the closed uncoupled cylindrical cell. The starting position of a 1000 m/z ion (zero rest energy) was incremented along the z-axis in order to accurately define the beginning potential energy of the ions. An excitation potential was applied to excite the ions to a final cyclotron radius of 50% of the cell radius. The z-component of the velocity (Vz) was recorded. This experiment was repeated with the excitation turned off in both cells. The quantity ΔVz was calculated by taking the difference in Vz recorded at the center between excitation on and off. The design of this simulation allowed us to show the change in Vz as a function of z-axis starting position.
FT-ICR MS Experimental Conditions
The spectra presented here were obtained from a constructed-in-house 3.0 T FTICR mass spectrometer. This novel instrument has been described in detail in a recent publication.35
Radial dependence of signal intensity and S/N ratio of detected FT-ICR signals were investigated with single frequency excitation of bradykinin [M + 2H]2+ ions. RIPT was used to transfer ion populations to the cell. A frequency of 87.4 kHz was applied for a total of 150 μs to induce coherent cyclotron motion of the ions. A total of 64K data points were collected at 160 kHz. For the investigation of radial dependence on the signal intensity, the following voltage profile was applied to the trapping rings during detection for both eTREC and TREC: −0.2, 0, 0.3, 0.8, 1.2, 1.6, 1.8, 2.4, 2.8, 3.2 V DC (all experiments). Ion accumulation was set to 250 ms. Excitation was applied for 150 μs duration and amplitude was varied in 3.0 Vp-p increments.
The sensitivity experiment was conducted on a set of bradykinin samples with concentrations of 10 nM, 50 nM, 100 nM, 300 nM, 500 nm, and 1 μM. For each cell configuration, the excite parameters were tuned independently to yield the most intense ion signal for the bradykinin [M + 2H]2+ over the data acquisition (64K data points @ 160 kHz/s) using 100 nM bradykinin. The ion accumulation time was set to 200 ms. To minimize sample carryover and ensure accuracy for the sensitivity determination, ESI solution (blank) was run in between successive bradykinin dilutions and 1000 spectra were summed to reveal if any carry over was present. This was repeated until no visible ion signal was detected for bradykinin [M + 2H]2+. In addition, samples were measured in order of lowest concentration to highest concentration. Since there was no need for hardware modification when switching cell states, data was acquired for all four cell states at each respective concentration without interruption or hardware modification.
The fine structure resolution data was obtained on the peptide MMMMG synthesized in-house. This peptide was purified by preparative reverse phase chromatography prior to mass spectrometric analysis. In this experiment, the instrument was set to accumulate ions for 200 ms. A chirp-based excitation was used over the frequency range of 20–250 kHz. The sweep rate was set to 360 Hz/μs and the amplitude was adjusted for maximum performance of the cell condition in use (eTREC or common (ex) 1.2 V). The 2 M data points were acquired at 160 kHz. This resulted in an overall time domain signal length of 6.55 s. Five single scan spectra were recorded in series. The five data sets were internally calibrated using the third isotope peak in the isotopic envelope of MMMMG.
All acquired FT-ICR data were analyzed with ICR-2LS.36 In all data presented, no zero-filling or apodization was performed on the acquired data, with the exception of high-resolution MMMMG spectra. In this case, Welch apodization was applied to the data set followed by a single zero-fill. Theoretical spectra of MMMMG were generated with the Mercury component37 in ICR-2LS.
Results
Previous studies of both EPIC and TREC demonstrated increased FT-ICR-MS performance.13,25,26 With EPIC, the cyclotron frequency and observable signal duration was very sensitive to the number of electrons in the electron beam. This initial observation of increased performance when effectively applying a negative potential in the center of the cell provided the impetus to develop TREC, a cell which allowed us to explore the effects of modulating radial electric fields during detection. In EPIC and TREC, the performance enhancement which was observed is directly related to decreasing the variation in radial electric fields during detection. The first generation TREC provided proof of principle in regard to the latter; however, the excitation fields in this cell are nonideal and lead to z-axis excitation and ion ejection. The nonideal excitation field has been addressed with the eTREC design while preserving the ability to modulate radial trapping fields during ion detection.
The eTREC design is shown in Figure 1. For a detailed description of eTREC, see the Experimental Section. SIMION modeling prior to implementation of eTREC aided in the determination of the most appropriate values for the capacitive voltage divider used. Multiple iterations of applied RF voltage to each arc segment was performed while minimizing the measured axial field to achieve an approximately linear excite field in the RF coupled model. See Supporting Information for a comparison of the eTREC electrode geometry in an uncoupled versus a coupled state.
Figure 1.
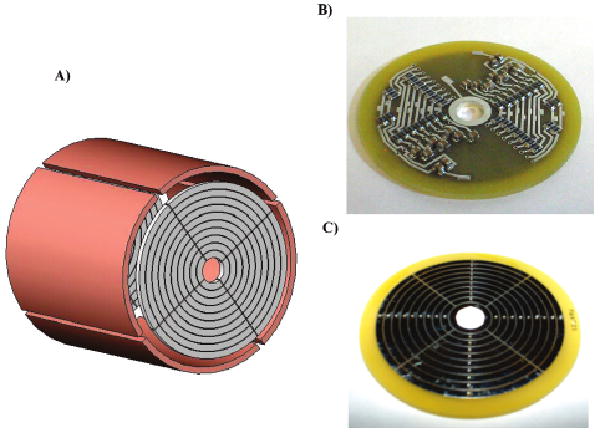
(A) A conceptual representation of the eTREC design. (B) A photograph of the backside of the eTREC trap plate including installed resistor and capacitor components. (C) A photograph of the front side of the eTREC trap plate revealing the conductor pattern for the rings and quadrants.
The z-axis ejection of ions during excitation is an observed limitation in the sensitivity of the closed cylindrical cell geometry. This limitation is considered most problematic during modern operation of FT-ICR instruments in which no collisional cooling or adiabatic lowering of trap potentials is utilized to control the kinetic energy of the ions in the cell. The ion population trapped in an actual experiment is likely to contain a broad distribution of energies and, thus, axial amplitudes. Z-axis ejection is dependent upon the energy and position of an ion during the excitation. These quantities dictate whether or not the ion remains trapped within the cell subsequent to the excitation event. To demonstrate that the eTREC design mitigates this known problem, a simulation of single ion excitation was performed (Figure 2). The excitation amplitude was held constant (Vp-p to achieve 50% cell radius) while initial z-axis displacement was incremented from 0 displacement to the boundary of the cell. The change in z-component of the velocity (ΔVz) was calculated by recording Vz with and without excitation at each z-displacement and taking the difference between the two values. A steady increase in the ΔVz was observed in the uncoupled cell as a function of starting z-axis oscillation amplitude. Eventually, the energy imparted to the ion during the excitation event was enough to overcome the potential well imposed by the trapping electrodes (1.0 V DC). The dashed line superimposed upon the plot represent the onset of the observation of z-axis ejection at a particular ΔVz and z-axis displacement in this SIMION model. In the case of eTREC, the trapping ring electrodes were all set to 1.0 V DC to establish the same potential well within both traps. Ideal excitation potentials should induce no increase in the ΔVz. The eTREC model performed such that z-axis ejection never occurred, even when the ion had z-axis displacement of 99% the cell length. This represents significant improvement over the closed cylindrical cell. However, eTREC did exhibit deviation from ideal behavior at z-axis displacement >50% cell length. This indicates that the eTREC excitation electrode geometry requires further refinement to achieve a maximum gain in sensitivity. The relative ease in design and fabrication of these trap plates permits further experimentation. SIMION modeling results show qualitative support for the excitation linearization and mitigation of z-axis ejection achieved by the eTREC technology experimentally.
Figure 2.
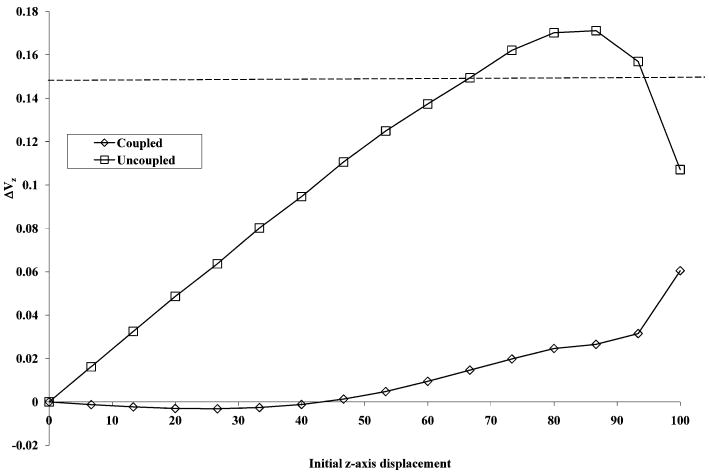
SIMION modeling data of velocity increase as a function of initial z-axis displacement. The dashed horizontal line represents the onset of the observation of z-axis ejection in this model.
The sensitivity of the detection process is of great importance in FT based mass analyzers, especially with the present popularity in RF ion trap-FT coupled instruments (LTQ-FT or LTQ-Orbitrap38) where the ion trap capacity is generally lower than that of the ICR or Orbitrap mass analyzers. Evaluation of the sensitivity of the four accessible cell configurations (common, TREC, common (ex), and eTREC) was carried out via a serial dilution experiment using Bradykinin [M + 2H]2+ as the analyte. The sweep based excitation amplitude was individually optimized for that cell configuration given the observation from the Supplemental Figure 4. Results from this experiment indicated that maximum sensitivity for each cell required different excitation amplitude (excited cyclotron radius). The percent gain in sensitivity has been quantified as a function of concentration in Figure 3. These data were generated by recording the signal amplitude of the first “beat” of Bradykinin [M + 2H]2+ in each time domain signal and averaging over technical triplicates. This method of recording the intensity of the first “beat” of the time domain signal allows one to minimize effects on the ion intensity which transpire over relatively longer time periods such as ion cloud dephasing. Therefore, differences in ion intensity must be attributed to z-axis ejection or proximity of the ion cloud to the detection electrodes. The ratio of both eTREC/TREC and common 1.2 V (ex)/common 1.2 V conditions are displayed. The latter two comparisons have been made in order to isolate the sensitivity gain achieved simply through the use of our excitation coupling geometry. The error bars represent ±σ. These data indicate >50% gain in sensitivity is achieved when excitation is enabled in both cell operation conditions (TREC or common 1.2 V). This direct experimental observation supports the observations made in silico using SIMION modeling which indicate that eTREC operation mitigates z-axis ejection.
Figure 3.
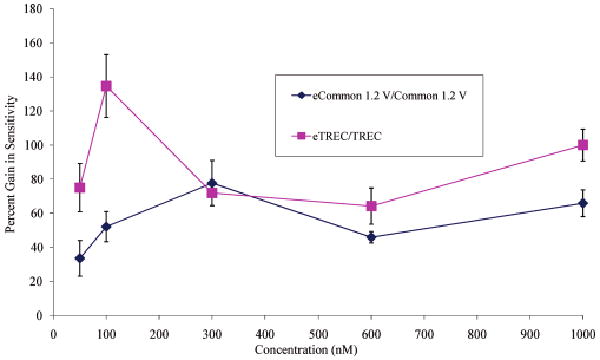
The percent gain in sensitivity calculated from the ratio of the first beat intensity for both eTREC/TREC and common (ex) 1.2 V/common 1.2 V cell conditions as a function of concentration. Greater than 50% gain in sensitivity is observed through the use of the excite coupling geometry of this cell.
The resolution achieved by the eTREC technology was demonstrated through the mass measurement of the peptide MMMMG. The third isotope peak in the isotopic envelope contains several possible elemental compositions; the two most abundant are 12C22H41N5O632S334S1 and 12C2013C2H41N5O632S4. The difference in mass between these two species is 10.9 mDa. To achieve baseline resolution of these ions, a resolving power of ∼200 000 is required. Figure 4 shows data acquired under eTREC conditions. The ability to resolve fine structure at modest magnetic field strength has been shown by others;39 however, prior examples involve adiabatic ramping of trapping potentials, minimization of ion population, collisional cooling, and other adjustments for minimization of the probability that these two distinct masses will be observed as a single mass. In the case of eTREC, none of these methods were utilized, which indicates that minimization of the radial field environment in the cell allows not only higher resolving power, but greater resolution. These data illustrate the fundamental difference between resolving power and resolution. In the previous report, it was shown that TREC technology increases phase coherence of ions and allows for observation of coherent ion motion for longer time periods; the observed frequency drift over long acquisition periods was also shown to be minimized as well. Minimization of the drift in frequency over the acquisition period is likely accountable for the increased resolution observed with eTREC. However, another possibility is that eTREC conditions reduce the probability of coalescence between two closely spaced m/z species, although no experimental data is provided to support this hypothesis. The ability to simultaneously detect very closely spaced molecular species is important for improving the depth of information40 obtained from analysis in all fields which utilize high-resolution mass spectrometry. In proteomics, isobaric amino acid substitutions (Met + Leu versus Pro + Phe) and peptide modifications (phosphorylation versus sulfation) exist in which the mass difference to be resolved is of the same order of magnitude.41
Figure 4.
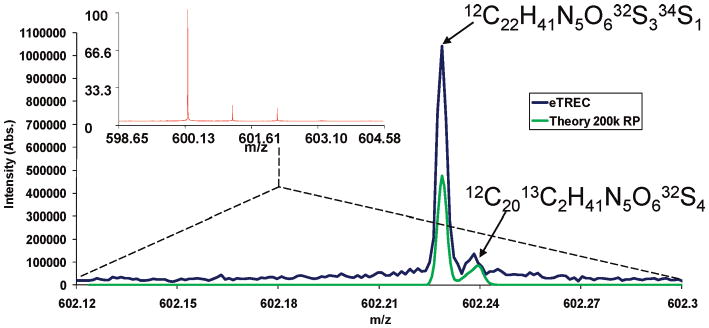
Five consecutively acquired single scans of MMMMG peptide [M + H]+ internally calibrated and summed. The mass range in view is centered about the third isotope peak in the envelope using eTREC.
Conclusions
The eTREC technology provides an efficient method for linearization of excitation fields. This first generation example of eTREC allowed for a >50% gain in sensitivity and a lower LOD, which has been primarily attributed to mitigation of z-axis ejection during ion excitation. The ability to manipulate the radial field environment within the cell was shown to be retained within this design, giving it the same increased detection performance as its predecessor, TREC. Fine structure resolution of the peptide MMMMG was observed using the eTREC technology at modest magnetic field strength, while using common (ex) 1.2 V conditions fine structure was not observed, although RP∼300 000 was achieved for both cell states. Direct experimental observation supports SIMION modeling results performed during conception of eTREC. However, deviation from excitation field ideality in close proximity to the trapping electrodes leaves room for improvement in the present design of eTREC. As a first generation design, eTREC has provided a straightforward and efficient vehicle toward proof of principle for this technology.
Supplementary Material
Acknowledgments
The authors acknowledge Alan Marshall and his group at the National High Magnetic Field Laboratory for the use of the MIDAS data station. We also thank Gordon Anderson for helpful discussion on implementation of electronics for eTREC. This material is based upon work supported by the Directorate of Biological Sciences, National Science Foundation under Grant No. 0352451; Murdock Charitable Trust; and Office of Science (BER), U.S. Department of Energy, Grant No. DE-FG02-04ER63924.
Footnotes
Supporting Information Available: Additional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Comisarow MB, Marshall AG. Chem Phys Lett. 1974;25:282–283. [Google Scholar]
- 2.He F, Emmett MR, Hakansson K, Hendrickson CL, Marshall AG. J Proteome Res. 2004;3:61–67. doi: 10.1021/pr034058z. [DOI] [PubMed] [Google Scholar]
- 3.Gabrielse G, Haarsma L, Rolston SL. Int J Mass Spectrom Ion Processes. 1989;88:319–332. [Google Scholar]
- 4.Williams DK, Hawkridge AM, Muddiman DC. J Am Soc Mass Spectrom. 2007;18:1–7. doi: 10.1016/j.jasms.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Wong RL, Amster IJ. J Am Soc Mass Spectrom. 2006;17:1681–1691. doi: 10.1016/j.jasms.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt A, Gehlenborg N, Bodenmiller B, Mueller LN, Campbell D, Mueller M, Aebersold R, Domon B. Mol Cell Proteomics. 2008;7:2138–2150. doi: 10.1074/mcp.M700498-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinner O, Seebacher J, Walzthoeni T, Mueller LN, Beck M, Schmidt A, Mueller M, Aebersold R. Nat Methods. 2008;5:315–318. doi: 10.1038/nmeth.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown SC, Kruppa G, Dasseux JL. Mass Spectrom Rev. 2005;24:223–231. doi: 10.1002/mas.20011. [DOI] [PubMed] [Google Scholar]
- 9.Han J, Danell RM, Patel JR, Gumerov DR, Scarlett CO, Speir JP, Parker CE, Rusyn I, Zeisel S, Borchers CH. Metabolomics. 2008;4:128–140. doi: 10.1007/s11306-008-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodgers RP, Schaub TM, Marshall AG. Anal Chem. 2005;77:20A–27A. doi: 10.1021/ac048766v. [DOI] [PubMed] [Google Scholar]
- 11.Marshall AG, Rodgers RP. Acc Chem Res. 2004;37:53–59. doi: 10.1021/ar020177t. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser NK, Skulason GE, Weisbrod CR, Wu S, Zhang K, Prior DC, Buschbach MA, Anderson GA, Bruce JE. Rapid Commun Mass Spectrom. 2008;22:1955–1964. doi: 10.1002/rcm.3574. [DOI] [PubMed] [Google Scholar]
- 13.Weisbrod CR, Kaiser NK, Skulason GE, Bruce JE. Anal Chem. 2008;80:6545–6553. doi: 10.1021/ac800535e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beu SC, Laude DA., Jr Anal Chem. 1992;64:177–180. [Google Scholar]
- 15.Bruce JE, Anderson GA, Lin CY, Gorshkov M, Rockwood AL, Smith RD. J Mass Spectrom. 2000;35:85–94. doi: 10.1002/(SICI)1096-9888(200001)35:1<85::AID-JMS910>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Brustkern AM, Rempel DL, Gross ML. ASMS poster presentation; Indianapolis, IN. 2008. [Google Scholar]
- 17.Caravatti P, Allemann M. Org Mass Spectrom. 1991;26:514–518. [Google Scholar]
- 18.Gabrielse G, MacKintosh FC. Int J Mass Spectrom Ion Processes. 1984;57:1–17. [Google Scholar]
- 19.Vartanian VH, Hadjarab F, Laude DA. Int J Mass Spectrom Ion Processes. 1995;151:175–187. [Google Scholar]
- 20.Wang M, Marshall AG. Anal Chem. 1989;61:1288–1293. doi: 10.1021/ac00186a021. [DOI] [PubMed] [Google Scholar]
- 21.Yin WW, Wang M, Marshall AG, Ledford EB., Jr J Am Soc Mass Spectrom. 1992;3:188–197. doi: 10.1016/1044-0305(92)87002-G. [DOI] [PubMed] [Google Scholar]
- 22.Guan S, Marshall AG. Int J Mass Spectrom Ion Processes. 1995;146/147:261–296. [Google Scholar]
- 23.Marshall AG, Hendrickson CL, Jackson GS. Mass Spectrom Rev. 1998;17:1–35. doi: 10.1002/(SICI)1098-2787(1998)17:1<1::AID-MAS1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser NK, Weisbrod CR, Webb BN, Bruce JE. J Am Soc Mass Spectrom. 2008;19:467–478. doi: 10.1016/j.jasms.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaiser NK, Bruce JE. Int J Mass Spectrom. 2007;265:271–280. [Google Scholar]
- 26.Kaiser NK, Bruce JE. Anal Chem. 2005;77:5973–5981. doi: 10.1021/ac050606b. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, Choi MC, Kim S, Hur M, Kim HS, Yoo JS, Blakney GT, Hendrickson CL, Marshall AG. Anal Chem. 2007;79:3575. doi: 10.1021/ac062016z. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell DW, Hearn BA, DeLong SE. Int J Mass Spectrom Ion Processes. 1993;125:95–126. [Google Scholar]
- 29.Wang M, Marshall AG. Anal Chem. 1990;62:515–520. doi: 10.1021/ac00204a017. [DOI] [PubMed] [Google Scholar]
- 30.Van der Hart WJ, Van de Guchte WJ. Int J Mass Spectrom Ion Processes. 1988;82:17–31. [Google Scholar]
- 31.Tolmachev AV, Robinson EW, Wu S, Kang H, Lourette NM, Pasa-Tolic L, Smith RD. J Am Soc Mass Spectrom. 2008;19:586–597. doi: 10.1016/j.jasms.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouki C, Westerberg L. Phys Scr, T. 2003;T104:107–108. [Google Scholar]
- 33.Senko MW, Canterbury JD, Guan S, Marshall AG. Rapid Commun Mass Spectrom. 1996;10:1839–1844. doi: 10.1002/(SICI)1097-0231(199611)10:14<1839::AID-RCM718>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 34.Easterling ML, Pitsenberger CC, Kulkarni SS, Taylor PK, Amster IJ. Int J Mass Spectrom Ion Processes. 1996;157/158:97–113. [Google Scholar]
- 35.Kaiser NK, Skulason GE, Weisbrod CR, Bruce JE. J Am Soc Mass Spectrom. 2009;20:755–762. doi: 10.1016/j.jasms.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson GA, Bruce JE, Smith RD. ICR-2LS. 2.18. Richland, WA: 1996. [Google Scholar]
- 37.Rockwood AL. Rapid Commun Mass Spectrom. 1995;9:103–105. [Google Scholar]
- 38.Makarov A, Denisov E, Kholomeev A, Balschun W, Lange O, Strupat K, Horning S. Anal Chem. 2006;78:2113–2120. doi: 10.1021/ac0518811. [DOI] [PubMed] [Google Scholar]
- 39.Solouki T, Emmett MR, Guan S, Marshall AG. Anal Chem. 1997;69:1163–1168. doi: 10.1021/ac960885q. [DOI] [PubMed] [Google Scholar]
- 40.Spengler B. J Am Soc Mass Spectrom. 2004;15:703–714. doi: 10.1016/j.jasms.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Bossio RE, Marshall AG. Anal Chem. 2002;74:1674–1679. doi: 10.1021/ac0108461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


