1. Introduction
Neisseria meningitidis is a major cause of meningitis and bacteremia, particularly in infants and children [1]. Efforts to develop a vaccine have been hampered by the difficulty in identifying antigens that elicit broadly protective antibody responses [2]. In particular, capsular polysaccharide-based vaccines are effective against most capsular groups but not group B, which expresses poly alpha 2,8 N-acetyl neuraminic acid (MBPS) [1], a polysaccharide chemically identical to polysialic acid (PSA) that is abundantly expressed in heart, brain and kidney of the developing fetus [3, 4]. MBPS is poorly immunogenic even when conjugated to a carrier protein [5-7]. The lack of MBPS immunogenicity may be the result of immune tolerance to a self antigen or the effects of sialylated glycans in modulating activation of B cells [8]. Recently, our laboratory showed that derivatives of MBPS containing de-N-acetylated neuraminic acid residues (Neu) were immunogenic when conjugated to a carrier protein and elicited antibodies that were reactive with Neu-containing polysialic derivatives (NeuPSA) [9]. The antibodies were protective against group B strains in vivo in an infant rat passive protection model of meningococcal bacteremia, but were not able to mediate bactericidal activity (BCA) with human complement against NmB strains in vitro [9].
The possible effects of non-human tissue components on expression of particular Nm antigens and assays used to evaluate antibody functional activity against Nm strains are poorly understood. For example, only recently was it shown that Nm strains express a factor H binding protein that facilitates survival of some Nm strains in human blood and is specific for human factor H [10, 11]. As a result, there can be large differences in the measurement of antibody functional activity between BCA assays that use human compared to non-human complement. Nm strains are commonly cultured in media containing bovine tissue extracts, such as Müller-Hinton media, and on plates containing bovine blood products, such as chocolate agar plates. Also, bovine serum albumin (BSA) is used as an irrelevant protein supplement in buffers for washing bacteria and diluting antibodies and serum. The bovine-derived supplements contain N-glycoyl neuraminic acid (Neu5Gc) sialic acid antigens, which are not found in human tissues [12]. Potentially, the presence of Neu5Gc sialic acid derivatives or other unknown components in bovine-derived blood products could affect the expression Nm antigens and the functional activity of antibodies targeting Nm antigens.
In the present study, we used two mAbs, SEAM 2 and SEAM 3 that are reactive with different NeuPSA epitopes and an anticapsular mAb, SEAM 12, to investigate the effect of culture conditions on the expression of NeuPSA epitopes by group B strains and factors that affect mAb mediated BCA. SEAM 2, 3 and 12 were produced using a derivative of MBPS that had been de-N-acetylated then re-N-acetylated with propionyl groups (NPr-MBPS) and conjugated to tetanus toxoid [13]. SEAM 2 and 3 are reactive with a variety of MBPS derivatives that contain Neu [9, 14, 15]. Specifically, SEAM 2 is reactive with NeuPSA derivatives having a degree of polymerization (Dp) greater than about 10 and containing between 40% and 60% Neu while SEAM 3 is reactive with NeuPSA derivatives containing as few as three residues and as little as a single Neu residue (Moe et al, in preparation).
2. Methods
2.1 mAbs
Murine mAbs SEAM 2, SEAM 3, and SEAM 12 were produced by immunizing a CD1 mouse with an N-Pr MBPS-tetanus toxoid conjugate vaccine [13]. The polysaccharide vaccine antigen contained ~16% Neu [16]. The mAbs were obtained from cell culture by precipitation with 50% (w/v) ammonium sulfate. The precipitated antibody was washed with ice cold 30% ammonium sulfate solution, dialyzed against PBS buffer, and purified by affinity chromatography as described below. Irrelevant isotype control mAbs were obtained from Southern Biotech (Birmingham, AL).
2.2 mAb purification
In previous studies [13, 14, 17, 18], partially purified preparations of anti-N-Pr MBPS mAbs SEAM 2 and 3 were used to measure binding and functional activity since standard purification methods such as affinity chromatography using Protein A or G or ion exchange chromatography resulted in low yields of SEAM 3. To separate the mAbs from other components present in the crude ammonium sulfate precipitated fraction, the mAbs were incubated for 30 min in PBS buffer containing 0.5 M Na2SO4. After removing precipitates by centrifugation (10,000 × g), the solution was loaded onto a Protein A column (HiTrap Protein A, GE Healthcare Bio-Sciences, Piscataway, NJ) in 20 mM histidine buffer, pH 6.5, containing 0.02% Tween 20 (Sigma-Aldrich, Saint Louis, MO) using an Äkta FPLC (GE Healthcare Bio-Sciences). For SEAM 3, most of the antibody is not retained on the column. The unbound antibody was found to consist of complex N-, O-, and sialylated glycoforms that showed no binding activity to the nominal N-Pr MBPS antigen or functional activity against group B strains (B. A. Flitter and G. R. Moe, unpublished). In contrast, the majority of SEAM 2 was retained on the column and the mAb did not appear to have the multiplicity of glycoforms observed for SEAM 3. The column was washed with the loading buffer and the bound antibody was eluted with 0.1 M histidine acetate buffer, pH 2.7 containing 0.02% Tween-20. Fractions containing eluted antibody were immediately adjusted to pH 6.5 with 1 M Tris•HCl buffer, pH 10. The buffer was then exchanged with lyophilization buffer (2 mM histidine, pH 6, containing 0.002% Tween 20 and 24 mM sucrose (Sigma-Aldrich) using a PD10 (GE Healthcare Bio-Sciences) size exclusion column and the fractions were lyophilized. The lyophilized mAb was stored at −80°C. Before use, the lyophilized mAb was reconstituted with water so that the final buffer contained 50 mM histidine, pH 6, 0.05% Tween-20, and 600 mM sucrose. The antibody concentration in reconstituted solutions was determined by comparison with a known concentration of a subclass-matched control mAb on an SDS-PAGE gel stained with Coomassie Brilliant Blue G-250 (Sigma-Aldrich).
2.3 Strains
Three wild-type NmB strains M986 (B:2a:P1.5,2:ST11) [7], NMB (B:2a:P1.5,2:ST8) [19], and NZ98/254 (B:4:P1.7-2,4:ST42) [20] used in this study were chosen based on their ability to survive in vivo in an infant rat model of meningococcal bacteremia and in vitro in human complement, plasma and whole blood.
2.4 Flow cytometry
Antibody binding to live bacteria was measured as previously described [9]. Bacteria used in the binding studies were cultured in Müller-Hinton (MH) media, Catlin 6 chemically defined (CDM) media, or CDM supplemented with 5% human serum (CDM HuS) that was heat inactivated at 56°C and depleted of IgG. HuS was prepared as described below. The composition of the CDM media was adapted from Fossa da Paz [21]. CDM was prepared fresh prior to each experiment by combining pre-made stock solutions of the amino acids, salts, glucose and iron. Recently it was observed that adding 0.2 mM glutamine (UCSF cell culture facility) to CDM decreased the doubling time of some strains and was, therefore, used routinely.
2.5 Bactericidal activity (BCA) assays
The ability of mAbs to mediate bacteriolysis in the presence of human complement was measured by BCA assay as described by Moe et al. [9]. Bacteria grown overnight on chocolate agar plates (Remel, Lenexa, KS) were cultured in either MH from OD620nm = 0.15 to 0.62, or in CDM HuS starting at OD620nm = 0.18 and grown to OD620nm = 0.7. The bacteria were pelleted by centrifugation and then washed in Dulbeccos PBS with Ca2+ and Mg2+ (DPBS), with or without 1% BSA or 1% heat-inactivated IgG-depleted human serum (HuS) prepared as described below. The human complement sources used in each assay were evaluated for intrinsic BCA against test strains in the presence of 30% complement.
2.6 Preparation of IgG-depleted human complement
To minimize the potential for confounding cross-reactive antibodies, human complement was depleted of IgG as follows. A 5 ml protein G column (HiTrap Protein G HP, GE Healthcare Bio-Sciences) was fitted with sterilized leur lock valves and two 10 ml syringes on the top and bottom of the column. 25 ml of sterile filtered DPBS, 0.5% glucose (DPBS+glu) was used to wash the column which was then chilled for 20 minutes at 4°C. Serum was removed from −80°C storage and thawed on ice. 5.5 ml of serum was loaded onto the column and was placed at 4°C for 5 minutes. DPBS+glu was then applied to the column to displace IgG depleted serum. Approximately 5 ml of the depleted serum was collected and immediately placed on ice, aliquoted and frozen at −80°C until used. An additional 5 ml of DPBS+glu was applied to the column until the flow through was completely clear. The protein G bound IgG was eluted from the column with 0.1 M histidine acetate, pH 2.7, 0.02% Tween 20.
2.7 Human plasma bactericidal assay
A human plasma bactericidal assay was used to determine if the mAbs could mediate complement-dependent bacteriolysis in the presence of 65% human plasma. Bacteria grown overnight on chocolate agar plates (Remel) were cultured in MH at a starting OD620nm of 0.15 and grown to an OD620nm of 0.62. The bacteria were washed in PBS, 1% BSA and resuspended in PBS containing 15% HuS to a concentration of ~5×105 bacterial cells per ml. The total volume of the reaction mixture was 100 μl consisting of 65 μl human plasma, 25 μl mAb and 10 μl diluted bacteria. Human plasma was obtained from freshly drawn blood from a donor that was known to lack antibodies that could mediate bacteriolysis or opsonophagocytosis of the test strain. Recombinant hirudin (Lepirudin (rDNA)) [Refludan®, Berlex]) was used as an anticoagulant. A solution of Lepirudin was drawn into a needle fitted to a 60 ml syringe such that the final concentration was 28 μg/ml in the drawn blood. The blood was transferred to 15 ml conical tubes and centrifuged to remove cells. The resulting plasma preparation was either used immediately or frozen at −80°C for later use. The assay was performed in a 96-well plate. Each well received 20 μl of polymorphonuclear leukocytes (PMNs; prepared as described below) diluted in human plasma and 45 μl of human plasma added to 25 μl of mAb and 10 μl of bacteria. At T=0, 50 μl, 10 μl and 1 μl from four control wells containing no mAb were diluted in PBS and spread on chocolate agar plates (Remel). To determine the CFU/ml remaining after 1.5 hr, the controls and test reactions were also plated on chocolate agar and all plates were incubated overnight at 37°C.
PMNs were isolated from whole human blood on the day of the assay. 20 ml of blood drawn in tubes containing EDTA were added to 200 ml of red cell lysis buffer (37 mM ammonium chloride, 4.75 mM sodium bicarbonate, pH 6.8, 0.6 mM EDTA) and left at room temperature for ten minutes. The cells were then centrifuged and washed twice with DPBS without Ca2+ and Mg2+ (Gibco) containing 1% BSA (Life Blood) and 0.5% glucose). If a large number of red blood cells remained in the pellet after the wash steps, the red cell lysis procedure was repeated. The white cell preparation was washed three times as above and the cell pellet finally resuspended in 1 ml of DPBS wash buffer. The viability and total number of cells were determined with a hemocytometer after staining with Turks and Trypan Blue. Additionally, the percentage of PMNs in the white cell preparation was measured using an ADVIA 120 Hematology System (Bayer Health Care, Tarrytown, NY). The final cell preparation was diluted in plasma to a concentration of 40 PMNs to 1 CFU of bacteria.
2.8 Whole human blood bacteremia assay
This assay was performed as described for the plasma bactericidal assay using Lepirudin as an anticoagulant except that 65 μl of whole human blood was substituted for plasma alone.
3. Results
3.1 Effect of culture conditions on anti-NeuPSA mAb SEAM 2 and 3 binding to live group B strains
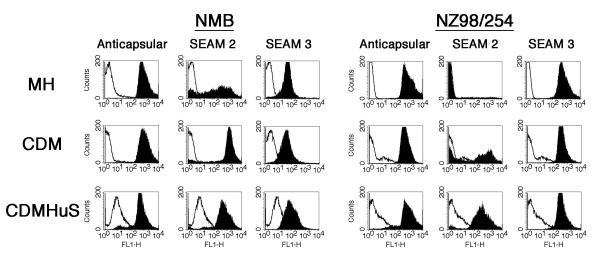
To determine what factors might affect the expression of antigens recognized by the anti-NeuPSA mAbs and resulting functional activity, we compared mAb binding to two Nm strains cultured in Müller-Hinton (MH), Catlin 6 chemically defined media (CDM), and CDM supplemented with 5% IgG-depleted, heat inactivated human serum (CDM HuS) measured by flow cytometry. Irrelevant, subclass matched murine mAbs were used as negative controls and the anti-group B capsular polysaccharide-reactive mAb SEAM 12 was used as a positive control. Binding is indicated by a shift to greater fluorescence compared to cells incubated in the presence of an irrelevant mAb. All binding experiments were performed using an antibody concentration of 20 μg/ml. As shown in Fig. 1, binding by the anticapsular mAb was the same for both Nm strains grown in MH and CDM but slightly decreased when grown in CDM HuS. SEAM 3 binding was similar under the three culture conditions although the relative fluorescence was higher for NZ98/254 compared to NMB. SEAM 2 showed strong binding to NMB and heterogeneous binding to NZ98/254 when cultured in CDM and strong binding to both strains cultured in CDM HuS. However, SEAM 2 binding was heterogeneous to strain NMB and was negative for NZ08/254 when the bacteria were cultured in MH media. Therefore, growth in MH media affected the expression of epitopes recognized by SEAM 2 but not SEAM 3 or the anticapsular mAb. The greatest binding by the anti-NeuPSA mAbs overall was to bacteria grown in CDM HuS.
Figure 1.
Antibody binding to Nesseria meningitidis group B strains determined by flow cytometry. Fluorescence in the presence of an irrelevant mAb is indicated by the unfilled histograms and the indicated test mAb by the filled histograms. The bacteria were cultured in Müller-Hinton (MH), Catlin 6 chemically defined media (CDM) or CDM supplemented with IgG depleted, heat inactivated human serum (CDM HuS) as indicated. [mAb]=20 μg/ml.
3.2 Human complement-dependent bactericidal activity (BCA) of SEAM 2 and 3 against NmB strains cultured in MH media
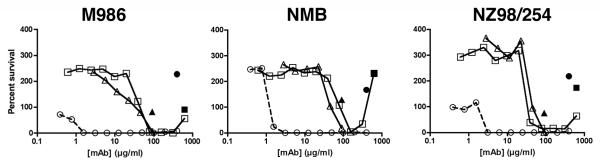
It has been established in several studies that antibody mediated complement-dependent bactericidal activity (BCA), as measured in vitro, correlates with protection against invasive meningococcal disease [1, 22]. Fig. 2 compares the concentration-dependence of the anticapsular mAb with anti-NeuPSA-reactive mAbs SEAM 2 and 3 in the BCA assay with IgG-depleted human complement against three NmB strains. The bacteria were cultured in Müller-Hinton (MH) media and buffers used to wash the bacteria and adjust the volume of the BCA reaction mixture contained 1% BSA. As a control for complement dependent killing, BCA was measured in the presence of the test antibody with heat-inactivated complement (filled symbols). The mAb concentrations required to kill 50% of the bacteria (BCA50) were 50 to 100-fold lower for the anticapsular mAb compared to SEAM 2 or 3 (Fig. 2 and Table 1).
Figure 2.
Concentration dependence of antibody mediated BCA against NmB strains cultured in Müller-Hinton media. IgG-depleted human serum (20% (v/v)) was used as the source of exogenous complement. The mAbs included anticapsular mAb SEAM 12 (circle) and anti-NeuPSA mAbs SEAM 2 (square) and SEAM 3 (triangle). Filled symbols indicate activity of the test mAb in the presence of heat-inactivated complement.
Table 1.
mAb concentrations (μg/ml) resulting in 50% bacteriolysis (BCA50) using human complement with bacteria grown and washed under the indicated conditionsa.
| Strain | Anticapsular | SEAM 2 | SEAM 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MH +BSA |
CDM HuS +BSA |
CDM HuS −BSA |
MH +BSA |
CDM HuS +BSA |
CDM HuS −BSA |
MH +BSA |
CDM HuS +BSA |
CDM HuS −BSA |
|
| M986 | 1 | 7 | 11 | 67 | 110 | 2 | 62 | >85 | >85 |
| NMB | 2 | 13 | 11 | 284 | >625 | 2 | 74 | >85 | >85 |
| NZ98/254 | 2 | 12 | 6 | 52 | >625 | 5 | 67 | >85 | >85 |
Bacteria were grown in MH or CDM HuS and were washed in buffer containing BSA (+BSA) or buffer containing HuS (−BSA).
3.3 Bactericidal activity of SEAM 2 and 3 against group B strains cultured in the presence of human serum
Since culturing the bacteria in CDM or CDM HuS resulted in increased binding of SEAM 2 to NmB strains compared to bacteria cultured in MH media, we measured BCA mediated by the mAbs against bacteria cultured in CDM HuS. IgG-depleted human serum was used as the media supplement and exogenous complement source to minimize the potential confounding effects of cross-reactive antibodies. In addition, our standard BCA protocol uses Dulbecco’s buffered saline (DPBS) containing 1% (w/v) bovine serum albumin (BSA) for washing and diluting the bacteria, for diluting the mAbs, and for maintaining a constant volume in the BCA reaction. Therefore, we compared the effect on BCA of bacteria cultured in CDM HuS, washed in DPBS-BSA or DPBS alone, and resuspended in buffer that contained BSA or only HuS without BSA, respectively. The anticapsular mAb was used as a positive control and subclass-matched irrelevant mAbs were used as negative controls. Also the duration of the reaction was increased from 60 min to 90 min to be consistent with BCA experiments using human plasma and whole blood described in the following sections.
As shown in Table 1, SEAM 2 mediated BCA against three strains tested when the bacteria were grown in CDM HuS but only when BSA was excluded from the wash buffer and reaction mixture. SEAM 3 did not mediate bacteriolysis at mAb concentrations up to 85 μg/ml in the absence or presence of BSA against any of the strains tested. In contrast to SEAM 2, removing BSA from the BCA assay had no significant effect on BCA mediated by the anticapsular mAb (Table 1). However, the BCA50 concentration of the anticapsular mAb was 3- to 10-fold higher in CDM HuS/DPBS than when the bacteria were grown in MH and the wash and reaction buffers contained BSA (Table 1). Therefore, leaving BSA out of the reaction mixture did not make the bacteria more susceptible to BCA since the bacteria were more resistant to SEAM 3 and anticapsular mediated bacteriolysis. The 50- to more than 100-fold difference in BCA activity of SEAM 2 was not the result of differences in SEAM 2 epitope expression or binding interference by BSA since SEAM 2 bound strongly to bacteria cultured in CDM HuS with BSA present in the binding reaction buffer (Fig. 1).
3.4 Bactericidal activity of SEAM 2 and 3 against group B strain NMB in human plasma and strain NZ98/254 in human plasma without and with polymorphonuclear leukocytes
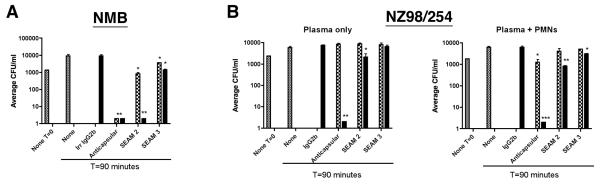
As shown in Table 1, human serum in culture media and excluding BSA from buffers resulted in an increase in the ability of SEAM 2 to mediate killing of group B strains. However, SEAM 3 was not able to mediate BCA under the same conditions. To determine how the presence of additional human blood components might affect the functional activity of the mAbs, we measured the BCA of SEAM 2 and 3 at two concentrations against two group B strains in the presence of 65% freshly prepared human plasma. The results are shown graphically in Fig. 3 for the mAbs tested at 2 μg/ml (checkered bars) and 20 μg/ml (filled bars). No antibody (gray bars) and an irrelevant murine IgG2b antibody tested at 20 μg/ml only were included as negative controls. The anticapsular antibody at concentrations of 2 μg/ml and 20 μg/ml was used as a positive control. In contrast to the BCA assay using 20% human complement, SEAM 3 was able to mediate limited but significant killing against strain NMB at both mAb concentrations tested but not against NZ98/254. Neither SEAM 2 or the positive control anticapsular mAb were able to mediate killing of NZ98/254 at antibody concentrations of 2 μg/ml but both had BCA at 20 μg/ml. To determine whether the mAbs could mediate opsonophagocytosis in addition to bacteriolysis against strain NZ98/254, the activity of the mAbs in plasma was measured in the presence of polymorphonuclear (PMN) leukocytes. As shown in Fig. 3, SEAM 2, 3 and the anticapsular mAb showed increased killing against strain NZ98/254 in the presence of PMNs compared to plasma alone.
Figure 3.
Effect of mAbs on the survival of Neisseria meningitidis group B strains NMB (A) in human plasma and NZ98/254 (B) in human plasma or plasma supplemented with PMNs. The bacteria were initially cultured in Müller-Hinton media before being diluted and added to the reaction mixture. mAbs were incubated with the bacteria in 65% human plasma at concentrations of 2 μg/ml (checkered bars) or 20 μg/ml (black bars) at 37°C for 90 minutes as indicated. The irrelevant IgG2b negative control mAb was tested only at 20 μg/ml. CFU/ml in the absence of antibody (gray bars) is shown at T=0 and T=90 minutes as indicated. ***, P>0.001. **, P=0.001 to 0.01. *, P=0.01 to 0.05.
3.5 Passive protection activity of SEAM 2 and 3 against NmB strain M986 in a whole human blood model of meningococcal bacteremia
The infant rat model of meningococcal bacteremia has been used to evaluate the ability of naturally acquired or vaccine-induced antibodies to mediate killing of meningococcal strains [9, 23-27]. SEAM 2 and 3 [9, 24-27] and polyclonal anti-NeuPSA [9] have been shown to mediate passive protection against NmB strain M986 bacteremia in the infant rat model. However, it was shown recently by Granoff et al. that Neisserial factor H binding protein (fHbp) can be important for survival of meningococcal strains when grown in whole human blood [11]. However, fHbp was shown to be relatively specific for human fH and, therefore, the ability of the anti-NeuPSA mAbs SEAM 2 and 3 to mediate protection against NmB strain M986 in the infant rat could be augmented by the inability of fHbp to bind rat fH. To determine whether SEAM 2 and 3 could mediate passive protection in the presence of human fH, we tested the mAbs in an ex vivo whole human blood model of meningococcal bacteremia [28]. NmB strain M986 was chosen for this experiment because it was used in the infant rat model experiments described above and survived particularly well in whole human blood. The anticapsular mAb was used as positive control and an irrelevant mAb as a negative control. As shown in Table 2, there was no protection against M986 in whole human blood afforded by an irrelevant mAb at 25 μg/ml. The positive control anticapsular mAb and SEAM 2 were completely protective at 5 μg/ml and 25 μg/ml. SEAM 3, which had no activity in the in vitro BCA assay at 85 μg/ml against M986 cultured in CDM HuS and washed in DPBS (Table 1) was completely protective at 25 μg/ml in whole human blood.
Table 2.
Passive protection against NmB strain M986 in a whole human blood model of meningococcal bacteremiaa.
| mAb | CFU/ml after 1.5 hr | ||
|---|---|---|---|
| 1 μg/ml | 5 μg/ml | 25 μg/ml | |
| Irrelevant IgG2b | NA | NA | 493 |
| Anticapsular | 220 | 0 | 0 |
| SEAM 2 | 560 | 0 | 0 |
| SEAM 3 | 580 | 200 | 0 |
Bacteria were grown in MH and were washed in buffer containing BSA.
4. Discussion
In this study we have investigated the effect of human serum on binding and functional activity against Neisseria meningitidis group B strains of two mAbs that recognize different NeuPSA epitopes. Based on reactivity with synthetic PSA derivatives, the mAb SEAM 3 binds to relatively short NeuPSA oligosaccharides (Dp<4) having as few as a single Neu residue whereas SEAM 2 binds to longer derivatives (Dp>10) containing approximately 50% Neu residues (Moe et al, in preparation). We found that culturing group B strains in MH media, which contains bovine tissue-derived components, decreased or eliminated the expression of epitopes recognized by SEAM 2 but had no measurable effect on the expression of epitopes recognized by SEAM 3 (Fig. 1). The SEAM 2 epitope was most highly expressed when the bacteria were cultured in CDM and CDM HuS (Fig. 1). Also, the presence of bovine serum albumin was found to have a considerable effect on decreasing BCA mediated by SEAM 2 (Table 1) even though BSA did not affect SEAM 2 binding when the bacteria were grown in CDM HuS. SEAM 3 mediated significant killing of group B strains only when tested in 65% human plasma, plasma supplemented with PMNs, or whole human blood (Tables 1 and 2). Taken together, the results show that the anti-NeuPSA antibodies had the greatest functional activity against NmB in a milieu of human blood components but had little or no activity when bacteria were grown and functional activity was measured in the presence of bovine blood components.
Humans are the obligate hosts for N. meningitidis, therefore, it is not surprising that some surface antigens expressed by the bacteria are human tissue specific with respect to control of expression, specificity of interactions, and functional activity particularly when the surface antigens have a role in promoting survival in the host. Sialic acids generally are known to have such role for meningococci. For example, sialic acid-containing capsule polysaccharides and sialylated LOS have been shown to be important for resistance to killing in human serum [29, 30]. Sialic acids are known to modulate complement activation by binding fH and preventing binding of mannose binding protein to mannose and fucose residues present on many bacterial pathogens [29-31].
Currently, little is known about how or why de-N-acetyl sialic acid is produced. However, Neu is unstable when not in polymeric form and, as a result, it has been suggested that de-N-acetyl sialic acid antigens are produced after Neu5Ac is linked through glycosidic bonds to other carbohydrates [32]. Based on anticapsular mAb binding, there was no difference in capsular polysaccharide production when NmB strains were grown in MH, CDM or CDM HuS. Therefore, it is possible that the loss of SEAM 2 reactivity and functional activity with bacteria cultured in MH media resulted from inhibition of PSA de-N-acetylase activity required to produce longer, more highly de-N-acetylated NeuPSA antigens. For example, humans express only Neu5Ac sialic acid antigens while other mammals express both Neu5Ac and N-glycoyl neuraminic acid (Neu5Gc)-containing antigens. The presence of Neu5Gc in MH media could conceivably have an effect on the production of antigens reactive with SEAM 2 by NmB strains. However, the loss of SEAM 2 functional activity when bovine serum albumin was present in the BCA assay did not result from an effect on antigen expression since SEAM 2 binding was the same in the presence or absence of BSA. Evidently, BSA or a contaminant in the BSA preparation modulates the ability of SEAM 2 to mediate bacteriolysis.
Although SEAM 2 and 3 reactive antigens were expressed by NmB strains when human serum factors were present, they were not required for expression since both mAbs showed strong binding when bacteria were cultured in CDM alone. Alternatively, it appears that factors present in serum resulted in group B strains being more susceptible to SEAM 2 and 3 mediated killing with the activity increasing as the composition of the exogenous complement source was closer to being whole human blood. The unknown serum factors were not likely to be serum antibodies reactive with NmB antigens since the same antibodies at the same concentrations were present under conditions where the anti-NeuPSA mAbs mediated and did not mediate BCA. For example the only difference between the conditions where SEAM 2 did or did not mediate bacteriolysis was the presence of BSA in the wash buffer and BCA reaction mixture (Table 1).
In summary, the results of this study show that factors present in human blood and bovine blood products have considerable but opposing effects on the expression of some NeuPSA epitopes and the activity of antibodies elicited by NeuPSA-based vaccines. While the mechanism has not yet been elucidated, the results are consistent with the fact that humans are obligate hosts for Neisseria meningitidis and the activity of anti-NeuPSA is best evaluated with human blood components in buffers and as a source of complement. The effects of MH media and BSA on SEAM 2-reactive epitope expression and BCA raise questions about how relevant bacteria grown in the presence of non-human blood components are to bacteria that cause invasive disease in humans. Recently, our laboratory has observed that Nm strains express binding activity for several human serum proteins, but binding is negatively affected by non-human blood components in growth media (Flitter et al, in preparation). The possible effect of human serum protein binding on complement activation mediated by anti-NeuPSA is currently being investigated as a possible explanation for the effects of bovine blood products on BCA described here.
Acknowledgement
This work was supported by grant AI46464 from the National Institute of Allergy and Infectious Disease of the National Institutes of Health (GRM) and was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number CO6 RR-16226 from the National Center for Research Resources, National Institutes of Health. BAF and JYI were supported in part by Jennifer Leigh Wells Family Fellowships.
Abbreviations
- BCA
complement mediated bactericidal activity
- CDM
chemically defined media Catlin 6
- Dp
degree of polymerization
- HuS
IgG-depleted heat inactivated human serum
- MH
Müller-Hinton
- MBPS
Neisseria meningitidis group B capsular polysaccharide
- Nm
Neisseria meningitidis
- N-Pr MBPS
N-propionyl MBPS
- Neu
neuraminic acid
- PSA
polysialic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Granoff DM, Feavers IM, Borrow R. Meningococcal Vaccines. In: Plotkin SAO, Walter A, editors. Vaccines. 4th ed Saunders; Philadelphia, Pa.: 2004. pp. 959–88. [Google Scholar]
- [2].Jodar L, Feavers I, Salisbury D, Granoff DM. Development of vaccines against meningococcal disease. Lancet. 2002;359:1499–508. doi: 10.1016/S0140-6736(02)08416-7. [DOI] [PubMed] [Google Scholar]
- [3].Hayrinen J, Jennings H, Raff HV, Rougon G, Hanai N, Gerardy-Schahn R, et al. Antibodies to polysialic acid and its N-propyl derivative: binding properties and interaction with human embryonal brain glycopeptides. J Infect Dis. 1995;171(6):1481–90. doi: 10.1093/infdis/171.6.1481. [DOI] [PubMed] [Google Scholar]
- [4].Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;2(8346):355–7. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- [5].Bruge J, Bouveret-Le Cam N, Danve B, Rougon G, Schulz D. Clinical evaluation of a group B meningococcal N-propionylated polysaccharide conjugate vaccine in adult, male volunteers. Vaccine. 2004;22(9-10):1087–96. doi: 10.1016/j.vaccine.2003.10.005. [DOI] [PubMed] [Google Scholar]
- [6].Jennings HJ, Roy R, Gamian A. Induction of meningococcal group B polysaccharide-specific IgG antibodies in mice by using an N-propionylated B polysaccharide-tetanus toxoid conjugate vaccine. J Immunol. 1986;137(5):1708–13. [PubMed] [Google Scholar]
- [7].Zollinger WD, Mandrell RE, Griffiss JM, Altieri P, Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979;63(5):836–48. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Courtney AH, Puffer EB, Pontrello JK, Yang ZQ, Kiessling LL. Sialylated multivalent antigens engage CD22 in trans and inhibit B cell activation. Proc Natl Acad Sci U S A. 2009;106(8):2500–5. doi: 10.1073/pnas.0807207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Moe GR, Bhandari TS, Flitter BA. Vaccines containing de-N-acetyl sialic acid elicit antibodies protective against neisseria meningitidis groups B and C. J Immunol. 2009;182(10):6610–7. doi: 10.4049/jimmunol.0803677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177(1):501–10. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Granoff DM, Welsch JA, Ram S. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun. 2009;77(2):764–9. doi: 10.1128/IAI.01191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci U S A. 1998;95(20):11751–6. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Granoff DM, Bartoloni A, Ricci S, Gallo E, Rosa D, Ravenscroft N, et al. Bactericidal monoclonal antibodies that define unique meningococcal B polysaccharide epitopes that do not cross-react with human polysialic acid. J Immunol. 1998;160(10):5028–36. [PubMed] [Google Scholar]
- [14].Moe GR, Dave A, Granoff DM. Epitopes recognized by a nonautoreactive murine anti-N-propionyl meningococcal group B polysaccharide monoclonal antibody. Infect Immun. 2005;73(4):2123–8. doi: 10.1128/IAI.73.4.2123-2128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moe GR, Dave A, Granoff DM. Molecular analysis of anti-N-propionyl Neisseria meningitidis group B polysaccharide monoclonal antibodies. Mol Immunol. 2006;43(9):1424–31. doi: 10.1016/j.molimm.2005.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Seid R, inventor; Chiron S.r.l., assignee. Neisseria meningitidis serogroup B Glycoconjugates. no. 6,638,513 US patent. 2003
- [17].Moe GR, Granoff DM. Molecular mimetics of Neisseria Meningitidis serogroup B polysaccharide. Intern Rev Immunol. 2001;20:201–21. doi: 10.3109/08830180109043034. [DOI] [PubMed] [Google Scholar]
- [18].Moe GR, Tan S, Granoff DM. Molecular mimetics of polysaccharide epitopes as vaccine candidates for prevention of Neisseria meningitidis serogroup B disease. FEMS Immunol Med Microbiol. 1999;26(3-4):209–26. doi: 10.1111/j.1574-695X.1999.tb01392.x. [DOI] [PubMed] [Google Scholar]
- [19].Stephens DS, Swartley JS, Kathariou S, Morse SA. Insertion of Tn916 in Neisseria meningitidis resulting in loss of group B capsular polysaccharide. Infect Immun. 1991;59(11):4097–102. doi: 10.1128/iai.59.11.4097-4102.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Martin DR, Ruijne N, McCallum L, O’Hallahan J, Oster P. The VR2 epitope on the PorA P1.7-2,4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB. Clin Vaccine Immunol. 2006;13(4):486–91. doi: 10.1128/CVI.13.4.486-491.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fossa da Paz M, Baruque-Ramos J, Hiss H, Vicentin MA, Leal MB, Raw I. Polysaccharide production in batch process of Neisseria meningitidis serogroup C comparing Frantz, modified Frantz and Catlin 6 cultivation media. Brazil J Micro. 2003;34:27–32. [Google Scholar]
- [22].Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129(6):1307–26. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Toropainen M, Kayhty H, Saarinen L, Rosenqvist E, Hoiby EA, Wedege E, et al. The infant rat model adapted to evaluate human sera for protective immunity to group B meningococci. Vaccine. 1999;17(20-21):2677–89. doi: 10.1016/s0264-410x(99)00049-3. [DOI] [PubMed] [Google Scholar]
- [24].Moe GR, Tan S, Granoff DM. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect Immun. 1999;67(11):5664–75. doi: 10.1128/iai.67.11.5664-5675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Welsch JA, Moe GR, Rossi R, Adu-Bobie J, Rappuoli R, Granoff DM. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J Infect Dis. 2003;188(11):1730–40. doi: 10.1086/379375. [DOI] [PubMed] [Google Scholar]
- [26].Moe GR, Zuno-Mitchell P, Hammond SN, Granoff DM. Sequential immunization with vesicles prepared from heterologous Neisseria meningitidis strains elicits broadly protective serum antibodies to group B strains. Infect Immun. 2002;70(11):6021–31. doi: 10.1128/IAI.70.11.6021-6031.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Moe GR, Zuno-Mitchell P, Lee SS, Lucas AH, Granoff DM. Functional activity of anti-Neisserial surface protein A monoclonal antibodies against strains of Neisseria meningitidis serogroup B. Infect Immun. 2001;69(6):3762–71. doi: 10.1128/IAI.69.6.3762-3771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Plested JS, Welsch JA, Granoff DM. Ex vivo model of meningococcal bacteremia using human blood for measuring vaccine-induced serum passive protective activity. Clin Vaccine Immunol. 2009;16(6):785–91. doi: 10.1128/CVI.00007-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jarvis GA, Vedros NA. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect Immun. 1987;55(1):174–80. doi: 10.1128/iai.55.1.174-180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kahler CM, Martin LE, Shih GC, Rahman MM, Carlson RW, Stephens DS. The (alpha2-->8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect Immun. 1998;66(12):5939–47. doi: 10.1128/iai.66.12.5939-5947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Varki A. Sialic acids as ligands in recognition phenomena. Faseb J. 1997;11(4):248–55. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- [32].Manzi AE, Sjoberg ER, Diaz S, Varki A. Biosynthesis and turnover of O-acetyl and N-acetyl groups in the gangliosides of human melanoma cells. J Biol Chem. 1990;265(22):13091–103. [PubMed] [Google Scholar]