Abstract
The alternative reading frame (ARF) tumor suppressor exerts both p53-dependent and p53-independent functions. The corepressor C-terminal binding protein (CtBP) interacts with ARF, resulting in proteasome-mediated degradation of CtBP. ARF can induce apoptosis in p53-null colon cancer cells, in a manner dependent on ARF interaction with CtBP. Bik was uniquely identified in an apoptotic gene array as coordinately upregulated in colon cancer cells after either CtBP2 knockdown or ARF overexpression. Validating the array findings, ARF induced Bik mRNA and protein expression, and this activity required an intact CtBP binding domain. Apoptosis induced by CtBP deficiency was substantially impaired when Bik expression was simultaneously silenced. An analysis of the Bik promoter revealed binding sites for the CtBP-interacting basic Kruppel-like factor (BKLF). A Bik promoter luciferase reporter was repressed by BKLF and CtBP2, and ARF reversed CtBP-associated repression. Chromatin immunoprecipitation analyses showed that CtBP was recruited to the Bik promoter largely by BKLF. Expression profiling of BH3-only gene expression in ARF-expressing or CtBP-deficient cells revealed that Bik was uniquely regulated by ARF/CtBP in colon cancer cells, whereas additional BH3-only proteins (Bim, Bmf) showed CtBP-dependent repression in osteosarcoma cells. ARF antagonism of CtBP repression of Bik and other BH3-only genes may have a critical role in ARF-induced p53-independent apoptosis and tumor suppression.
Keywords: ARF, CtBP, Bik, BH3, apoptosis
The tumor suppressor alternative reading frame (ARF) is a product of the INK4a/ARF locus1 that can act through p53-dependent or independent pathways.2,3 ARF is frequently inactivated in a wide spectrum of human cancer types, including colorectal, breast, and pancreatic adenocarcinomas, malignant glioma, melanoma, and non-Hodgkin’s lymphoma.4,5 Germline homozygous knockout of Arf in mice results in the development of lymphomas and sarcomas similar to those observed in p53-deficient mice.3 Simultaneous inactivation of p53 and Arf results in a broader tumor spectrum and more aggressive tumors than are observed with either knockout alone,3 suggesting an additional mechanism for ARF tumor suppression apart from its canonical activation of p53.
The transcription regulator C-terminal binding protein (CtBP) has been identified as a specific target of the ARF tumor suppressor relevant to the ability of ARF to induce apoptosis in cells lacking p53.6 ARF binds to CtBP, resulting in proteasome-mediated degradation and inactivation of CtBP.6 In vertebrates, CtBP-family proteins (CtBP1 and CtBP2) are highly conserved and show sequence and functional similarity to D-isomer-specific 2-hydroxy acid dehydrogenases.7 CtBP proteins act as transcriptional repressors in conjunction with a wide range of DNA-binding transcription factors, and are regulated and activated as repressors by nicotinamide adenine dinucleotide (NADH) binding to their dehydrogenase domains.8
CtBP1/2-null mouse embryo fibroblasts (MEFs) are hypersensitive to apoptosis in response to a wide variety of stimuli.9 Microarray analysis has shown that epithelial-specific and proapoptotic genes are upregulated in these MEFs,9 although the precise mechanism that links CtBP to the suppression of proapoptotic gene expression is not known. Separate evidence suggests that small interfering RNA (siRNA)-mediated CtBP knockdown in human tumor cell lines is sufficient to induce apoptosis in the absence of additional stress.6 Although not yet proven, CtBP is likely to be linked to tumor progression, as it promotes both cell survival and epithelial-mesenchymal transition by repressing the transcription of both proapoptotic and epithelial genes.9
The effects of CtBP on cell survival have been linked specifically to its repression of proapoptotic Bcl-2 homology domain 3 (BH3)-only genes, of which Noxa and Puma were identified in the microarray comparison of wild-type and CtBP1/2 knockout MEFs.9 It is noteworthy that the proapoptotic BH3-only proteins are critical mediators of death induced by cytokine deprivation, activated oncogenes, and various DNA-damage stresses.10 Their presumptive mechanism of action is to dissociate bax or bak from antiapoptotic bcl-2 family proteins, allowing them to translocate to the outer mitochondrial membrane to form pores that allow the cytoplasmic release of cytochrome c.11,12 Of the eight known BH3-only genes, Bid is a critical mediator of apoptosis mediated by death receptor signaling,13 Bim is the determinant of taxane responsiveness,14 Puma and Noxa are central mediators of p53-induced apoptosis,15 and Bad regulates apoptosis mediated by growth factor/cytokine signaling.16 In contrast, the cellular apoptotic stimuli that act through Nbk/Bik, and the biologic functions of this gene in mammals, is not yet known.17
In this study, the BH3-only protein Bik was identified as an ARF and BKLF/CtBP-regulated gene, and a critical mediator of ARF/CtBP-induced p53-independent apoptosis in colon cancer cells. CtBP repression of Bik was directly antagonized by ARF, and CtBP was recruited to the Bik promoter through BKLF. Apoptosis induced by CtBP deficiency in the absence of p53 was substantially impaired when Bik expression was also reduced by RNA interference, and stress-induced p53-independent apoptosis was potentiated by ARF. Other BH3-only family members besides Bik were co-regulated by CtBP in other cell types to suggest that p53-independent ARF tumor suppression may involve regulation of different sets of BH3-only proteins, dependent on tissue origin.
Results
Bik is upregulated after CtBP2 depletion or ARF overexpression
To identify the mediators of ARF/CtBP2-induced p53-independent apoptosis,6 a human cDNA apoptosis microarray was interrogated with mRNA obtained from HCT116 p53−/− cells infected with control or ARF adenovirus,6 or treated with control or CtBP2 siRNA. Genes with more than a twofold change (as compared with control) after either CtBP2 depletion or ARF overexpression were considered for further analysis. Although a number of TNF pathway genes were induced after both ARF expression and CtBP2 silencing, none were common between the two conditions (Figure 1a). The BH3-only gene Bik was the only common gene upregulated under both conditions (Figure 1a).
Figure 1.
Bik is upregulated upon ARF overexpression or CtBP depletion in p53-null human colon cancer cells. (a) Total RNA isolated from HCT116 p53−/− cells after either ARF overexpression or CtBP knockdown was subjected to an apoptotic gene array (SABiosciences, Frederick, MD, USA) analysis. The relative expression level of genes relevant to apoptosis was estimated by comparing signal intensities of spots of cDNA for each relevant gene with the intensity of spots of housekeeping genes. The fold changes in gene expression level after ARF overexpression or CtBP knockdown was calculated by using GEArray Expression Analysis Suite (SABiosciences). (b) ARF/CtBP regulates Bik at the mRNA level. Quantitative real-time PCR using RNA prepared from HCT116 p53−/− cells infected with either vector (Ev) or hARF retrovirus, or transfected with either control (siCon) or CtBP2 (siCtBP2) siRNA, was carried out using Bik- and GAPDH-specific primers. The bars represent GAPDH-normalized average fold change of Bik in the treated cells. The error bars represent ±1 S.D. (c) CtBP2 regulates Bik expression. HCT116 p53−/− cells were treated with control (siCon) or CtBP2 (siCtBP2) siRNA duplexes, and CtBP2, Bik, and GAPDH levels were determined by immunoblotting 24 h after transfection. (d) CtBP2 interaction with ARF is required for regulation of Bik expression. HCT116 p53 −/− cells were infected with vector, hARF, or hARF (L50D) mutant lentiviruses for 24 h. Cell lysates were analyzed for Bik, GAPDH, and ARF levels using immunoblotting
To more quantitatively assess the effects of CtBP2 depletion and ARF overexpression on Bik expression, Bik mRNA levels were analyzed in HCT116 p53−/− cells at 24 h after either depletion of CtBP2 using siRNA or ARF overexpression by retroviral infection (Figure 1b). Real-time polymerase chain reaction (PCR) analysis confirmed that Bik expression was increased upon either CtBP2 depletion (2.6-fold) or ARF over-expression (2.3-fold) in HCT116 p53−/− cells (Figure 1b). Similarly, the protein levels of Bik were found to be upregulated in CtBP2 siRNA-treated cells as compared with control siRNA-treated cells (Figure 1c).
CtBP2 interaction is required for ARF regulation of Bik
As the ability of ARF to interact with CtBP correlates with its ability to induce apoptosis in p53-null cells,6 Bik expression was analyzed in cells in which ARF/CtBP interaction was either intact or abrogated. HCT116 p53−/− cells infected with ARF, ARFL50D (CtBP interaction was defective6, but p53 stabilization function and nucleolar localization were similar to those of wild-type p14ARF; Supplementary Figures S1A–C), or control retrovirus were analyzed for Bik protein levels using western blotting (Figure 1d). Both ARF and ARFL50D were expressed at similar levels (Figure 1d). Bik was induced in ARF-expressing cells, but not in ARFL50D-expressing cells, in which Bik levels were similar to those in empty virus-infected cells (Figure 1d). Thus, the ability of ARF to interact with CtBP was required for its induction of Bik expression.
Bik depletion rescues ARF/CtBP-induced p53-independent apoptosis
To further analyze the hypothesis that Bik functions as an important mediator of ARF/CtBP-induced apoptosis, Bik and CtBP2 were individually or simultaneously knocked down in HCT116 p53−/− cells induced to undergo apoptosis by ultraviolet (UV) treatment. Despite comprehensive screening for effective Bik-specific siRNA and shRNA sequences, the best knockdown of Bik that could be achieved was only partial, although two independent shRNA sequences were obtained (Supplementary Figure S2a). Annexin V and Trypan blue stains documented apoptotic fraction (Figure 2a) and viability (Figure 2b), and cell lysates were immunobloted to monitor Bik and CtBP2 levels and assess poly (ADP-ribose) polymerase (PARP) and caspase 3 cleavage (Supplementary Figure S2b).
Figure 2.
p53-independent stress-induced apoptosis through an ARF/CtBP/Bik pathway in colon cancer cells. (a) Apoptosis assay: HCT 116: p53−/− cells were stably infected with control (shCon), shBik1, or shBik2 lentiviruses and transiently transfected with either control (siCon) or CtBP2 (siC2) siRNA. All cells were exposed to 20 J/m2 UV-C. After 24 h of transfection and UV treatment, apoptosis was determined by annexin V-PE/7-AAD staining. (b) Cell viability assay: the percentage of non-viable cells was determined after treatment as in (a) by staining cells with 0.4% Trypan blue. (c) ARF/CtBP2 regulation of stress-induced apoptosis. HCT116 p53−/− cells infected with empty, ARF, or L50D retroviruses were exposed to 20 J/m2 UV-C or hypoxia (0.5% O2), and the level of apoptosis determined after 24 h using annexin V-PE/7-AAD staining. All experiments were performed in triplicate and the results were expressed as Mean±1 S.D., *P<0.05 for (a, b) siC2/shCon versus siCon/shCon; (c) ARF versus Ev. ** P<0.05 for (a, b) siC2/shBik1 versus siC2/shCon; †P = 0.07 for (a, b) siC2/shBik2 versus siC2/shCon
As expected, siRNA-mediated CtBP2 depletion led to increased Bik levels in cells with control shRNA, and even in Bik shRNA-expressing cells (Supplementary Figure S2b). The basal apoptosis rate in low-dose (20 J/m2) UV-treated cells with control siRNA and shRNA was 7%, and the overall non-viability rate was 12% (Figures 2a and b). Treatment with siCtBP2 induced a more than doubling of the apoptotic fraction to 16% (P =0.04) and non-viability to 27% (P = 0.01). This effect was partially abrogated by shBik1, with reduction of apoptotic fraction to 11% (7% basal level, P = 0.02) and non-viability to 15% (12% basal level, P = 0.02; Figures 2a and b). A second Bik shRNA (shBik2) yielded essentially similar effects in all assays, although the P-value only indicated a trend to significance for both apoptosis and viability (P = 0.07), possibly because of the less effective knockdown achieved with this shRNA sequence (Figures 2a and b, Supplementary S2a). Results with annexin and Trypan blue staining were mirrored in the abundance of PARP and caspase 3 cleavage products (Supplementary Figure S2b). These data strongly support the hypothesis that Bik has an important role in the induction of apoptosis after CtBP depletion in colon cancer cells. The partial rescue of apoptosis by Bik knockdown would be consistent with the partial knockdown of its expression by shRNA, suggesting that a more robust knockdown might have further suppressed apoptosis closer to baseline levels. However, the contribution of other proapoptotic proteins (BH3-only or other) to apoptosis in CtBP2-deficient cells cannot be completely ruled out.
Potentiation of environmental stress-induced apoptosis by ARF/CtBP interaction
ARF is not generally considered to participate in stress-induced apoptosis, such as that caused by UV, as it is not expressed at detectable levels in normal cells and is induced mainly by oncogenic, and not environmental, stress signals.2,4 However, the ability of ARF to inhibit CtBP might potentiate the apoptotic affect of environmental stress, and this could be of importance in situations in which oncogenically activated and p53-deficient (thus ARF-expressing) cells are exposed to mutagenic (UV) or tumor-promoting (hypoxia37) stimuli. To gauge the affect of ARF expression and ARF/CtBP complex formation on stress-induced apoptosis in cells lacking p53, HCT116 p53−/− cells were transduced with empty, ARF or ARFL50D-expressing retroviruses, and exposed to UV (20 J/m2) or hypoxia (0.5% O2), followed by determination of apoptotic fraction. ARF expression led to a 2.7-fold and 1.7-fold increase, respectively, in the apoptotic fraction after UV or hypoxia treatment as compared with cells transduced with control virus (P<0.05 for both conditions; Figure 2c). ARFL50D expression in UV-treated or hypoxic cells, by contrast, did not induce additional apoptosis over the background level observed with empty virus in each condition (Figure 2c). These findings show that ARF’s interaction with, and inhibition of, CtBP can potentiate p53-independent apoptosis induced by two disparate and potent cancer-related cellular stressors.
ARF/CtBP regulation of the Bik promoter through BKLF recognition elements
In silico analysis of the Bik promoter for recognition sites relevant to transcription factors that recruit CtBP as a corepressor, revealed five sites with an exact match to BKLF (KLF8/ZNF741/BKLF3) recognition elements, including a tandem repeat18 (Figure 3a). Examination of the upstream (−1 to −3000) promoter regions of the other seven known BH3-only genes revealed obvious BKLF sites upstream of the Noxa, Puma, Bmf, and Bim genes (Figure 3a). To test the hypothesis that the recruitment of CtBP by BKLF represses Bik promoter activity, Bik promoter luciferase reporters containing either all wild-type BKLF-binding sites, or with the two tandem sites mutated, were transfected into U2OS cells with BKLF, ARF, and CtBP2 expression vectors, as indicated. Either BKLF or CtBP2, alone, repressed the wild-type promoter by approximately 2.5-fold (P<0.01; Figure 3b), whereas there was no effect on the mutant reporter (Supplementary Figure S3). CtBP2/BKLF coexpression further repressed Bik promoter activity by another twofold (P<0.05; Figure 3b). Overexpression of ARF had no effect on BKLF repression of Bik promoter activity (compare first and third grey bars; Figure 3b), but when ARF was co-transfected with CtBP2 and BKLF, ARF caused a near-complete reversal of CtBP2-associated repression (compare second and fourth grey bars; P<0.05; Figure 3b). Reversal of CtBP2-mediated repression by ARF is consistent with the finding that ARF degrades and/or sequesters CtBP in the nucleolus, abrogating its repressor activities.6 Thus, BKLF elements are crucial for CtBP/BKLF-mediated repression of the Bik promoter. Consistent with the known effects of ARF on CtBP, ARF reversed CtBP2/BKLF-mediated repression of the Bik promoter, but had no effect on BKLF-mediated repression in the absence of CtBP2.
Figure 3.
ARF/CtBP regulates the Bik promoter through BKLF recognition elements. (a) BH3-only genes contain BKLF recognition elements. (Left) Diagram of BKLF recognition elements (BK) in the Bik promoter and (right) alignment and BKLF element localization in the Noxa, Puma, Bim, Bmf, and Bik promoters. (b) ARF antagonizes CtBP/BKLF repression of the Bik promoter. A Bik promoter luciferase reporter plasmid (pGL3-Bikluc) was co-transfected with or without expression constructs for ARF, BKLF, or CtBP2 into U2OS cells along with a control reporter plasmid expressing Renilla luciferase (pRL-TK). Normalized firefly luciferase activity from three independent experiments was averaged, and error bars indicate ± 1 S.D. *P<0.05 for CtBP2 +BKLF versus BKLF; **P<0.05 for ARF + CtBP2 +BKLF versus CtBP2 + BKLF
CtBP2 is recruited to the Bik promoter
Previous studies have shown that CtBP2 binds to BKLF and regulates expression of genes downstream of BKLF recognition elements.19 To address whether CtBP is directly recruited to the Bik promoter, CtBP2 chromatin immunoprecipitation (ChIP) using chromatin from H1299 lung carcinoma cells was performed (Figure 4). The PS1 PCR primer set amplified a fragment adjacent to a single BKLF-binding site in the distal Bik promoter, whereas the PS2 primer set amplified a fragment encompassing two tandem BKLF-binding sites critical for regulation of the promoter (Figure 3) and adjacent to two additional sites in the proximal Bik promoter (Figure 4a). NS negative control primers amplified a fragment located 10 kb upstream of the Bik promoter, whereas the E-cadherin promoter was used as a positive control (Figure 4b).20 CtBP2 did not localize to the promoter region interrogated by the PS1 primers, possibly because of the presence of only a single nearby BKLF-binding site (Figure 4a), but robust binding was observed to the E-cadherin promoter (Figure 4b). CtBP2 did show strong binding to the PS2 fragment (Figures 4a and b), correlating with luciferase reporter data showing that most of the transcriptional regulation by BKLF/CtBP operates through the two tandem BKLF sites within PS2 (Figure 3b and Supplementary Figure S3). Adding further specificity to these data, CtBP2 did not localize to the NS control fragment, and the control IgG ChIPs with PS1 and PS2, and a no-antibody ChIP with PS2 also did not yield any signal (Figures 4a and b).
Figure 4.
BKLF-mediated recruitment of CtBP to the Bik promoter. (a) ChIP assay of the Bik promoter was performed using chromatin prepared from H1299 cells and CtBP2 or control IgG antibody. Immunoprecipitated and input DNAs were amplified by PCR using PS1 and PS2 primer sets specific for fragments of the Bik promoter indicated in the graphic, and a negative control primer set (NS) that amplifies a fragment 10 kb upstream of the Bik promoter. PCR products were electrophoresed in an agarose gel and stained with ethidium bromide. (b) ChIP assay for CtBP2. H1299 chromatin was immunoprecipitated with or without CtBP2 antibody. Immunoprecipitated and input DNAs were amplified by PCR using NS, specific Bik PS2 primers, or positive control E-cadherin promoter primers20. (c) Efficacy of BKLF shRNA. shRNA targeting BKLF or GFP was stably expressed in H1299 cells. RT-PCR was performed to determine the knockdown of BKLF mRNA with GAPDH mRNA level as an internal control. RT-PCR products were electrophoresed in an agarose gel and stained with ethidium bromide. (d) BKLF regulation of Bik expression. shBKLF or shGFP were stably expressed in HCT116 p53−/− cells, and protein levels of GAPDH and Bik were determined using immunoblotting. (e) CtBP2 recruitment to the Bik promoter requires BKLF. CtBP2 or control (no antibody) ChIP was performed with chromatin obtained from shGFP or shBKLF-expressing cells. Immunoprecipitated DNA was analyzed by PCR using Bik PS2, NS, and E-cadherin primers as in (a). (f) A model of BKLF/CtBP2-mediated transcription regulation of Bik expression
To test whether CtBP2 recruitment to the Bik promoter required BKLF, a CtBP2 ChIP assay was performed using chromatin from cells in which BKLF was stably depleted using shRNA (knockdown ~50% by RT-PCR; Figure 4c) and Bik PS2, control NS, and E-cadherin primers. When compared with control, BKLF shRNA expression led to increased Bik expression as determined using immunoblot, and as predicted using the reporter assays (Figures 3b and 4d). As expected, CtBP2 was present at the Bik and E-cadherin promoters in control shRNA-expressing cells, but in the absence of BKLF, CtBP2 was no longer recruited to the Bik promoter, despite remaining present at the E-cadherin promoter (Figure 4e). The specificity of the ChIP signals was bolstered by the lack of signal either in the control (no antibody) ChIP or in the CtBP2 ChIP with NS primers (Figure 4e). Thus, CtBP2 is recruited to the Bik promoter largely through BKLF (Figure 4f).
Differential regulation of BH3-only proapoptotic genes by ARF/CtBP
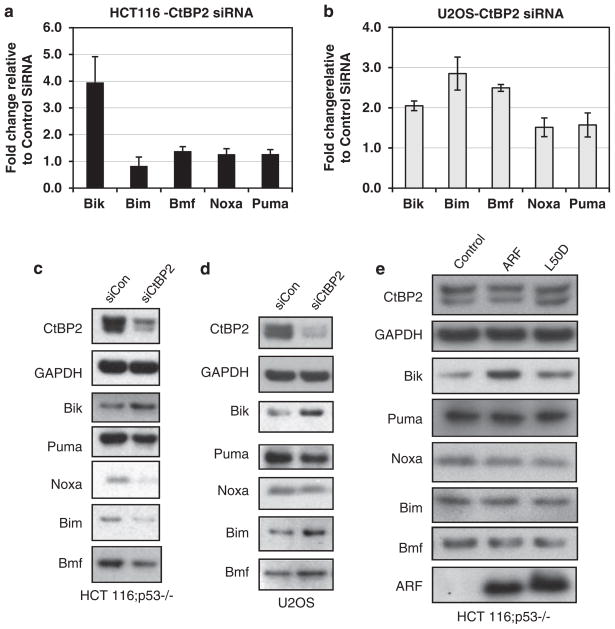
Though Bik has an important role in ARF-induced p53-independent apoptosis in HCT116 p53−/− cells, several other BH3-only genes have been previously shown by genomic techniques (in MEFs) to be regulated by CtBP1 and CtBP2.9 To study the potential regulation of the family of BH3-only genes by CtBP in cells of either epithelial or mesenchymal lineage, the mRNA and protein levels of Bik, Bim, Bmf, Noxa, and Puma were determined in HCT116 p53−/− colon carcinoma cells and U2OS osteosarcoma cells after treatment with control or CtBP2 siRNA (Figure 5). As predicted by the apoptosis gene array (Figure 1), Bik was the only BH3 gene that was induced >twofold in HCT116 p53−/− cells, whereas U2OS cells were more permissive for BH3-only gene expression, with a 2- to 2.5-fold induction of Bik, Bim, and Bmf, and a 1.5-fold induction of Puma and Noxa mRNAs, after CtBP2 knockdown (Figures 5a and b). When mRNA induction was ≥2-fold, protein expression was also increased, as observed for Bik in HCT116 p53−/− cells, and for Bik, Bim, and Bmf in U2OS cells (Figures 5c and d).
Figure 5.
Regulation of BH3-only genes by ARF and CtBP. RNA isolated after CtBP2 or control siRNA treatment of HCT116 p53−/− (a), or U2OS (b) cells was subjected to RQ-PCR using GAPDH, β-actin and Bik, Bim, Bmf, Puma, and Noxa primers. Cell lysates from HCT116 p53−/− (c), or U2OS (d) cells treated with control or CtBP2 siRNA and HCT116 p53−/− cells infected with either empty, ARF, or ARFL50D retroviruses (e) were analyzed for GAPDH, Bik, Bim, Bmf, Puma, and Noxa protein levels using immunoblotting. siCon, control siRNA; siCtBP2, CtBP2 siRNA
ARF expression would be expected to phenocopy CtBP2 knockdown for regulation of BH3-only gene expression, based on its antagonism of CtBP.6 Indeed, ARF expression resulted in Bik induction as previously observed (Figure 1), but had little effect on protein levels of the other BH3-only proteins (Bim, Bmf, Puma, and Noxa) in HCT116 p53−/− colon carcinoma cells (Figure 5e). ARFL50D expression had little effect on the abundance of any BH3-only protein, including Bik, as has been already noted (Figures 1 and 5e). Thus, ARF expression phenocopies the specific affect of CtBP2 depletion on BH3-only gene expression in colon cancer cells, supporting the hypothesis that ARF/CtBP complexes directly control BH3-only gene expression and thus, p53-independent apoptosis, in a cell-type specific manner.
Discussion
ARF overexpression, or depletion of CtBP2, induced mRNA and protein expression of the proapoptotic BH3-only gene Bik. Induction of Bik required ARF/CtBP interaction, and the induction of apoptosis by UV and CtBP2 depletion required physiological levels of Bik. ARF potentiated UV- or hypoxia-induced apoptosis in a manner dependent on CtBP interaction. CtBP2 was recruited to the Bik promoter by the transcription factor BKLF, and ARF abrogated CtBP2/BKLF repression of the Bik promoter. Furthermore, the pattern of BH3-only gene regulation by CtBP seemed to depend on the cell-type context, suggesting that p53-independent tumor suppression by ARF may be more relevant to certain tumor types than others.
Bik is a proapoptotic protein of the ‘BH3-only’ family. Expression of Bik triggers apoptosis in breast, lung, prostate, and colon carcinoma cells, as well as in glioma and melanoma-derived cell lines.12,21–24 Consistent with a role for Bik in tumor suppression, chromosome 22p13.3, which contains Bik, is commonly deleted in human colorectal and breast cancers,25 and Bik mutations have been identified in renal cell carcinoma.26
In contrast, Bik functions in non-malignant cells may overlap with those of other BH3-only proteins, as Bik is not essential for normal development.27 In vitro, Bik knockout mouse T and B cells did not show an apoptotic defect, although epithelial cells were not examined in that study.27 The absence of a mouse phenotype does not necessarily exclude a role in native tumor suppression, as the Bik knockout mice and cells were not exposed to an oncogenic stress to reveal a more subtle tumor or apoptotic phenotype.27
Functionally, Bik is not a direct initiator of apoptosis, but acts upstream of the pro-survival Bcl-2-family members.28 Recent studies also suggest that Bik has a role in oxidative stress-induced apoptosis.29 Bik binds directly to Bcl-2 or Bcl-XL through its BH3 domain, and inactivates their antiapoptotic functions. Therefore, an increase in Bik levels lowers the cellular apoptotic threshold by blocking the antiapoptotic function of Bcl-2-family proteins.30 A competing hypothesis suggests that Bik might also activate the downstream effectors Bak or Bax directly to cause apoptosis.31 Further study on the apoptotic pathway downstream of Bik will be required to fully understand the role of Bik in ARF/CtBP2-induced p53-independent apoptosis.
CtBP has been described as a transcriptional regulator of apoptosis by repressing multiple proapoptotic genes, such as Noxa, Puma, and PERP.9 Many of these genes are also the known transcriptional targets of p53. However, CtBP regulation of Bik, as shown in the current work, is p53 independent, and likely acting through BKLF instead of p53. BKLF can recruit mCtBP2, through its PXDLS motif32 to the β-globin promoter element, resulting in repression.33 We have observed that CtBP2 is also recruited by BKLF to tandem CACCC elements in the Bik promoter, as knockdown of BKLF abrogated CtBP2 recruitment to the Bik promoter in colon cancer cells, and mutation of the tandem repeat abrogated CtBP2 repression of a Bik reporter. The involvement of other related KLF transcription factors (KLF1/EKLF: erythroid Kruppel-like factor, KLF2/LKLF: lung Kruppel-like factor, KLF4/GKLF: gut-enriched, KLF5/IKLF: intestinal-enriched, KLF7/UKLF: ubiquitous KLF, among others) in CtBP2 recruitment cannot be ruled out in other cell contexts, as many KLFs function in a tissue-dependent manner.34
CtBP senses the metabolic state of the cell because of a requirement for NADH binding to its dehydrogenase domain to activate repressor function.8 CtBP has been linked to the hypoxic activation of cell migration, and this effect may be because of its repression of other non-apoptosis pathway genes, such as phopshin and tensin homolog chromosome 10 (PTEN).9,35 As hypoxia is fundamentally linked to tumor progression, CtBP may serve as a critical oncogenic link by which hypoxia leads to activation of key malignant characteristics, such as enhanced cell survival and increased motility and invasion.35–37 On the basis of this hypothesis, the current data would support the idea that Bik might be especially important in tumor suppression in hypoxic cells, and moreover, ARF loss or mutation should specifically enhance cell survival in hypoxia by release of CtBP from any negative control. This idea is certainly consistent with ARF’s enhancement of apoptosis caused by hypoxia (Figure 2c). This hypothesis may also explain why ARF can so profoundly affect tumor progression in vivo, with its loss promoting increased tumor aggressiveness.36,38,39
This work raises the possibility that tumorigenesis is enhanced in the absence of ARF because of an apoptotic defect in a parallel ARF-regulated tumor surveillance system that is completely independent of p53. With a further understanding of the cellular consequences of ARF/CtBP interaction, there is the distinct possibility of manipulating this pathway either through ARF-mimetics or CtBP inhibitors for therapeutic benefit in the substantial fraction of tumors that lack p53 and/or ARF function.
Materials and Methods
Cell culture and transfection
HCT116 human colon cancer cells (ARF silenced)40 with targeted deletion of p53 were grown in McCoy’s 5A medium (Invitrogen, Carlsbad, CA, USA). U2OS (human osteosarcoma) and H1299 (human lung cancer) cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg of streptomycin, and incubated in humidified 5% CO2 at 37°C. Mammalian expression plasmids were transfected using Fugene,20 and siRNA duplexes were transfected with Oligofectamine (Invitrogen), with an siRNA concentration of 40 nM. After retro/lentiviral infections, the cells were selected using puromycin at 2 μg/ml.
Plasmids, siRNAs, shRNA, and viral expression vectors
V5-tagged CtBP2 expression plasmid pcDNA-V5-CtBP2 has been described earlier.6 PcDNA-T7ARF was generated by insertion of a PCR-amplified ARF coding sequence with a T7 tag sequence embedded in the 5′ primer into pCDNA3. The expression plasmid containing the CtBP2-binding defective allele of L50D of ARF was generated from PcDNA-T7ARF using PCR as per the Quikchange protocol (Stratagene, La Jolla, CA, USA). pBabe-Puro ARF and pBabe-ARFL50D were used to generate retroviruses. pLenti-ShGFP, pLenti-ShBik, pLenti-Puro-hARF, and pLenti-Puro-hARFL50D were generated using the Gateway cloning system (Invitrogen). Retro/lentiviruses were produced in HEK293T cells by transfecting ARF constructs along with packaging constructs (pol/gag and VSVG). The siRNA sequence for human CtBP2 was AAGCGCCUUGGUCAGUAAUAG; shBKLF: CTGGTCGATATGGATAAACTCA; shBik1: GGAGAAATGTCTGAAGTAA; and shBik2: ACACTTAAGGAGAACATAA.
Immunoblotting and immunofluorescence
Cells were lysed in lysis buffer (20 mM HEPES pH 7.4, 0.5% Triton X-100, 2 mM MgCl2, 10 μM ZnCl2, 2 mM N-ethylmaleimide, 1 mM phenylmethylsulfonyl fluoride, and 240 mM NaCl) containing protease inhibitor tablets.20 Antibodies used were as follows: CtBP2 (BD Biosciences, San Jose, CA, USA), hARF (Novus Biologicals, Littleton, CO, USA), T7 tag (Novagen, Madison, WI, USA), GAPDH (Advanced Immuno Chemical, Long Beach, CA, USA), Noxa (Imgenex, San Diego, CA, USA), Bik, Puma, Cleaved Caspase (Cell Signaling, Danvers, MA, USA) PARP (Santa Cruz, Santa Cruz, CA, USA). Anti-rabbit IgG-horseradish peroxidase and anti-mouse IgG-horseradish peroxidase conjugates (Jackson Immunoresearch) were used with enhanced chemiluminescence detection (GE Healthcare, Piscataway, NJ, USA) for immunoblotting. Immunofluorescent detection of ARF and ARFL50D was performed as described6 using anti-hARF antibody.
cDNA array analysis of apoptosis-associated genes
Total RNA was extracted from HCT116 p53−/− cells after CtBP2 knockdown or ARF infection using RNeasy (Qiagen, Valencia, CA, USA). Biotin labeled cDNA probes were generated with 5 μg of total RNA using TrueLabeling-AMP 2.0 kit (SuperArray) according to the manufacturer’s instructions. cDNA probes were purified using an ArrayGrade cDNA cleanup kit (SABiosciences). Biotinylated-cDNA probes were denatured, hybridized to GEarray Human Apoptosis microarray (OHS-012, SuperArray) as per the manufacturer’s instructions. After overnight incubation at 60°C, the membranes were washed successively in 2 × SSC-1% sodium dodecyl sulfate (SDS) and 0.1 × SSC-0.5% SDS for 15 min each. The arrays were developed using chemiluminescent detection (SABiosciences) and the acquired images were analyzed using GE Array Expression Analysis Suite 1.1 (SABiosciences). The basic raw data were normalized for empty spot and housekeeping genes (GAPDH and β-actin).
Real-time quantitative PCR
mRNA transcripts for human Bik, Puma, Noxa, and GAPDH were analyzed by real-time quantitative reverse transcription-PCR (RQ-PCR) using SYBR green (Applied Biosystems, Foster City, CA, USA) and an ABI 7300 (Applied Biosystems). Relative amounts of the mRNA transcripts were calculated using the ΔΔCT method with GAPDH and β-actin mRNA as internal references. The primer sets used were Bik (sense: 5′-TCCTATGGCT CTGCAATTGTCA-3′, antisense: 5′-GGCAGGAGTGAATGGCTCTTC-3′), Bim (sense: 5′-GCCCCTACCTCCCTACAGAC-3′, antisense: 5′-ACTGTCGTATGGA AGCCATTG-3′), Bmf (sense: 5′-CCACCAGCCAGGAAGACAAAG-3′, antisense: 5′-TGCTCCCCAATGGGCAAGACT-3′), Noxa (sense: 5′-CTGCAGGACTGTT CGTGTTCA-3′, antisense: 5′-GGAACCTCAGCCTCCAACTG-3′), Puma (sense: 5′-GGGCCCAGACTGTGAATCC-3′, antisense: 5′-CGTCGCTCTCTCTAAACC TATGC-3′), and β-actin (sense: 5′-GCTCCTCCTGAGCGCAAGT-3′, antisense: 5′-TCGTCATACTCCTGCTTGCTGAT-3′). RT-PCR for BKLF was performed using the following BKLF primers: sense BKLF 5′-AGGTGGCTCAATGCAGGTAT-3′ and antisense BKLF 5′-CATGGGCAGAGACTGCACTA-3′. GAPDH primers were sense 5′-ATCACCATCTTCCAGGAGCGA-3′ and antisense 5′-GCCAGTGAGCTTCC CGTTCA-3′.
Apoptosis and cell viability assays
For viability analysis, cells were trypsinized and mixed 1: 1 with Trypan blue solution and counted using a hemocytometer. For apoptosis analysis, cells were trypsinized after 48 h of transfection, washed with phosphate buffered saline (PBS) and stained with annexin V-phycoerythrin and 7-aminoactinomycin D (AAD) according to the manufacturer’s instructions (BD Biosciences, USA).
Bik promoter luciferase reporter assay
A 1.9-kb region of human Bik promoter (−1710 to + 203) was amplified by PCR from pBLCAT218 and inserted into the firefly luciferase reporter pGL3 (Promega, Madison, WI, USA). U2OS cells were transfected with pGL3-Bik and a control plasmid expressing the Renilla luciferase (pRL-TK), CtBP2, ARF, and BKLF using Fugene.20 The expression of reporter genes was determined using Dual Luciferase assay (Promega) after 36 h of transfection.
Chromatin immunoprecipitation assay (ChIP)
Cells were plated for 24 h and approximately 108 cells were used for each ChIP assay. Cells were washed once in PBS and were treated with 1% formaldehyde in cold PBS for 10 min at 4°C with continuous shaking. Glycine (final concentration of 125 mM) was added to quench the formaldehyde for 5 min at 4°C with continuous shaking. Cells were then harvested and washed twice with ice-cold PBS. Nuclei were isolated by incubating the cells in nucleus isolation buffer (5 mM PIPES pH 8.0, 85 mM KCl, and 0.5% NP-40) for 20 to 30 min on ice. The nuclei were harvested at 4°C by centrifuging the cell suspension at 7000 × g for 5 min and resuspended in 2 ml of RIPA buffer (150 mM NaCl, 1% v/v NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris pH 8. 0, and 5 mM ethylenediamine tetra-acetic acid (EDTA)) containing protease inhibitors. Chromatin was fragmented to approximately 200–700 bp by sonication. Nuclear debris was removed by centrifuging the lysates at 4°C for 15 min at 14 000 r.p.m. The lysate was precleared by incubation with the protein G Sepharose beads for 30 min at 4°C. Immunoprecipitation was performed overnight at 4°C with the respective antibody. Protein G Sepharose beads were added and the immunocomplexes were allowed to bind to the beads for 2 h at 4°C. The beads were washed once each with RIPA buffer, RIPA buffer with 500 mM NaCl, immunoprecipitation wash buffer (10 mM Tris Cl pH 8.0, 250 mM LiCl, 0. 5% NP40, 0. 5% Sodium deoxycholate, and 1 mM EDTA), and finally with Tris-EDTA. Beads were resuspended in 200 μl of elution buffer (50 mM Tris Cl, pH 8.0, 10 mM EDTA, and 1% SDS) with Proteinase K and incubated overnight at 55°C. DNA was extracted using phenol–chloroform, precipitated in the presence of glycogen by ethanol, allowed to air dry, and dissolved in TE buffer pH 8.0. Immunoprecipitated DNA was diluted 10-fold to keep the PCR in the linear range of amplification. The following set of primers was used to amplify different regions of the genes indicated: Bik primer set 1 for the promoter region (−1504 to −1647), sense 5′-CTGC TAATGTTTACTGAACATCTC-3′ and antisense 5′-AAATTGAGACAGGGT GGTAAAG-3′ Bik primer set 2 for the promoter region (−551 to −693 bp) of Bik in which BKLF-binding sites are present; Bik primer set 2, sense 5′TATACC AGGGCTGGAGTTAGGTCC3′and antisense 5′-CTCACGTGCAGACCTGGT GAGA-3′; non-specific primers (−9.5 to −9.3 kb) upstream of BKLF-binding sites 5′CCTAAGAAGCTGGCCACAGCTC3′ and 5′CCATCATGTTGGCCAGAATGGT CTC3′; E-Cadherin primers 5′ TAGCCTGGCGTGGTGGTGTGCACCTG3′ and 5′GTGCGTGGCTGCAGCCAGGTGAGCC3′.20
Supplementary Material
Acknowledgments
We thank Drs. T Kowalik for high-titer Ad-hARF and Ad-lacZ, E Campeau for lentiviral shRNA vectors, and G Chinnadurai for pBLCAT2 reporter construct. SRG was supported by an American Cancer Society Research Scholar Award and the Worcester Foundation for Biomedical Research. SP was supported by Our Danny Cancer Fund.
Abbreviations
- ARF
alternative reading frame
- CtBP
C-terminal binding protein
- BH3
Bcl-2 homology domain 3
- ChIP
chromatin immunoprecipitation
- siRNA
small interfering RNA
- shRNA
short hairpin RNA
- PCR
polymerase chain reaction
- BKLF
basic Kruppel-like factor
- MEF
mouse embryo fibroblast
- PARP
poly (ADP-ribose) polymerase
- SDS
sodium dodecyl sulfate
- 7-AAD
7-aminoactinomycin D
- UV
ultraviolet
- PBS
phosphate buffered saline
- EDTA
ethylenediamine tetra-acetic acid
- NADH
nicotinamide adenine dinucleotide
- NP-40
Nonidet P-40
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
References
- 1.Quelle DE, Zindy F, Ashmun RA, Sherr CJ. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 2.Lowe SW, Sherr CJ. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev. 2003;13:77–83. doi: 10.1016/s0959-437x(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 3.Weber JD, Jeffers JR, Rehg JE, Randle DH, Lozano G, Roussel MF, et al. p53-independent functions of the p19(ARF) tumor suppressor. Genes Dev. 2000;14:2358–2365. doi: 10.1101/gad.827300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharpless NE, DePinho RA. The INK4A/ARF locus and its two gene products. Curr Opin Genet Dev. 1999;9:22–30. doi: 10.1016/s0959-437x(99)80004-5. [DOI] [PubMed] [Google Scholar]
- 5.Burri N, Shaw P, Bouzourene H, Sordat I, Sordat B, Gillet M, et al. Methylation silencing and mutations of the p14ARF and p16INK4a genes in colon cancer. Lab Invest. 2001;81:217–229. doi: 10.1038/labinvest.3780230. [DOI] [PubMed] [Google Scholar]
- 6.Paliwal S, Pande S, Kovi RC, Sharpless NE, Bardeesy N, Grossman SR. Targeting of C-terminal binding protein (CtBP) by ARF results in p53-independent apoptosis. Mol Cell Biol. 2006;26:2360–2372. doi: 10.1128/MCB.26.6.2360-2372.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaeper U, Boyd JM, Verma S, Uhlmann E, Subramanian T, Chinnadurai G. Molecular cloning and characterization of a cellular phosphoprotein that interacts with a conserved C-terminal domain of adenovirus E1A involved in negative modulation of oncogenic transformation. Proc Natl Acad Sci USA. 1995;92:10467–10471. doi: 10.1073/pnas.92.23.10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V, Carlson JE, Ohgi KA, Edwards TA, Rose DW, Escalante CR, et al. Transcription corepressor CtBP is an NAD(+)-regulated dehydrogenase. Mol Cell. 2002;10:857–869. doi: 10.1016/s1097-2765(02)00650-0. [DOI] [PubMed] [Google Scholar]
- 9.Grooteclaes M, Deveraux Q, Hildebrand J, Zhang Q, Goodman RH, Frisch SM. C-terminal-binding protein corepresses epithelial and proapoptotic gene expression programs. Proc Natl Acad Sci USA. 2003;100:4568–4573. doi: 10.1073/pnas.0830998100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 11.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Germain M, Mathai JP, Shore GC. BH-3-only BIK functions at the endoplasmic reticulum to stimulate cytochrome c release from mitochondria. J Biol Chem. 2002;277:18053–18060. doi: 10.1074/jbc.M201235200. [DOI] [PubMed] [Google Scholar]
- 13.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 14.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 15.Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 16.Datta SR, Ranger AM, Lin MZ, Sturgill JF, Ma YC, Cowan CW, et al. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev Cell. 2002;3:631–643. doi: 10.1016/s1534-5807(02)00326-x. [DOI] [PubMed] [Google Scholar]
- 17.Gillissen B, Essmann F, Hemmati PG, Richter A, Richter A, Oztop I, et al. Mcl-1 determines the Bax dependency of Nbk/Bik-induced apoptosis. J Cell Biol. 2007;179:701–715. doi: 10.1083/jcb.200703040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma S, Budarf ML, Emanuel BS, Chinnadurai G. Structural analysis of the human pro-apoptotic gene Bik: chromosomal localization, genomic organization and localization of promoter sequences. Gene. 2000;254:157–162. doi: 10.1016/s0378-1119(00)00276-6. [DOI] [PubMed] [Google Scholar]
- 19.van Vliet J, Turner J, Crossley M. Human Kruppel-like factor 8: a CACCC-box binding protein that associates with CtBP and represses transcription. Nucleic Acids Res. 2000;28:1955–1962. doi: 10.1093/nar/28.9.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Wang SY, Nottke AC, Rocheleau JV, Piston DW, Goodman RH. Redox sensor CtBP mediates hypoxia-induced tumor cell migration. Proc Natl Acad Sci USA. 2006;103:9029–9033. doi: 10.1073/pnas.0603269103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong Y, Yang Q, Vater C, Venkatesh LK, Custeau D, Chittenden T, et al. The pro-apoptotic protein, Bik, exhibits potent antitumor activity that is dependent on its BH3 domain. Mol Cancer Ther. 2001;1:95–102. [PubMed] [Google Scholar]
- 22.Oppermann M, Geilen CC, Fecker LF, Gillissen B, Daniel PT, Eberle J. Caspase-independent induction of apoptosis in human melanoma cells by the proapoptotic Bcl-2-related protein Nbk/Bik. Oncogene. 2005;24:7369–7380. doi: 10.1038/sj.onc.1208890. [DOI] [PubMed] [Google Scholar]
- 23.Han J, Sabbatini P, White E. Induction of apoptosis by human Nbk/Bik, a BH3-containing protein that interacts with E1B 19 K. Mol Cell Biol. 1996;16:5857–5864. doi: 10.1128/mcb.16.10.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniel PT, Pun KT, Ritschel S, Sturm I, Holler J, Dorken B, et al. Expression of the death gene Bik/Nbk promotes sensitivity to drug-induced apoptosis in corticosteroid-resistant T-cell lymphoma and prevents tumor growth in severe combined immunodeficient mice. Blood. 1999;94:1100–1107. [PubMed] [Google Scholar]
- 25.Castells A, Ino Y, Louis DN, Ramesh V, Gusella JF, Rustgi AK. Mapping of a target region of allelic loss to a 0.5-cM interval on chromosome 22q13 in human colorectal cancer. Gastroenterology. 1999;117:831–837. doi: 10.1016/s0016-5085(99)70341-0. [DOI] [PubMed] [Google Scholar]
- 26.Sturm I, Stephan C, Gillissen B, Siebert R, Janz M, Radetzki S, et al. Loss of the tissue-specific proapoptotic BH3-only protein Nbk/Bik is a unifying feature of renal cell carcinoma. Cell Death Differ. 2006;13:619–627. doi: 10.1038/sj.cdd.4401782. [DOI] [PubMed] [Google Scholar]
- 27.Coultas L, Bouillet P, Stanley EG, Brodnicki TC, Adams JM, Strasser A. Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed death. Mol Cell Biol. 2004;24:1570–1581. doi: 10.1128/MCB.24.4.1570-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 29.Ritchie A, Gutierrez O, Fernandez-Luna JL. PAR bZIP-bik is a novel transcriptional pathway that mediates oxidative stress-induced apoptosis in fibroblasts. Cell Death Differ. 2009 doi: 10.1038/cdd.2009.13. [DOI] [PubMed] [Google Scholar]
- 30.Shimazu T, Degenhardt K, Nur EKA, Zhang J, Yoshida T, Zhang Y, et al. NBK/BIK antagonizes MCL-1 and BCL-XL and activates BAK-mediated apoptosis in response to protein synthesis inhibition. Genes Dev. 2007;21:929–941. doi: 10.1101/gad.1522007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathai JP, Germain M, Shore GC. BH3-only BIK regulates BAX, BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J Biol Chem. 2005;280:23829–23836. doi: 10.1074/jbc.M500800200. [DOI] [PubMed] [Google Scholar]
- 32.Quinlan KG, Verger A, Kwok A, Lee SH, Perdomo J, Nardini M, et al. Role of the C-terminal binding protein PXDLS motif binding cleft in protein interactions and transcriptional repression. Mol Cell Biol. 2006;26:8202–8213. doi: 10.1128/MCB.00445-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner J, Crossley M. Cloning and characterization of mCtBP2, a co-repressor that associates with basic Kruppel-like factor and other mammalian transcriptional regulators. EMBO J. 1998;17:5129–5140. doi: 10.1093/emboj/17.17.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dang DT, Pevsner J, Yang VW. The biology of the mammalian Kruppel-like family of transcription factors. Int J Biochem Cell Biol. 2000;32:1103–1121. doi: 10.1016/s1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paliwal S, Kovi RC, Nath B, Chen YW, Lewis BC, Grossman SR. The alternative reading frame tumor suppressor antagonizes hypoxia-induced cancer cell migration via interaction with the COOH-terminal binding protein corepressor. Cancer Res. 2007;67:9322–9329. doi: 10.1158/0008-5472.CAN-07-1743. [DOI] [PubMed] [Google Scholar]
- 36.Chen YW, Paliwal S, Draheim K, Grossman SR, Lewis BC. p19Arf inhibits the invasion of hepatocellular carcinoma cells by binding to C-terminal binding protein. Cancer Res. 2008;68:476–482. doi: 10.1158/0008-5472.CAN-07-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernards R. Cancer: cues for migration. Nature. 2003;425:247–248. doi: 10.1038/425247a. [DOI] [PubMed] [Google Scholar]
- 38.Kelly-Spratt KS, Gurley KE, Yasui Y, Kemp CJ. p19Arf suppresses growth, progression, and metastasis of Hras-driven carcinomas through p53-dependent and -independent pathways. PLoS Biol. 2004;2:E242. doi: 10.1371/journal.pbio.0020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger JH, Bardeesy N. Modeling INK4/ARF tumor suppression in the mouse. Curr Mol Med. 2007;7:63–75. doi: 10.2174/156652407779940477. [DOI] [PubMed] [Google Scholar]
- 40.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.