Abstract
A variety of lactic acid bacteria contain rudimentary electron transport chains that can be reconstituted by the addition of heme and menaquinone to the growth medium. These activated electron transport chains lead to higher biomass production and increased robustness, which is beneficial for industrial applications, but a major concern when dealing with pathogenic lactic acid bacteria.
Introduction and context
Many lactic acid bacteria (LAB) have been used for millennia for the production of a wide variety of fermented foods. Lactic acid fermentation increases the shelf-life of foods, while simultaneously making the foods easier to digest and improved in flavor [1,2]. Since they lack a heme biosynthesis pathway, this group of anaerobic Gram-positive bacteria cannot form endogenous electron transport chains (ETCs) and, thus, have long been considered as obligate fermentative bacteria.
Major recent advances
For some LAB - that is, Lactococcus lactis, Lactobacillus plantarum, Streptococcus agalactiae and Enterococcus faecalis - it has been shown in some detail that the ETC activity can be reconstituted by addition of menaquinone and/or heme to the culture [3-9]. Below we describe this phenomenon in more detail.
Aerobic respiration in L. lactis and S. agalactiae
L. lactis and S. agalactiae show a doubling of biomass when cultivated in the presence of heme alone [4,7,9] or heme and menaquinone combined [6]. This can be explained in three ways: first, the oxidation of NADH by the ETC affects the redox-balance and, in L. lactis, results in a shift from homo-lactic to a mixed-acid fermentation with acetate production, resulting in increased substrate-level ATP generation; second, a reduced acidification occurs when acetate and acetoin are produced instead of lactate, resulting in a more complete utilization of the available sugars; and third, extra energy is conserved by proton pumping, potentially conserving 2/3 (0.66) ATP molecules for each NADH molecule oxidized [7-9]. Together, these effects add up to give a dramatic improvement in growth [4,6,8,9].
Tolerance to different stresses is also much improved in the respiratory cell-state [10,11]. The reconstituted cytochrome bd shows active oxygen consumption, leading to low intracellular oxygen levels under aerated conditions and protection against oxidative stress [11]. This protection against oxidative stress during respiration was clearly shown in a microarray study that reported down-regulation of many stress-related genes [12]. Interestingly, a respiration-negative mutant (a cydA mutant) of S. agalactiae showed not only a lack of respiratory behaviour but also a decrease in virulence (as determined in a rat-model) and a decreased ability to grow in blood [6,13].
Anaerobic respiration in E. faecalis and Lb. plantarum
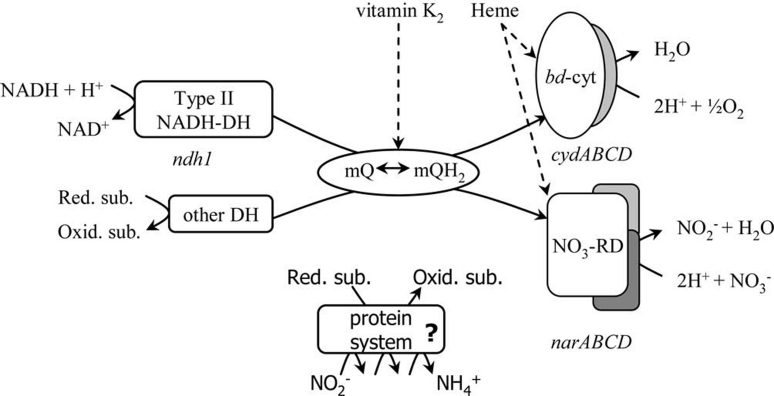
As described above, several LAB show aerobic respiration via a rather simple and non-redundant ETC consisting of a NADH dehydrogenase, a menaquinol pool and a bd-type cytochrome [7,11,14] (Figure 1). E. faecalis and Lb. plantarum also contain components for anaerobic respiration. In E. faecalis the existence of a branched ETC that terminates not only in a cytochrome bd-type but also in a fumarate reductase has been suggested [15]. An association of the E. faecalis frdA gene with fumarate reduction was shown, although the complete frdACBD gene cluster, typical for fumurate reductase in Escherichia coli [16], was missing. Many LAB contain homologues of frdA, suggesting that this single gene may also be functional in other LAB [15].
Figure 1. Model of the branched ETC of Lb. plantarum WCFS1.
bd-cyt, bd-type cytcochrome complex; DH, dehydrogenase; mQ/mQH2, oxidized/reduced menaquinone; NO3-RD, nitrate reductase; oxid., oxidised; red., reduced; sub., substrate.
Comparative genomics and genome-wide transcriptome analysis revealed that the narGHJI genes, encoding a nitrate-reductase A complex, are present and expressed in several lactobacilli; Lb. plantarum is able to reduce nitrate to nitrite only when both heme and menaquinone are supplied. Typically, reduction of nitrate by Lb. plantarum coincides with (transient) formation of nitrite and further reduction to ammonia, and a significant increase in biomass [17]. Lb. plantarum carrying a deleted ndh1 gene failed to respire in the presence of heme and menaquinone under aerobic conditions [17]. However, this mutation did not abolish the nitrate reductase activity. This indicates that the ETC of Lb. plantarum is also branched at the level of electron donation (Figure 1).
Distribution of respiration in LAB
As described above, some LAB demonstrate respiratory behaviour when menaquinone and/or heme are added to the culture. In fact, many species of LAB have the cydABCD genes present on their genome that encode the bd-type aerobic cytochrome. Of the 45 completely sequenced LAB genomes, these cyd-genes were found in about half the species [5]. In a recent screening activity (increased biomass formation during aerobic growth on media containing heme and menaquinone) it was shown that there are potential respirators among Lactococcus, Streptococcus, Lactobacillus, Carnobacterium, Enterococcus, Oenococcus and Leuconostoc spp. [5]. However, very few LAB besides Lb. Plantarum - only Lb. reuteri and Lb. fermentum - contain the necessary narGHJI genes for anaerobic nitrate respiration [17].
Industrial and ecological relevance
Enhanced biomass production by adding heme-containing compounds to aerated cultures of LAB is of interest for industrial processes, especially with regard to starter culture production [10,18]. Anaerobic ETC activity, with nitrate or fumarate as terminal electron acceptors, could also be implemented in industrial fermentation. It can be argued that nitrate respiration may even be a normal activity for several strains of Lb. plantarum, Lb. reuteri and Lb. fermentum in the human gastrointestinal (GI) tract. Heme is present in all foods that are of eukaryal origin (heme can be found in all mitochondria) as well as in many bacteria but not LAB. Also, menaquinones are produced by many prokaryotes in the GI tract. In fact, most of the human nutritional vitamin K2 requirement is covered by the gut microbiota [19,20]. In addition, nitrate and nitrite are part of the normal human diet [21-23] and derive from the consumption of green leafy vegetables or various meat products where these nitrogen compounds are used as preservatives [24].
Future directions
Many LAB have components of ETCs that enable them to use various intra- and extracellular electron donor and acceptor components for improved bioenergetics. The fact that these ETCs need the presence of exogenous cofactors such as heme and menaquinones to operate does not preclude that their activity is part of the normal lifestyle of LAB. Moreover, the presence of functional ETCs allows enhanced growth and functionality on a wider variety of substrates, which may provide new applications and even new health benefits by using LAB in the food, feed and pharmaceutical industries. Heme-sequestering by LAB should lead to a reduced risk of colonic cancer associated with eating red meat [22,25]. The reduction of nitrate via nitrite to ammonia in the GI tract by lactobacilli could lead to the transient production of nitric oxide. In fact, increased nitric oxide levels have been shown in the GI tract by combined dietary supplementation of nitrate and lactobacilli [26]. Nitric oxide production is known to counteract colonic inflammation [27] and has been implicated as a host defence mechanism against pathogens [28]. A final functional and health benefit of this induced nitrate reduction would be to use this process for lowering or completely removing nitrate (and nitrite) in foods such as cheese, which contain unacceptable levels in some countries.
Acknowledgments
The Kluyver Centre is supported by the Netherlands Genomics Initiative (NGI).
Abbreviations
- ETC
electron transport chain
- GI
gastrointestinal
- LAB
lactic acid bacteria
Competing interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://F1000.com/Reports/Biology/content/1/34
References
- 1.Kalantzopoulos G. Fermented products with probiotic qualities. Anaerobe. 1997;3:185–90. doi: 10.1006/anae.1997.0099. [DOI] [PubMed] [Google Scholar]
- 2.Leroy F, De Vuyst L. Lactic acid bacteria as functional starters for the fermentation industry. Trends Food Sci Technol. 2004;15:67–78. doi: 10.1016/j.tifs.2003.09.004. [DOI] [Google Scholar]
- 3.Pritchard GG, Wimpenny JW. Cytochrome formation, oxygen-induced proton extrusion and respiratory activity in Streptococcus faecalis var. zymogenes grown in the presence of haematin. J Gen Microbiol. 1978;104:15–22. doi: 10.1007/BF02069035. [DOI] [PubMed] [Google Scholar]
- 4.Sijpesteijn AK. Induction of cytochrome formation and stimulation of oxidative dissimilation by hemin in Streptococcus lactis and Leuconostoc mesenteroides. Antonie Van Leeuwenhoek. 1970;36:335–48. doi: 10.1007/BF02069035. [DOI] [PubMed] [Google Scholar]
- 5.Brooijmans RJW. PhD Thesis. University of Wageningen; 2008. Electron transport chains of lactic acid bacteria. [Google Scholar]
- 6.Yamamoto Y, Poyart C, Trieu-Cuot P, Lamberet G, Gruss A, Gaudu P. Respiration metabolism of Group B Streptococcus is activated by environmental haem and quinone and contributes to virulence. Mol Microbiol. 2005;56:525–34. doi: 10.1111/j.1365-2958.2005.04555.x. [DOI] [PubMed] [Google Scholar]
- 7.Brooijmans RJ, Poolman B, Schuurman-Wolters GK, de Vos WM, Hugenholtz J. Generation of a membrane potential by Lactococcus lactis through aerobic electron transport. J Bacteriol. 2007;189:5203–9. doi: 10.1128/JB.00361-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koebmann BJ, Blank LM, Solem C, Petranovic D, Nielsen LK, Jensen PR. Increased biomass yield of Lactococcus lactis during energetically limited growth and respiratory conditions. Biotechnol Appl Biochem. 2008;50:25–33. doi: 10.1042/BA20070132. [DOI] [PubMed] [Google Scholar]
- 9.Duwat P, Sourice S, Cesselin B, Lamberet G, Vido K, Gaudu P, Le Loir Y, Violet F, Loubière P, Gruss A. Respiration capacity of the fermenting bacterium Lactococcus lactis and its positive effects on growth and survival. J Bacteriol. 2001;183:4509–16. doi: 10.1128/JB.183.15.4509-4516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudu P, Vido K, Cesselin B, Kulakauskas S, Tremblay J, Rezaïki L, Lamberret G, Sourice S, Duwat P, Gruss A. Respiration capacity and consequences in Lactococcus lactis. Antonie Van Leeuwenhoek. 2002;82:263–9. doi: 10.1023/A:1020635600343. [DOI] [PubMed] [Google Scholar]
- 11.Rezaïki L, Cesselin B, Yamamoto Y, Vido K, van West E, Gaudu P, Gruss A. Respiration metabolism reduces oxidative and acid stress to improve long-term survival of Lactococcus lactis. Mol Microbiol. 2004;53:1331–42. doi: 10.1111/j.1365-2958.2004.04217.x. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen MB, Garrigues C, Tuphile K, Brun C, Vido K, Bennedsen M, Møllgaard H, Gaudu P, Gruss A. Impact of aeration and heme-activated respiration on Lactococcus lactis gene expression: identification of a heme-responsive operon. J Bacteriol. 2008;190:4903–11. doi: 10.1128/JB.00447-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Poyart C, Trieu-Cuot P, Lamberet G, Gruss A, Gaudu P. Roles of environmental heme, and menaquinone, in Streptococcus agalactiae. Biometals. 2006;19:205–10. doi: 10.1007/s10534-005-5419-6. [DOI] [PubMed] [Google Scholar]
- 14.Blank LM, Koebmann BJ, Michelsen O, Nielsen LK, Jensen PR. Hemin reconstitutes proton extrusion in an H+-ATPase-negative mutant of Lactococcus lactis. J Bacteriol. 2001;183:6707–9. doi: 10.1128/JB.183.22.6707-6709.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Jacky Snoep 14 Nov 2001
- 15.Huycke MM, Moore D, Joyce W, Wise P, Shepard L, Kotake Y, Gilmore MS. Extracellular superoxide production by Enterococcus faecalis requires demethylmenaquinone and is attenuated by functional terminal quinol oxidases. Mol Microbiol. 2008;42:729–40. doi: 10.1046/j.1365-2958.2001.02638.x. [DOI] [PubMed] [Google Scholar]
- 16.Cecchini G, Schröder I, Gunsalus RP, Maklashina E. Succinate dehydrogenase and fumarate reductase from Escherichia coli. Biochim Biophys Acta. 2002;1553:140–57. doi: 10.1016/S0005-2728(01)00238-9. [DOI] [PubMed] [Google Scholar]
- 17.Brooijmans RJW, de Vos WM, Hugenholtz J. The electron transport chains of Lactobacillus plantarum WCFS1. Appl Environ Microbiol. 2009;(Apr 3) doi: 10.1128/AEM.00147-09. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duwat P, Sourice S, Gruss A. Process for preparing starter cultures of lactic acid bacteria. French patent application FR9809463. 1998
- 19.Conly JM, Stein K. The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog Food Nutr Sci. 1992;16:307–43. [PubMed] [Google Scholar]
- 20.Conly JM, Stein K, Worobetz L, Rutledge-Harding S. The contribution of vitamin K2 (menaquinones) produced by the intestinal microflora to human nutritional requirements for vitamin K. Am J Gastroenterol. 1994;89:915–23. [PubMed] [Google Scholar]
- 21.Dich J, Järvinen R, Knekt P, Penttilä PL. Dietary intakes of nitrate, nitrite and NDMA in the Finnish Mobile Clinic Health Examination Survey. Food Addit Contam. 1996;13:541–52. doi: 10.1080/02652039609374439. [DOI] [PubMed] [Google Scholar]
- 22.Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R. Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme. Cancer Res. 1999;59:5704–9. [PubMed] [Google Scholar]
- 23.Ysart G, Miller P, Barrett G, Farrington D, Lawrance P, Harrison N. Dietary exposures to nitrate in the UK. Food Addit Contam. 1999;16:521–32. doi: 10.1080/026520399283669. [DOI] [PubMed] [Google Scholar]
- 24.White JW., Jr Relative significance of dietary sources of nitrate and nitrite. J Agric Food Chem. 1975;23:886–91. doi: 10.1021/jf60201a034. [DOI] [PubMed] [Google Scholar]
- 25.Pierre F, Peiro G, Tache S, Cross AJ, Bingham SA, Gasc N, Gottardi G, Corpet DE, Gueraud F. New marker of colon cancer risk associated with heme intake: 1,4-dihydroxynonane mercapturic acid. Cancer Epidemiol Biomarkers Prev. 2006;15:2274–9. doi: 10.1158/1055-9965.EPI-06-0085. [DOI] [PubMed] [Google Scholar]
- 26.Sobko T, Huang L, Midtvedt T, Norin E, Gustafsson LE, Norman M, Jansson EA, Lundberg JO. Generation of NO by probiotic bacteria in the gastrointestinal tract. Free Radic Biol Med. 2006;41:985–91. doi: 10.1016/j.freeradbiomed.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Lamine F, Fioramonti J, Bueno L, Nepveu F, Cauquil E, Lobysheva I, Eutamène H, Théodorou V. Nitric oxide released by Lactobacillus farciminis improves TNBS-induced colitis in rats. Scand J Gastroenterol. 2004;39:37–45. doi: 10.1080/00365520310007152. [DOI] [PubMed] [Google Scholar]
- 28.Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997;99:2818–25. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]