Abstract
Background
Exocrine tissue is commonly cotransplanted with islets in autografting and allotransplantation of impure preparations. Proteases and insulin are released by acinar cells and islets, respectively, during pretransplantation culture and also systemically after transplantation. We hypothesized that released proteases could cleave insulin molecules and that addition of alpha 1 antitrypsin (A1AT) to impure islet cultures would block this cleavage, improving islet recovery and function.
Methods
Trypsin, chymotrypsin, and elastase (TCE) activity and insulin levels were measured in culture supernates of pure (n = 5) and impure (n = 5) islet fractions, which were isolated from deceased donors. SDS-PAGE was used to detect insulin after incubation with proteases. We assessed the effects of A1AT supplementation (0.5 mg/mL; n = 4] on TCE activity, insulin levels, culture recovery, and islet quality. The ultrastructure of islets exposed to TCE versus control medium was examined using electron microscopy (EM).
Results
Protease (TCE) activity in culture supernates was directly proportional to the percentage purity of islets: pure, impure, or highly impure. Increasingly lower levels of insulin were detected in culture supernates with higher protease activity levels. Insulin levels measured in supernates of 2000 IE aliquots of impure and highly impure islet preparations were 61 ± 23.7% and 34 ± 33% of that in pure preparations, respectively. Incubation with commercially available proteases (TCE) or exocrine acinar cell supernates cleaved insulin molecules as assessed using SDS-PAGE. Addition of A1AT to impure islet preparations reduced protease activity and restored normal insulin levels as detected using enzyme-linked immunosorbent assay (ELISA) and SDS-PAGE of culture supernates. A1AT improved insulin levels to 98% ± 1.3% in impure and 78% ± 34.2% in highly impure fractions compared with pure islet fractions. A1AT supplementation improved postculture recovery of islets in impure preparations compared with nontreated controls (72% ± 9% vs 47% ± 15%). Islet viability as measured using membrane integrity assays was similar in both the control (98% ± 2%) and the A1AT-treated groups (99% ± 1%). EM results revealed reduction or absence of secretory granules after exposure to proteases (TCE).
Conclusion
Culture of impure human islet fractions in the presence of A1AT prevented insulin cleavage and improved islet recovery. A1AT supplementation of islet culture media, therefore, may increase the proportion of human islet products that meet release criteria for transplantation.
Impure human islet preparations are commonly transplanted as autografts and allografts.1 In clinical allotransplantation, pure and impure islets are cultured in identical medium for 48–72 hours prior to transplantation. When islets are cultured in the presence of exocrine cells (impure preparations), they are exposed to potentially harmful proteolytic enzymes—trypsin, chymotrypsin, and elastase (TCE)—which are released from cocultured acinar cells. We observed that released proteases cleaved intact insulin molecules in culture. To optimize the survival of islets cocultured with acinar cells, we supplemented the culture medium with a protease inhibitor (alpha-1 antitrypsin [A1AT]) to block insulin degradation and improve islet culture recovery and function.
Methods
Human allograft clinical preparations2 of pure and impure islets were cultured in CMRL-supplemented medium overnight. Culture supernates were obtained to measure proteases (TCE)3 and insulin content enzyme-linked immunosorbent assay [ELISA]. Islet recoveries were monitored on the day of transplantation. Independently, pure and impure islet research culture were supplemented with A1AT (0.5 mg/mL). Supernates were obtained for TCE and insulin measurements after overnight culture. Islet recovery and quality estimated from the A1AT-supplemented group were compared with the untreated group. The effects of proteases on insulin molecules were studied using SDS-PAGE. The ultrastructure of islets exposed to TCE or to control medium was examined using electron microscopy (EM).
Statistical Analysis
Results were calculated as mean values ± SD. Statistical significance was evaluated using the paired sample Student t test with P value < .05 considered statistically significant.
Results
Among clinical islet preparations, the average islet culture recovery after 48–72 hours for pure materials (n = 10) showed 86% recovery (P < .0001) versus 70% recovery for impure preparations (n = 8; P < .01). We designed this study to address the reason for poor islet culture recovery in impure preparations. Protease (TCE) levels in culture supernates were directly proportional to the percentage islet purity. Elastase levels were negligible in all 3 preparations. Insulin levels were low when there was high protease activity Although 2000 IEQ were uniformly present in pure impure and highly impure preparations the detected insulin levels in impure materials (n = 5) was 58% and in highly impure (n = 5) was 32% compared with pure ones. Incubation of insulin with commercial pure proteases (TCE) or exocrine acinar cell culture supernates was associated with degradation of intact insulin molecules, as detected in SDS-PAGE gels (Fig 1). EM showed a reduction in or absence of secretory granules with cellular damage, possibly due to exposure to TCE when compared with nontreated controls. Supplementation of impure islet preparations with A1AT was associated with reduced protease levels, restoring normal insulin levels in culture supernates, as measured using ELISA and using SDS-PAGE. Compared with pure islet fractions A1AT improved insulin levels to 98% ± 1.3% for impure and 78% ± 34.2% for highly impure preparations. A1AT supplementation improved postculture islet recovery in impure preparations compared with nontreated controls (72% ± 9% vs 47% ± 15%). Islet viability measured using membrane integrity tests was similar for both the control (98% ± 2%) and the A1AT-treated group (99% ± 1%).
Fig 1.
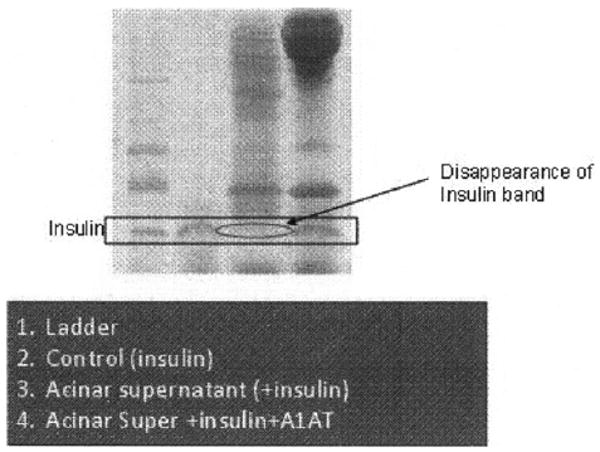
Proteases (TCE) or exocrine acinar cell supernate cleaves the insulin molecule as assessed using SDS-PAGE gel. Disappearance of insulin band was detected after exposure of insulin to acinar cell culture supernate.
Discussion
The 2008 annual report of The Collaborative Islet Transplant Registry (CITR) indicated that the average islet purity among the 489 worldwide transplanted preparations was 63.4% ± 17.5%,1 indicating that islets had been cultured and transplanted in the presence of exocrine acinar cells. When islets are cultured in the presence of exocrine acinar cells (impure preparations) they may be exposed to potentially harmful proteolytic enzymes—TCE. Islet recovery from impure preparations is commonly lower than pure islet cultures, presumably due to negative effects of proteases. A1AT is a broad spectrum, clinical-grade, serine-protease inhibitor.4 With A1AT supplementation, culture recovery was improved from impure preparations. In this study, we observed that released proteases cleaved insulin molecules in culture, potentially causing poor islet recovery. The supplementation of culture media with A1AT improved the recovery and restored the insulin levels in impure preparations, presumably by inhibiting protease (TCE) activity. A1AT supplementation of islet culture media, therefore, may increase the proportion of human islet products that meet release criteria for transplantation.
Acknowledgments
The authors would like to thank Jeff Ansite, Thomas Gilmore, Muhammad Abdulla, Bob Konz, and Brian Perrault for excellent technical support. Special thanks to Josh Whilem for manuscript editing.
Supported in part by grants from the National Institutes of Health grants R01 DK56963, U42 RR16598, and U01 AI65193) and the Juvenile Diabetes Research Foundation (JDRF 4-2004-372).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alejandro R, Barton FB, Hering BJ, et al. 2008 Update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86:1783. doi: 10.1097/TP.0b013e3181913f6a. [DOI] [PubMed] [Google Scholar]
- 2.Balamurugan AN, Breite AG, Anazawa T. Successful human islet isolation and transplantation indicating the importance of class 1 collagenase and collagen degradation activity assay. Transplantation. doi: 10.1097/TP.0b013e3181d21e9a. in press. [DOI] [PubMed] [Google Scholar]
- 3.Kawabata S, Miura T, Morita T, et al. Highly sensitive peptide-4-methylcoumaryl-7-amide substrates for blood-clotting proteases and trypsin. Eur J Biochem. 1988;172:17. doi: 10.1111/j.1432-1033.1988.tb13849.x. [DOI] [PubMed] [Google Scholar]
- 4.Ray MB, Desmet VJ, Gepts W. Alpha-1-antitrypsin immunoreactivity in islet cells of adult human pancreas. Cell Tissue Res. 1977;185:63. doi: 10.1007/BF00226668. [DOI] [PubMed] [Google Scholar]


