SUMMARY
During B cell development, immature B cell fate is determined by whether the B cell antigen receptor is engaged in the bone marrow. Immature B cells that are non-autoreactive continue maturation and emigrate from the marrow whereas autoreactive immature B cells remain and are tolerized. However, the microenvironment where these events occur and the chemoattractants responsible for immature B cell trafficking within and out of the bone marrow remain largely undefined. Sphingosine 1-phosphate (S1P) is a chemoattractant that directs lymphocyte trafficking and thymocyte egress and in this study we investigated whether S1P contributed to B cell development, egress and positioning within the bone marrow. Our findings show that immature B cells are chemotactic towards S1P but that this response is dependent on antigen receptor specificity: non-autoreactive, but not autoreactive, immature B cells migrate towards S1P and are shown to require S1P3 receptor for this response. Despite this response, S1P3 is shown not to facilitate immature B cell egress but is required for normal B cell development including the positioning of transitional B cells within bone marrow sinusoids. These data indicate that S1P3 signaling directs immature B cells to a bone marrow microenvironment important for both tolerance induction and maturation.
Keywords: Immature B cells, Autoreactive, Sphingosine 1-phosphate, Migration
INTRODUCTION
In the bone marrow of adults developing immature B cells must assemble an antigen receptor that is capable of signaling but does not react with self-antigens [1, 2]. The fate of an immature B cell is thus determined by whether the B cell antigen receptor (BCR) is engaged in the bone marrow microenvironment. If an immature B cell expresses a BCR that does not engage in the marrow or recognizes a self-antigen generating a weak BCR signal, the non-autoreactive immature B cell continues maturation and emigrates from the bone marrow. However, if an immature B cell assembles a BCR that recognizes a cognate antigen that signals above a certain threshold, maturation is arrested and the autoreactive cell is rendered tolerant in the bone marrow microenvironment [3–5].
In contrast to the relatively well-defined migration of developing thymocytes within the thymus [6, 7], the movements of developing B lineage cells within the bone marrow is much less understood. However, it is clear chemoattractants and adhesion molecules play important roles in orchestrating this cell migration [8–10] similar to that documented for mature lymphocytes to and from lymphoid organs and subsequent tissue localization [11–13]. Indeed, the CXCL12 chemokine is produced by bone marrow stromal cells and interacts with its receptor CXCR4 on early B lineage cells to retain these developing cells in the appropriate bone marrow microenvironment [14, 15]. Recently, using in vivo cell labeling and imaging, newly generated B cells located in the marrow parenchyma were shown to migrate to the sinusoids as immature and transitional B cells [16]. The cannabinoid receptor 2, a G-protein coupled receptor (GPCR) whose ligand is a monoacylglycerol lipid, was further shown to be required for retention of immature B cells in the sinusoids [16], although the chemoattractants that guide immature B cells to the sinusoids or out of the marrow remain to be identified.
Sphingosine 1-phosphate (S1P) is a lysophospholipid that has been well documented to direct lymphocyte migration by signaling through cognate GPCRs [17–22]. This lipid is present in the low micromolar range in blood, low- to mid-nanomolar range in lymph and at approximately 5–10 nanomolar in the thymus and lymph node tissues [23, 24]. This concentration gradient between tissue and circulatory systems is thought to be important in directing the recirculation of lymphocytes through tissue [23].
There are five identified S1P receptors, S1P1-5, and during thymic development thymocytes express increasing levels of S1P receptor 1 (S1P1), which is also required for thymocyte egress [19, 25, 26]. In contrast, immature B cell egress from the bone marrow is not dependent on S1P1 [19, 27] suggesting either S1P responsiveness is not required for egress or, alternatively, immature B cells rely on a distinct S1P receptor for migration. Indeed, while both B and T cells require S1P1 to exit secondary lymphoid organs in vivo [19], T cells rely on S1P1 [19, 25, 26] and marginal zone B cells on S1P3 [20] to chemotactically respond to S1P in vitro. Besides lymphocyte homing, S1P is also important for B cell positioning in secondary lymphoid organs. Thus, although S1P is an important chemoattractant for T cell development and mature B and T cell homeostatic recirculation and tissue localization, a role for S1P in B cell development has not been yet identified.
Because autoreactive immature B cells remain in the bone marrow to be tolerized whereas non-autoreactive cells exit the bone marrow, we hypothesized that only non-autoreactive immature B cells should be able to respond to an egress signal. In this study, we used immunoglobulin transgenic mice in which the IgH and IgL chain variable gene segments encoding a characterized specificity were targeted to their endogenous loci, allowing us to distinguish between autoreactive and non-autoreactive immature B cells. With this system we investigated whether immature B cell antigen receptor specificity dictates S1P responsiveness. We report here that non-autoreactive and autoreactive bone marrow immature B cells demonstrate a differential dependence on S1P3 for migration to S1P but that this responsiveness is not required for immature B cell egress from the marrow. Instead, S1P3 is required for normal B cell development and positioning of non-autoreactive immature B cells in bone marrow sinusoids as S1P3−/− mice harbor a significantly reduced transitional B cell sinusoidal population and an increased frequency of Igλ+ cells.
RESULTS
Non-autoreactive immature B cells migrate to S1P in vitro
S1P serves as a chemoattractant for a variety of cells types including T cells [18, 19, 25, 26], marginal zone B cells [20, 21, 28], NK cells [22] and dendritic cells [17, 29] and responsiveness to this lysophospholipid chemoattractant guides leukocyte recirculation and thymocyte egress [19]. However, despite the many similarities in the development of B and T lymphocytes, S1P1 is not required for the egress of immature B cells from bone marrow [19, 27]. We nevertheless speculated that immature B cells may still respond to S1P using a different S1P receptor, and we hypothesized that antigen receptor specificity influences this response. Thus, we initially sought to determine the extent of autoreactive and non-autoreactive immature B cell migration to S1P in vitro.
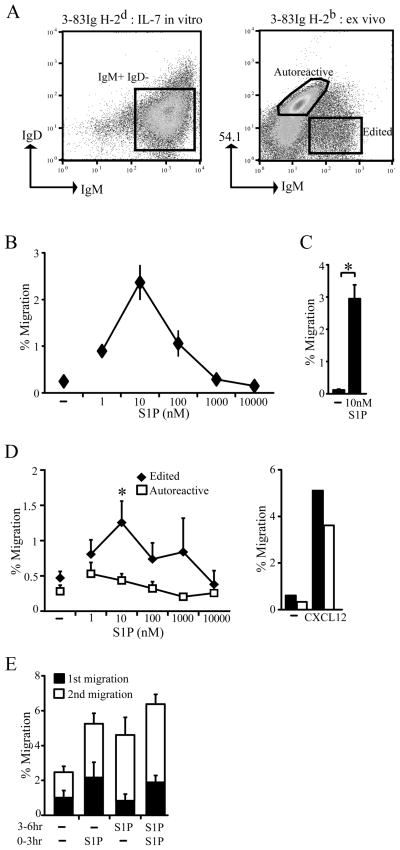
The immature B cell population in the bone marrow of wild type mice is comprised of immature B cells with both autoreactive and non-autoreactive specificities [30]. In order to discriminate between these two populations, we took advantage of immunoglobulin knock-in mice in which all immature B cells express the same specificity encoded by the 3–83 IgH and IgL chain [5, 31]. B cells from these 3-83Igi mice express a B cell antigen receptor (BCR) that recognizes the ubiquitously expressed major histocompatibility class I molecule, H-2Kb but not H-2Kd [32]. Consequently, immature B cells from 3-83Ig, H-2d mice are non-autoreactive whereas immature B cells from 3-83Igi, H-2b mice are autoreactive. Non-autoreactive immature B cells were generated in vitro by culturing bone marrow cells from 3-83Igi, H-2d mice in the presence of IL-7 for 4 to 5 days and after which approximately 90% of all remaining cells are IgM+ IgD− immature B cells (Fig. 1A). These non-autoreactive immature B cells were then tested for their ability to migrate towards S1P. The results from these experiments demonstrated that a small but significant percent of non-autoreactive immature B cells were able to migrate to S1P in vitro in a concentration dependent manner with maximal migration at 10 nM S1P (Fig. 1B,C).
Figure 1.
Immature B cells with non-autoreactive antigen receptors migrate towards S1P. A) Representative flow cytometric analysis of IgM and IgD expression by non-autoreactive immature B cells generated by IL-7 in vitro culture of bone marrow cells (left panel) and 3–83 idiotype (54.1) and IgM expression by ex vivo bone marrow immature B cells gated as B220+ IgDlow/− cells (right panel) from 3-83Igi, H-2d mice. B) Chemotaxis of non-autoreactive immature B cells towards differing concentrations of S1P or media (−). Data are representative of at least 4 independent experiments and are shown as mean with SD for duplicate wells. C) Cumulative results as for the experiment in (B) at 10nM S1P, mean ± SEM of 18 independent experiments; *p<0.05 in a Student’s one-tailed unpaired t-test. Non-autoreactive immature B cells were generated from IL-7 bone marrow cultures using 3-83Igi, H-2d mice (B, C). D) B220+ bone marrow cells were isolated from 3-83Igi, H-2b mice and migration to the indicated concentrations of S1P or 0.5μg/mL CXCL12 by autoreactive (IgMlow 3-83low IgD−) and edited IgM+ 3-83−IgD−cells measured. Data are mean ± SEM of 4–8 independent experiments; *p<0.05 in a Student’s one-tailed unpaired t-test. CXCL12 migration is representative of 4 independent experiments. E) Non-autoreactive immature B cells from IL-7 bone marrow cultures were allowed to migrate towards media (−) or 10 nM S1P during an initial migration period (0–3 hr) after which non-responding cells were again assayed for migration towards media or S1P after a second 3 hour migration period (3–6 hr). Data are mean ± SEM of 3 independent experiments.
To evaluate the S1P chemotactic response of autoreactive immature B cells, we again used 3-83Igi mice but this time analysis was ex vivo using bone marrow immature B cells isolated from the autoreactive H-2b genetic background. These autoreactive immature B cells can be easily identified by low expression of the 3–83 Ig on the cell surface using the 54.1 anti-3–83 idiotypic antibody (Fig. 1A and ref. [33]). The reduced surface Ig expression has been shown to be a consequence of receptor downmodulation upon autoantigen engagement [34]. However, 3-83Igi, H-2b bone marrow also carry non-autoreactive cells that have undergone receptor editing [31, 35] and that are 3–83 idiotype negative (54.1−) IgM+ (Fig. 1A). Thus, the use of ex vivo immature B cells from this autoreactive mouse strain allows the direct comparison of autoreactive immature B cells to receptor edited, non-autoreactive immature B cells from the same bone marrow. Interestingly, at all concentrations of S1P tested, autoreactive immature B cells were unable to migrate to S1P although the edited, and presumably non-autoreactive, immature B cells from the same bone marrow were able to respond to S1P (Fig. 1D). Importantly, edited non-autoreactive immature B cells isolated ex-vivo displayed comparable S1P chemotaxis compared with non-autoreactive immature B cells generated in vitro (compare Fig. 1B and D) indicating that IL-7 did not significantly influence S1P chemoattraction. Further, autoreactive immature B cells were not inherently unable to chemotax, as they migrated to CXCL12 similarly to non-autoreactive cells (Fig. 1D) indicating specificity in the lack of chemotactic response to S1P by autoreactive cells. S1P concentrations in vivo range from micromolar in the plasma to nanomolar in the lymph with estimates of approximately 5–10 nM in primary and secondary lymphoid organ tissues [23, 24]. Thus, taken together we conclude that non-autoreactive, but not autoreactive, immature B cells migrate to S1P at physiological S1P concentrations.
Even at optimal concentrations of S1P, the percentage of non-autoreactive immature B cells migrating to S1P was lower than the proportion responsive to CXCL12. We noted however that this migration frequency to S1P for non-autoreactive immature B cells is similar to that previously reported for the analogous T lineage developmental counterparts, single positive thymocytes [19, 25]. Nevertheless, this result led us to examine whether S1P responsiveness by non-autoreactive immature B cells was limited by the duration of the assay or, alternatively, only a subset of the non-autoreactive immature B cell population was capable of responding to S1P. To discriminate between these possibilities we performed a serial migration assay where after an initial migration assay (0–3 hr), the cells that did not migrate during this period were harvested and assessed for S1P chemotactic potential in a second migration assay (3–6 hr). As shown in Fig. 1E, S1P responsiveness by the non-autoreactive immature B cell population was not limited to the first responding fraction. An additional fraction of non-autoreactive immature B cells migrated to S1P during the second migration assay regardless of whether the initial S1P responding population was present (first migration assay to media alone) or not (first migration assay to S1P). In these experiments we reproducibly observed that cells exposed to S1P in the initial migration assay displayed an increased migration frequency in the second migration period whether exposed to S1P again or media (Fig. 1E). While the basis for this remains unclear we envision continued S1P3 signaling by those cells previously engaged with S1P could account for this increased migration. Regardless, together these findings demonstrate that non-autoreactive immature B cell migration to S1P is able to develop with time and is not limited to an initial responding population.
Non-autoreactive and autoreactive immature B cells express S1P1, S1P3, and S1P4 receptors
There are five identified S1P receptors (S1P1-5) [36] and we next determined the S1P receptors expressed by immature B cells. Because S1P receptor-specific antibodies compatible with flow cytometric analysis are currently unavailable, we used quantitative PCR to assess S1P receptor expression. Total RNA was prepared from non-autoreactive and autoreactive immature B cells sorted from bone marrow of 3-83Igi, H-2d (non-autoreactive) and 3-83Igi, Rag1−/−, H-2b (autoreactive) mice, respectively, as B220+CD43−CD23−IgD− cells. Because 3-83Igi, H-2b mice harbor both autoreactive and receptor edited cells (Fig. 1A), the 3-83Igi H-2b mice were bred onto a Rag1−/− genetic background. The Rag1-deficiency in the 3-83Igi, H-2b mice ensures that the autoreactive immature B cells do not undergo receptor editing in vivo and thus, all immature B cells remain autoreactive [31]. The results from this analysis (Fig. 2A) demonstrated that both non-autoreactive and autoreactive immature B cells express considerable levels of S1P1 and relatively reduced but significant levels of the S1P3 and S1P4 receptors whereas S1P2 and S1P5 expression was negligible. This pattern of S1P receptor expression by immature B cells closely resembles that of mature follicular B cells previously reported [20].
Figure 2.
Immature B cell migration to S1P in vitro requires S1P3. A) S1P receptor mRNA expression relative to HPRT of non-autoreactive and autoreactive immature B cells was measured by quantitative PCR. Data are mean ± SEM of 4 independent experiments. B) Non-autoreactive immature B cells from IL-7 bone marrow cultures were tested for migration to 10 nM S1P, 0.5 μg/mL CXCL12 or media in the presence or absence of 100 μM of the CAY10444 S1P3 antagonist. Data are mean ± SEM of 3 independent experiments with *p=0.05 in a Student’s one-tailed unpaired t-test. C) Non-autoreactive immature B cells from IL-7 cultures were tested for migration to SEW2871, an S1P1 agonist, VPC24191, an S1P1 and S1P3 agonist, VPC23153, an S1P4 agonist, 10 nM S1P or media (−). Data are representative of 3 independent experiments. D) B220+ bone marrow cells were isolated from S1P3+/− or S1P3−/− mice and tested ex vivo for migration of immature B220+ IgM+ IgD−B cells to the indicated concentrations of S1P, 0.5 μg/mL CXCL12 or media. IgM+ IgDhigh mature cells were excluded from analysis. Data are mean ± SEM of 4 independent experiments; *p<0.05 Student’s one-tailed unpaired t-test.
S1P3 mediates immature B cell migration to S1P
Given that non-autoreactive immature B cells are able to migrate to S1P, it was important to determine which S1P receptor was responsible for this migration. Immature B cells express S1P receptors S1P1, S1P3 and S1P4 and S1P3 is not only responsible for marginal zone B cell migration to S1P [20, 21] but non-autoreactive immature B cells also display modestly increased expression of S1P3. Thus, we hypothesized that S1P3 was responsible for non-autoreactive immature B cell chemotaxis to S1P and initially used pharmacological S1P receptor agonists and antagonists to test this hypothesis. Specifically, we used the CAY10444 selective S1P3 antagonist [37], VPC24191, an S1P1 and S1P3 agonist [38–40], SEW2871, a selective S1P1 agonist [41], and VPC23152, an S1P4 agonist [42]. Treatment of non-autoreactive immature B cells with CAY10444 led to a significant (p = 0.05) inhibition of S1P migration (Fig. 2B); migration to CXCL12 was slightly inhibited by this S1P3 antagonist but this inhibition was not significant (p = 0.12). A specific S1P3 agonist is not currently commercially available, thus, non-autoreactive immature B cells were treated with the S1P1/S1P3 agonist, VPC24191 [38–40]. These data show that VPC24191 was able to promote moderate, but reproducible migration of non-autoreactive immature B cells in a dose-dependent manner (Fig. 2C). Although the immature B cell migration frequency to the S1P1/S1P3 agonist was reduced compared to S1P in this experiment, it was within the range observed for non-autoreactive immature B cell migration to 10 nM S1P. In contrast, the SEW2871 selective S1P1 agonist did not promote chemotaxis at any concentration tested (Fig. 2C) but was biologically functional as indicated by its ability to downregulate CD69 expression on thymocytes in vitro (data not shown) and as previously observed [26]. Finally, non-autoreactive immature B cells migrated weakly to VPC23152, an S1P4 agonist [42], and at a frequency ( <0.5%) that was less than 20% of that to S1P (Fig. 2C).
These in vitro results suggested S1P3 was the S1P receptor promoting immature B cell migration to S1P and we examined S1P3−/− immature B cells [43] to confirm these findings. The results from these experiments clearly demonstrated that S1P3 mediates immature B cell migration to S1P (Fig. 2D). Specifically, S1P3+/− immature B cells migrated to S1P whereas S1P3−/− immature B cells were unable to migrate to S1P at all concentrations of S1P tested (Fig. 2D). S1P3−/− immature B cells could nevertheless migrate to CXCL12 comparably to immature B cells from heterozygous littermates (Fig. 2D, right). We noted, however, that the concentration of S1P3 at which S1P3+/− heterozygous immature B cells displayed maximum migration was increased relative to the non-autoreactive Ig knock-in immature B cells. The basis for this difference is not clear but may reflect either that S1P3 expression may be reduced in S1P3+/− heterozygous B cells, all the immature B cells in the Ig knock-in were uniform in specificity, and/or genetic strain differences. Regardless, we conclude from these results that the S1P3 receptor is responsible for immature B cell migration to S1P.
S1P3 deficient and sufficient immature B cells egress from bone marrow with similar kinetics
Non-autoreactive immature B cells use S1P3 to migrate to S1P in vitro, thus we questioned whether S1P3 responding to S1P might contribute to immature B cell exit from bone marrow. Newly-generated B cells leave the bone marrow as CD93+ transitional B cells [44] and migrate to the spleen where they complete maturation to mature B cells. To address whether S1P3 contributes to immature B cell egress we compared the number and frequency of B220+CD93+ immature/transitional B cells in spleens of S1P3-deficient and S1P3+/− littermate control mice. Results from these analyses demonstrated a significant reduction in the number and frequency of immature/transitional B cells in spleens of S1P3−/− mice compared to control littermates (Fig. 3A) and is similar to that reported for an independently generated S1P3-deficient mouse strain [21]. Furthermore, S1P3+/+ mice harbored similar frequencies of splenic B lineage cells compared with heterozygous S1P3+/− mice excluding potential S1P3 dosage effects in populating the peripheral pool (data not shown).
Figure 3.
S1P3 deficient and sufficient immature B cells have similar bone marrow egress. A) Absolute (right panel) and relative (left panel) numbers of B220+ CD93+ (immature/transitional) and B220+ CD93+ (mature) B cells were determined in spleens of 11 week old S1P3−/− (n = 6) and S1P3+/− (n = 6) littermate mice. Data are mean ± SEM of 3 independent experiments; *p<0.05 Student’s one-tailed unpaired t-test. B) S1P3+/+ (wt, n = 8) or S1P3−/− (n = 8) mice were injected i.p. with 1 mg of BrdU every 24 hours for the duration of the experiment. On day four, bone marrow (BM), blood (Bld) and spleen (Spl) cells were harvested and the percentage of BrdU+ cells for each B cell subset was determined by flow cytometry. Data are mean ± SEM of 3 independent experiments. C) B cell frequencies were determined in chimeric mice (n = 17, total) reconstituted with a mixture of lineage depleted bone marrow cells from S1P3−/− and B6.SJL wild type mice. S1P3−/− B cells were identified as CD45.1- and wild type B cells were identified as CD45.1+. B cell subsets in the bone marrow were defined as B220+ IgM+ IgD− (immature) and B220+ IgM+ IgDmid (transitional) and in the blood and spleen as B220+ CD93high (immature) and B220+ CD93mid (transitional). Data are mean ±SEM of 3 independent experiments.
These data suggest S1P3 participates in facilitating immature B cell egress from the bone marrow. To more directly test whether S1P3 contributed to immature B cells bone marrow egress, wild typeand S1P3−/− mice were treated in vivo with BrdU and the percent of BrdU+ immature B cells measured after 2 and 4 days. These results showed that BrdU incorporation was similar between S1P3-sufficient and deficient immature B cells in the bone marrow, blood and spleen after 2 to 4 days (Fig. 3B and data not shown) and suggesting similar developmental kinetics and bone marrow egress. To evaluate egress by another approach we measured S1P3−/− immature B cell exit from bone marrow in competition with wild type B cells using mixed bone marrow chimeras. Lineage depleted bone marrow cells from S1P3−/− (CD45.2+) and B6.SJL (CD45.1+) mice were mixed 1:1 and transferred into lethally irradiated C57BL/6 recipient mice. Six weeks after transfer, the percentage of each donor immature and transitional cells in recipient mice was determined. These experiments revealed that the percentage of S1P3−/− and control immature and transitional B cells remained constant in bone marrow, blood and spleen (Fig. 3C) again indicating that S1P3-deficient cells exit the bone marrow normally. We note, however, that in 3 independent experiments (n = 17 chimeric mice), S1P3−/− B lineage cells reconstituted at a higher frequency relative to wild type B lineage cells. The basis for this increased reconstitution is not clear but we postulate may result from hybrid vigor as donor S1P3−/− cells were from a mixed C57BL/6 and 129/Sv genetic background, whereas donor wild type cells were from C57BL/6 genetic background. Thus, despite that S1P3−/− mice harbor fewer splenic immature B cells, we conclude that S1P3 does not participate in immature B cells egress from bone marrow, nor migration to spleen.
Impaired B cell localization and development in bone marrow of S1P3−/− mice
Our data indicate that S1P3 is not required for immature B cells to leave the bone marrow, yet non-autoreactive, but not autoreactive, immature B cells rely on S1P3 to migrate towards S1P in vitro. Thus, we hypothesized that S1P-mediated migration by non-autoreactive immature B cells might be necessary to localize these cells in a bone marrow microenvironment that facilitates further maturation and/or survival. To address this, we enumerated B cells within the distinct developmental subsets in the bone marrow of S1P3−/− and littermate control mice and found that, compared to controls, S1P3−/− mice had a significant reduction in the number of bone marrow pre-B, immature and transitional B cells (Fig. 4A). In contrast, we did not detect significant differences in the number of bone marrow pro-B or mature B cells between these genotypes. We considered the reduction in S1P3-deficient developing B cell populations might reflect a difference in cell survival between mutant and control cells and directly measured cell survival between genotypes during a 3 day in vitro culture. The results from these experiments showed similar numbers of viable S1P3−/− and control immature B cells throughout the culture period (data not shown) indicating that S1P3 does not participate in immature B cell survival.
Figure 4.
Abnormal B cell development in bone marrow of S1P3−/− mice. A) Absolute numbers of B cell subsets were determined in bone marrow of 11 week old S1P3−/− (n = 6) and S1P3+/− (n = 6) littermate mice. B cell subsets in bone marrow were defined as B220+ CD2−IgM− (pro), B220+ CD2+ IgM− (pre), B220+ IgM+ IgD− (immature), B220+ IgM+ IgDmid (transitional) and B220+ IgM+ IgDhigh (mature). Data are mean ±SEM of 3 independent experiments; *p<0.05 Student’s one-tailed unpaired t-test. B) Absolute and relative numbers of bone marrow sinusoidal B cells were determined in S1P3−/− (n = 8) and S1P3+/− or S1P3+/+ (n = 7) control mice injected i.v. with 1 μg anti-CD19-PE for 2 minutes. Sinusoidal (CD19-PE+) B cell subsets in bone marrow were defined as in (A). Data are mean ± SEM of 3 independent experiments; *p<0.05 Student’s one-tailed unpaired t-test. C) Percentages of bone marrow sinusoidal (CD19-PE+) B cells were determined in 3-83Igi, H-2b (n = 6) and 3-83Igi, H-2d (n = 6) mice injected i.v. with 1 μg anti-CD19-PE for 2 minutes. B cell subsets were defined as B220+ IgMlow 3-83low IgD− (immature autoreactive), B220+ IgM+ 3-83−IgD− (immature receptor edited), B220+ IgM+ 3-83−IgDmid (transitional receptor edited) in 3-83Igi, H-2b (n = 6) mice and B220+ IgM+ 3-83+ IgD− (immature non-autoreactive) and B220+ IgM+ 3-83+ IgDmid (transitional non-autoreactive) in 3-83Igi, H-2d (n = 6) mice. Data are mean ± SEM of 3 independent experiments; *p<0.05 Student’s one-tailed unpaired t-test. D) Percentage of Igλ+ B cells in total bone marrow in S1P3−/− (n = 8) and S1P3+/− or S1P3+/+ (n = 7) control mice. B cells subsets were defined as described in (A). Data are mean ± SEM of 3 independent experiments; *p<0.05 Student’s one-tailed unpaired t-test.
To examine the impact of S1P3 on immature B cell localization in bone marrow, we used a recently described method for selectively labeling lymphocytes located within bone marrow sinusoids [16]. Specifically, S1P3−/− and control mice were injected i.v. with a PE-coupled anti-CD19 monoclonal antibody (anti-CD19-PE) and 2 minutes later mice were sacrificed and bone marrow cells harvested. During this brief treatment only B cells in the sinusoids are labeled with anti-CD19-PE whereas B cells in the parenchyma remain unlabeled. Consistent with previously published results [16], we found that 5.7% of immature B cells and 21.9% of transitional B cells reside in the sinusoids of wild type mice (Fig. 4B). In contrast, while S1P3−/− immature B cell sinusoidal localization was similar to controls, S1P3−/− transitional B cell positioning in the sinusoids was significantly reduced compared to control mice (Fig. 4B). Thus, these data demonstrate that S1P3 is required for normal localization of transitional B cells to the bone marrow sinusoids.
Considering the differential response of autoreactive and non-autoreactive immature B cells to S1P (Fig. 1), we next similarly examined the bone marrow localization of immature and transitional B cells in 3-83Igi, H-2d (non-autoreactive) and 3-83Igi, H-2b (autoreactive) mice. These results clearly demonstrated that only non-autoreactive immature and transitional B cells are located in the sinusoids, whereas immature B cells that express an autoreactive BCR remained unlabeled indicating these cells are restricted to the parenchyma (Fig. 4C). Importantly, in the autoreactive mouse strain, the immature B cells rescued by receptor editing were also found in the sinusoids at similar frequency compared with non-autoreactive immature B cells (Fig. 4C). Since autoreactive immature B cells are not found in the sinusoids (Fig. 4C) and are unable to migrate to S1P (Fig. 1D), we conclude that S1P3 is required for efficient localization of non-autoreactive transitional B cells in the bone marrow sinusoids.
Immature and transitional B cells have been shown to migrate from the bone marrow parenchyma to the sinusoids [16] and likely exit the marrow from this vascular compartment. Our results indicate that autoreactive immature B cells are restricted to the bone marrow parenchyma whereas a considerable frequency of non-autoreactive transitional B cells are found in the sinusoids. Because transitional B cell localization to the sinusoids is also reduced in the absence S1P3, we questioned whether developing S1P3-deficient B cells might be retained in a microenvironment conducive for ongoing Ig gene rearrangement and/or tolerance induction. To assess this we measured Igλ light chain usage by S1P3−/− bone marrow B cells and found an increased proportion of Igλ+ cells in the immature, transitional and mature recirculating B cell subsets compared to control populations (Fig. 4D). These data demonstrate that S1P3 helps shape the B cell repertoire and likely by directing developing non-autoreactive immature and/or transitional B cells from the parenchyma to sinusoids.
DISCUSSION
S1P is a lysophospholipid that has emerged as an important chemoattractant that regulates leukocyte homeostatic trafficking and T lymphocyte development [12, 45]. The S1P1 receptor is expressed by both mature B and T lymphocytes and is responsible for signaling the movement of these cells through secondary lymphoid organs in addition to guiding single-positive immature thymocytes out of the thymus [19]. Despite the many parallels between B and T lymphopoiesis [46–49], and that immature B cells like immature thymocytes also express S1P1, immature B cell egress from bone marrow is not dependent on this S1P receptor. However, whether S1P acting via alternate S1P receptors participates in B cell egress and development remains unknown. In this study we demonstrate that immature B cells migrate towards S1P but that this responsiveness depends on the S1P3 receptor. Importantly, this responsiveness is also dependent on antigen receptor specificity: non-autoreactive, but not autoreactive, immature B cells migrate towards S1P. We further document that whereas S1P3 is not required for immature B cell egress from the marrow, S1P3 is nevertheless necessary for normal B cell development. In the absence of S1P3, developing B cells do not efficiently localize in bone marrow sinusoids and immature and transitional B cells display an altered repertoire as indicated by increased Igλ usage.
The immature bone marrow B cell population is comprised of cells expressing either non-autoreactive or autoreactive specificities [30, 50] and, at present, markers have not been identified that distinguish between these two populations. Thus, defining how antigen receptor specificity influences immature B cell function in mice with a wild type antigen receptor repertoire is not readily amenable to investigation. Immunoglobulin knock-in mice, whose bone marrow B cell development is relatively physiological with regards to the kinetics and level of antigen receptor expression, affords a system to assess how BCR specificity affects function. We have used such a model system to measure S1P receptor expression and response by non-autoreactive or autoreactive immature B cells expressing identical antigen receptor specificities but either in the presence or absence of self-antigen. We show that non-autoreactive and autoreactive immature B cells express the same set of S1P receptors (S1P1, S1P3 and S1P4) as previously reported for both follicular and marginal zone mature B cell populations [20]. Yet, only the S1P3 receptor was required for immature B cell S1P migration whereas S1P1 did not appear to signal migration to this chemoattractant and S1P4 did so only minimally. These findings are similar to that previously reported for marginal zone B cells which use S1P3, and not S1P1, for chemotaxis to S1P [20]. Thus, despite significant expression of S1P1, and in contrast to thymocytes [19, 25, 26], S1P1 does not play a role in mediating immature B cell migration. A potential caveat to this interpretation is that S1P1 transcript levels may not accurately reflect surface expression [51] and determining whether immature B cells express S1P1 protein on the surface and, if so, the role of this receptor on these cells clearly deserves further attention.
Interestingly, autoreactive immature B cells also expressed S1P3, although at reproducibly and modestly lower levels compared to non-autoreactive cells, yet did not demonstrate chemotaxis to a wide range of S1P concentrations. These data suggest that S1P3 signaling is attenuated as a consequence of antigen receptor specificity. In mature B cells BCR-mediated signaling inhibits chemokine and S1P driven chemotaxis [28, 52–54] and this is also true of CXCL12 chemotaxis by bone marrow immature B cells acutely stimulated via the BCR [55]. However, while autoreactive (and chronically BCR-stimulated) immature B cells do not migrate towards S1P they retain CXCL12 responsiveness suggesting specificity in the inhibition of S1P3-mediated migration. The molecular basis for how antigen receptor specificity regulates S1P chemotaxis and whether S1P3 may signal other functions in addition to migration remains to be established.
Having determined that non-autoreactive immature B cells responded to S1P via S1P3 we evaluated whether S1P3 was also required for immature B cell egress. However, a direct examination of wild type and S1P3-deficient immature B cell egress using in vivo BrdU-labeling and mixed S1P3−/− and control bone marrow chimeras indicated equivalent immature B cell bone marrow exit between S1P3-deficient and sufficient cells. Thus, we conclude that S1P3 is not required for immature B cell exit from the marrow. If S1P3-mediated migration to S1P is not required for egress why do non-autoreactive immature B cells display S1P chemotaxis? While still fragmentary, recent progress has been made on identifying microenvironmental niches within the bone marrow that promote the maturation of distinct B cell developmental populations. In particular, in vivo imaging and labeling have recently shown that immature and transitional B cells migrate from the bone marrow parenchyma to the sinusoids prior to leaving the bone marrow [16]. Using a similar labeling technique our results establish the importance of S1P3 in promoting the localization of transitional B cells to the sinusoids as evidenced by the significant reduction in the percent of S1P3-deficient cells able to reach this location. In contrast, S1P3−/− immature B cells localized to the sinusoids similar to control cells implying that transitional, but not immature B cells require S1P3 to enter sinusoids. We note, however, that the proportion of immature B cells that locate to sinusoids was considerably reduced relative to transitional B cells. Given that the sinusoidal microenvironment may be a specialized niche important for immature B cell development [16], we consider that S1P3 could be mediating immature B cell migration to sinusoids and that the inability of cells to reach this niche results in impaired maturation to the transitional stage. As developing S1P3−/− B cells do reach sinusoids albeit in reduced numbers, S1P3 is not the only receptor capable of guiding cells to the sinusoids. We speculate that other receptors, in particular S1P receptors S1P1 and S1P4 could contribute to this positioning.
The marrow microenvironment where immature B cells are interrogated for autoreactivity remains to be identified. Our data demonstrate that autoreactive immature B cells are restricted to the marrow parenchyma indicating that the parenchyma is the predominant site where the immature BCR is assessed for self-reactivity. Tolerance induction by receptor editing is reflected by increased Igλ usage [56, 57] and we find that B cells in S1P3-deficient mice display an elevated percent of Igλ+ cells. While speculative, we suggest that as a consequence of inefficient migration to the sinusoids non-autoreactive S1P3−/− immature B cells are retained in the parenchymal microenvironment where either Ig genes undergo rearrangement and/or immature B cells are rendered tolerant. With this in mind, it will be of interest to more accurately determine where autoreactive and non-autoreactive, as well as S1P3-deficient and sufficient, immature B cells localize in the bone marrow and is an area under current investigation.
Importantly, our data do not formally establish the intrinsic requirement of S1P3 on immature B cells to maintain proper bone marrow B cell numbers. It has been reported by others that the decrease in marginal zone B cell numbers in the spleen of S1P3 deficient mice is due to disordered positioning of MAdCAM-1+ endothelial cells [21]. So it is possible the defects in immature B cell numbers and localization that we observe in S1P3 deficient mice is due to disorder in the stromal microenvironment of the bone marrow. However, our data indicate that expression of S1P3 on immature B cells is necessary for their migration to S1P and it remains unknown if bone marrow stromal cells express S1P3.
Despite that S1P3 does not play a role in immature B cell egress from bone marrow, our results clearly establish a requirement for S1P3 in facilitating normal B cell development. We demonstrate that non-autoreactive immature B cells migrate to S1P in vitro via S1P3 and in the absence of this S1P receptor, developing B cells do not efficiently localize in bone marrow sinusoids and display increased Igλ usage as immature and mature B cells. Thus, we suggest that S1P3 directs non-autoreactive immature B cells to a bone marrow microenvironment important for their development and maturation.
MATERIALS and METHODS
Mice
S1P3−/− mice [43] were used on a mixed C57BL/6 and 129/Sv genetic background. S1P3+/− mice were generated from interbreeding of S1P3+/+ and S1P3−/− mice. Mice used for determining B cell numbers were littermates from F1 crosses between S1P3−/− and S1P3+/− mice. 3-83Igi (Igh3-83/3-83 Igκ3–83/3–83), H-2d and 3-83Igi, Rag1−/−, H-2b mice are on a BALB/c background and have been described previously [31, 58]. B6.SJL-PtprcaPepcb/BoyJ mice were kindly provided by Dr. Philippa Marrack (NJH). In all experiments, age matched (6–12 weeks) mice were used and were housed and bred in specific pathogen free conditions at the Biological Resource Center at National Jewish Health. Experiments were performed using protocols approved by the Institutional Animal Care and Use Committee.
Flow Cytometry and Antibodies
Cells were stained for surface marker expression using the following antibodies: B220 (RA3-6B2; BD Biosciences), IgM (R33.24.12), IgD (1.35), CD2 (RM2-5; eBioscience), Igλ (polyclonal; Southern Biotechnology Associates), CD3 (145-2C11; BD Biosciences), CD49b (DX5; BD Biosciences), CD93 (AA4.1; eBioscience), CD43 (S7; BD Biosciences), 54.1 (anti 3–83 idiotype; [33]) and CD23 (B3B4; Biolegend). Stained cells were analyzed on a FACSCalibur or LSRII (BD Biosciences) and data analyzed with FlowJo v8 (Tree Star) software.
IL-7 bone marrow cultures
Briefly, 3-83Ig H-2d or 3-83Ig H-2b Rag1−/− bone marrow cells were cultured in IMDM (Invitrogen) supplemented with 10% fetal calf serum, 1000 units/mL penicillin, 100 μg/mL streptomycin, Glutamax and 50 μM β-mercaptoethanol in the presence of 50–100 units/mL of IL-7 for 4 to 5 days as described [59]. IL-7 was removed from the culture before assays were performed.
Migration assays
Migration assays were performed as previously described [28]. Briefly, S1P (Avanti Polar Lipids; cat no. 860492P) or recombinant murine CXCL12 (R&D Systems) were added to the bottom well of a 5 μm pore Costartranswell plate in a total of 500 μL of migration medium (IMDM supplemented with 1% fatty-acid-free BSA, Glutamax, 1000 units/mL penicillin and 100 μg/mL streptomycin). Cells were washed twice with migration medium and added to the upper insert of the transwell at a concentration of 106 cells/100 μl. After 3 hr incubation at 37°C, cells were removed from the upper and lower wells and relative cells counts were acquired using a FACSCalibur. Non-autoreactive and autoreactive immature B cells were identified by flow cytometric analysis as described in figure legends. Migration assays were performed in duplicate and percent migration was calculated as (number of cells in lower well)/(number of cells in lower + upper well) × 100.
For serial migration assays, after 3 hr incubation at 37°C (first migration; 0–3hr), cells were removed from the lower wells, and the upper insert of transwells containing non-migrating cells were transferred to a second transwell plate containing fresh migration medium and chemoattractant in the bottom well. After a second 3 hr incubation (second migration; 3–6hr), cells were removed from the upper and lower wells and relative cells counts enumerated. Migration assays were performed in duplicate and percent migration for first migration was calculated as (number of cells in lower well 0–3hr)/(number of cells in lower well 0–3hr + lower well 3–6hr + upper well) × 100. Percent migration for second migration was calculated as (number of cells in lower well 3–6hr)/(number of cells in lower well 3–6hr + upper well) × 100.
Cell sorting and quantitative PCR
Bone marrow cells were isolated from 3-83Ig, H-2d or 3-83Ig, Rag1−/−, H-2b mice and sorted as B220+ IgD− CD23− CD43− using a MoFlow sorter. RNA was isolated using TRIzol (InvitrogenLife Technologies), and trace amounts of DNA were removed usinga DNA-free kit (Ambion). cDNA was prepared from equivalent amounts of RNA using a SuperScript III First-Strand Synthesis Systemfor RT-PCR (Invitrogen Life Technologies). Quantitative PCR amplification was performed using Platinum SYBR Green qPCR SuperMix-UDG(Invitrogen Life Technologies) and detected on an MJ ResearchDNA Engine Opticon 2 real-time PCR machine. Primers for S1P receptors and HPRT were previously described [20].
S1P receptor pharmacological reagents
CAY10444, a selective S1P3 antagonist, and SEW2871, an S1P1 agonist, were purchased from Cayman Chemical. VPC24191, an S1P1 and S1P3 agonist, and VPC23152, an S1P4 agonist, were purchased from Avanti Polar Lipids.
In vivo BrdU labeling
Mice were injected i.p. with 1mg of BrdU (Sigma) in 100 μl of PBS every 24 hours and sacrificed 4 days later. Surfaced stained bone marrow, blood and spleen cells were fixed, permeabilized, DNAse treated and stained with anti-BrdU FITC (BD) according to the Alternate Protocol of Tough et al. [60].
Bone marrow chimeras
Bone marrow cells were isolated from S1P3−/− (CD45.2+) and B6.SJL-PtprcaPepcb/BoyJ (CD45.1+) mice and depleted of CD3+ IgM+ and CD49b+ cells by magnetic-activated cell separation (Miltenyi). Lineage-depleted cells were resuspended in PBS at a 1:1 ratio and a total of 2 × 106 cells were injected i.v. into C57BL/6 recipient mice lethally irradiated with 1000 rads. Six to seven weeks after transfer, recipient mice were sacrificed and bone marrow, blood and spleen cells were stained with CD45.1-FITC (A20; BD Biosciences). B cell subsets were identified by flow cytometric analysis as described in figure legends.
CD19 labeling of bone marrow sinusoidal cells
Mice were injected i.v. with 1 μg of anti-CD19-PE (1D3; BD Biosciences) in 200 μl of PBS and sacrificed 2 minutes after injection as previously described [26]. Bone marrow cells from one femur and one tibia of each mouse were harvested and B cell subsets were identified phenotypically by flow cytometry as described in figure legends.
Acknowledgments
This work was supported by the National Institutes of Health (AI052310 to R.P. and AI052157 to R.T.) and an NIAID training grant (T32-AI07405) award to E.E.D. The authors wish to thank Dr. Richard Proia (NIH) for the gift of S1P3-deficient mice, Kevin Lynch for reagents and advice, and Josh Loomis and Shirley Sobus for help with cell sorting. We also thank members of the R&R lab for useful comments throughout this work and Peter Henson for comments on the manuscript.
Abbreviations used in this paper
- S1P
Sphingosine1-phosphate
Footnotes
CONFLICT OF INTEREST
The authors declare no financial or commercial conflict of interest.
References
- 1.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 2.Meffre E, Casellas R, Nussenzweig MC. Antibody regulation of B cell development. Nat Immunol. 2000;1:379–385. doi: 10.1038/80816. [DOI] [PubMed] [Google Scholar]
- 3.Monroe JG, Dorshkind K. Fate decisions regulating bone marrow and peripheral B lymphocyte development. Adv Immunol. 2007;95:1–50. doi: 10.1016/S0065-2776(07)95001-4. [DOI] [PubMed] [Google Scholar]
- 4.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Pelanda R, Torres RM. Receptor editing for better or for worse. Curr Opin Immunol. 2006;18:184–190. doi: 10.1016/j.coi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Weinreich MA, Hogquist KA. Thymic emigration: when and how T cells leave home. J Immunol. 2008;181:2265–2270. doi: 10.4049/jimmunol.181.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ladi E, Yin X, Chtanova T, Robey EA. Thymic microenvironments for T cell differentiation and selection. Nat Immunol. 2006;7:338–343. doi: 10.1038/ni1323. [DOI] [PubMed] [Google Scholar]
- 8.Glodek AM, Honczarenko M, Le Y, Campbell JJ, Silberstein LE. Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis. J Exp Med. 2003;197:461–473. doi: 10.1084/jem.20021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Le Y, Zhu BM, Harley B, Park SY, Kobayashi T, Manis JP, Luo HR, Yoshimura A, Hennighausen L, Silberstein LE. SOCS3 protein developmentally regulates the chemokine receptor CXCR4-FAK signaling pathway during B lymphopoiesis. Immunity. 2007;27:811–823. doi: 10.1016/j.immuni.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev. 2003;195:58–71. doi: 10.1034/j.1600-065x.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 12.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 13.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 14.Kawabata K, Ujikawa M, Egawa T, Kawamoto H, Tachibana K, Iizasa H, Katsura Y, Kishimoto T, Nagasawa T. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc Natl Acad Sci U S A. 1999;96:5663–5667. doi: 10.1073/pnas.96.10.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 16.Pereira JP, An J, Xu Y, Huang Y, Cyster JG. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat Immunol. 2009;10:403–411. doi: 10.1038/ni.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czeloth N, Bernhardt G, Hofmann F, Genth H, Forster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J Immunol. 2005;175:2960–2967. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- 18.Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. Faseb J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- 19.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 20.Cinamon G, Matloubian M, Lesneski MJ, Xu Y, Low C, Lu T, Proia RL, Cyster JG. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5:713–720. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 21.Girkontaite I, Sakk V, Wagner M, Borggrefe T, Tedford K, Chun J, Fischer KD. The sphingosine-1-phosphate (S1P) lysophospholipid receptor S1P3 regulates MAdCAM-1+ endothelial cells in splenic marginal sinus organization. J Exp Med. 2004;200:1491–1501. doi: 10.1084/jem.20041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, Jacques Y, Baratin M, Tomasello E, Vivier E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 23.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 24.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1- phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 25.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 26.Alfonso C, McHeyzer-Williams MG, Rosen H. CD69 down-modulation and inhibition of thymic egress by short- and long-term selective chemical agonism of sphingosine 1-phosphate receptors. Eur J Immunol. 2006;36:149–159. doi: 10.1002/eji.200535127. [DOI] [PubMed] [Google Scholar]
- 27.Halin C, Scimone ML, Bonasio R, Gauguet JM, Mempel TR, Quackenbush E, Proia RL, Mandala S, von Andrian UH. The S1P-analog FTY720 differentially modulates T-cell homing via HEV: T-cell-expressed S1P1 amplifies integrin activation in peripheral lymph nodes but not in Peyer patches. Blood. 2005;106:1314–1322. doi: 10.1182/blood-2004-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubtsov A, Strauch P, Digiacomo A, Hu J, Pelanda R, Torres RM. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity. 2005;23:527–538. doi: 10.1016/j.immuni.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Czeloth N, Schippers A, Wagner N, Muller W, Kuster B, Bernhardt G, Forster R. Sphingosine-1 phosphate signaling regulates positioning of dendritic cells within the spleen. J Immunol. 2007;179:5855–5863. doi: 10.4049/jimmunol.179.9.5855. [DOI] [PubMed] [Google Scholar]
- 30.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 31.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol. 2004;5:645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- 32.Lang J, Jackson M, Teyton L, Brunmark A, Kane K, Nemazee D. B cells are exquisitely sensitive to central tolerance and receptor editing induced by ultralow affinity, membrane-bound antigen. J Exp Med. 1996;184:1685–1697. doi: 10.1084/jem.184.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 34.Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A, Goodnow CC. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 35.Pelanda R, Schwers S, Sonoda E, Torres RM, Nemazee D, Rajewsky K. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7:765–775. doi: 10.1016/s1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 37.Koide Y, Hasegawa T, Takahashi A, Endo A, Mochizuki N, Nakagawa M, Nishida A. Development of novel EDG3 antagonists using a 3D database search and their structure-activity relationships. J Med Chem. 2002;45:4629–4638. doi: 10.1021/jm020080c. [DOI] [PubMed] [Google Scholar]
- 38.Jongsma M, van Unen J, van Loenen PB, Michel MC, Peters SL, Alewijnse AE. Different response patterns of several ligands at the sphingosine-1-phosphate receptor subtype 3 (S1P(3)) Br J Pharmacol. 2009 doi: 10.1111/j.1476-5381.2009.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brizuela L, Rabano M, Gangoiti P, Narbona N, Macarulla JM, Trueba M, Gomez-Munoz A. Sphingosine-1-phosphate stimulates aldosterone secretion through a mechanism involving the PI3K/PKB and MEK/ERK 1/2 pathways. J Lipid Res. 2007;48:2264–2274. doi: 10.1194/jlr.M700291-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Kimura A, Ohmori T, Kashiwakura Y, Ohkawa R, Madoiwa S, Mimuro J, Shimazaki K, Hoshino Y, Yatomi Y, Sakata Y. Antagonism of sphingosine 1-phosphate receptor-2 enhances migration of neural progenitor cells toward an area of brain. Stroke. 2008;39:3411–3417. doi: 10.1161/STROKEAHA.108.514612. [DOI] [PubMed] [Google Scholar]
- 41.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 42.Clemens JJ, Davis MD, Lynch KR, Macdonald TL. Synthesis of benzimidazole based analogues of sphingosine-1-phosphate: discovery of potent, subtype-selective S1P4 receptor agonists. Bioorg Med Chem Lett. 2004;14:4903–4906. doi: 10.1016/j.bmcl.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 43.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, Yamashita T, Proia RL. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 44.Rolink AG, Andersson J, Melchers F. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur J Immunol. 1998;28:3738–3748. doi: 10.1002/(SICI)1521-4141(199811)28:11<3738::AID-IMMU3738>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 45.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borst J, Jacobs H, Brouns G. Composition and function of T-cell receptor and B-cell receptor complexes on precursor lymphocytes. Curr Opin Immunol. 1996;8:181–190. doi: 10.1016/s0952-7915(96)80056-2. [DOI] [PubMed] [Google Scholar]
- 47.Muljo SA, Schlissel MS. Pre-B and pre-T-cell receptors: conservation of strategies in regulating early lymphocyte development. Immunol Rev. 2000;175:80–93. [PubMed] [Google Scholar]
- 48.Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109(Suppl):S45–55. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann R, Bruno L, Seidl T, Rolink A, Melchers F. Rules for gene usage inferred from a comparison of large-scale gene expression profiles of T and B lymphocyte development. J Immunol. 2003;170:1339–1353. doi: 10.4049/jimmunol.170.3.1339. [DOI] [PubMed] [Google Scholar]
- 50.Grandien A, Fucs R, Nobrega A, Andersson J, Coutinho A. Negative selection of multireactive B cell clones in normal adult mice. Eur J Immunol. 1994;24:1345–1352. doi: 10.1002/eji.1830240616. [DOI] [PubMed] [Google Scholar]
- 51.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 52.Bleul CC, Schultze JL, Springer TA. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. J Exp Med. 1998;187:753–762. doi: 10.1084/jem.187.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guinamard R, Signoret N, Ishiai M, Marsh M, Kurosaki T, Ravetch JV. B cell antigen receptor engagement inhibits stromal cell-derived factor (SDF)-1alpha chemotaxis and promotes protein kinase C (PKC)-induced internalization of CXCR4. J Exp Med. 1999;189:1461–1466. doi: 10.1084/jem.189.9.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casamayor-Palleja M, Mondiere P, Verschelde C, Bella C, Defrance T. BCR ligation reprograms B cells for migration to the T zone and B-cell follicle sequentially. Blood. 2002;99:1913–1921. doi: 10.1182/blood.v99.6.1913. [DOI] [PubMed] [Google Scholar]
- 55.Brauweiler A, Merrell K, Gauld SB, Cambier JC. Cutting Edge: Acute and chronic exposure of immature B cells to antigen leads to impaired homing and SHIP1-dependent reduction in stromal cell-derived factor-1 responsiveness. J Immunol. 2007;178:3353–3357. doi: 10.4049/jimmunol.178.6.3353. [DOI] [PubMed] [Google Scholar]
- 56.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hertz M, Nemazee D. BCR ligation induces receptor editing in IgM+IgD- bone marrow B cells in vitro. Immunity. 1997;6:429–436. doi: 10.1016/s1074-7613(00)80286-1. [DOI] [PubMed] [Google Scholar]
- 58.Braun U, Rajewsky K, Pelanda R. Different sensitivity to receptor editing of B cells from mice hemizygous or homozygous for targeted Ig transgenes. Proc Natl Acad Sci U S A. 2000;97:7429–7434. doi: 10.1073/pnas.050578497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Melamed D, Kench JA, Grabstein K, Rolink A, Nemazee D. A functional B cell receptor transgene allows efficient IL-7-independent maturation of B cell precursors. J Immunol. 1997;159:1233–1239. [PubMed] [Google Scholar]
- 60.Tough DF, Sprent J, Stephens GL. Measurement of T and B cell turnover with bromodeoxyuridine. Curr Protoc Immunol. 2007;Chapter 4(Unit 4):7. doi: 10.1002/0471142735.im0407s77. [DOI] [PubMed] [Google Scholar]