Abstract
Non-alcoholic fatty liver disease (NAFLD) is defined as fat accumulation in the liver, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH). Although it used to be considered a benign condition, nowadays it is known to be associated with liver injury and the development of end-stage liver disease. NAFLD is the hepatic manifestation of metabolic syndrome (MS) with an incidence rising in accordance with the increased prevalence of MS, the latter being considered the most common cause of liver enzyme elevation in Western countries. To date, no medications or surgical procedures have been approved for effective treatment of NAFLD, and all of the therapies tested so far must still be regarded as experimental. It is expected that, based on the large amount of data produced in the last few years and the ongoing large multicenter clinical trials, the effective treatment(s) for NASH will soon be defined. Meanwhile, lifestyle interventions and behavior therapy, the only treatments shown to be effective, must be introduced in daily clinical practice and, if possible, supported by public health programs.
Introduction and context
Although non-alcoholic fatty liver disease (NAFLD) was first described by Zelman [1] more than 65 years ago, only in 1980 did Ludwig and colleagues [2] define a new entity characterized by a type of fatty liver with inflammation, ballooned hepatocytes, and fibrosis, naming it ‘non-alcoholic steatohepatitis’ (NASH). NAFLD is now defined as fat accumulation in the liver exceeding 5-10% by weight in subjects without significant alcohol consumption (<20 g/day, the equivalent of less than two or three glasses of wine per day) and without any other known causes of chronic liver disease. In clinical practice, the diagnosis of NAFLD is usually reached by ultrasonography that allows the detection of moderate to severe steatosis with a fair sensitivity and specificity, but only when fat on the liver biopsy exceeds 20-30%.
NAFLD and NASH are strictly correlated with the presence of insulin resistance (IR), which is often associated with central obesity, type 2 diabetes, and dyslipidemia, rendering liver steatosis the hepatic manifestation of metabolic syndrome (MS). The incidence of NAFLD/NASH is rapidly rising in adults and children due to the increased prevalence of obesity, type 2 diabetes, and metabolic diseases, and is now considered the most frequent cause of elevated liver enzymes [3,4].
Population studies estimate that 20-25% of adults have NAFLD, and about 10% may progress to NASH, cirrhosis, and ultimately hepatocellular carcinoma over a period of 30-40 years [5]. In contrast, the overall survival due to increased risk for both cardiovascular and liver disease mortality is lower [6,7]. Since hepatologists worldwide are facing an epidemiological explosion of this disease, several efforts are aimed at the early identification of subjects affected by NAFLD/NASH and at finding an effective treatment.
Recent advances
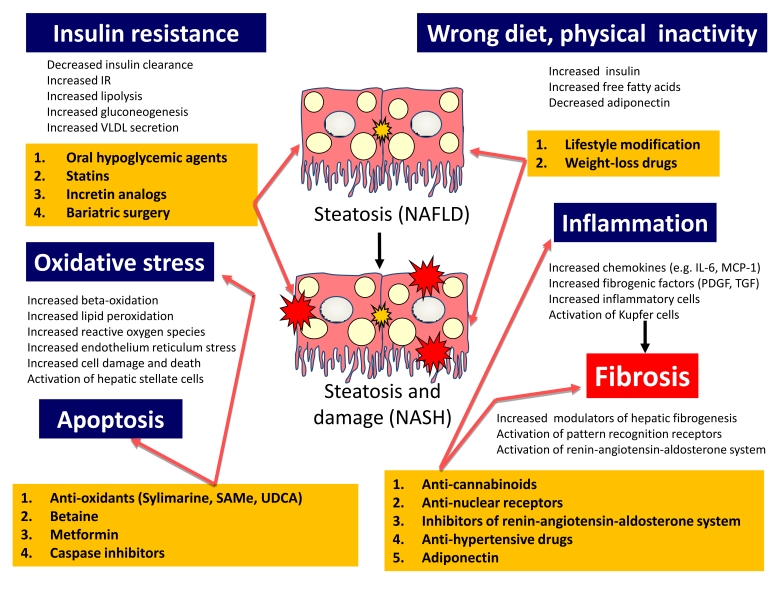
Progress in developing potential therapies for NAFLD/NASH has been made mainly by addressing the singular step involved in the pathogenesis of the diseases (Figure 1). Agents reducing oxidative stress and/or apoptosis or showing cytoprotective properties have been evaluated with inconclusive results since the studies were conducted in small uncontrolled trials. At present, lifestyle interventions and behavior therapy, together with drugs used to treat the associated components of the MS (hypertension, diabetes, or dyslipidemia), represent the only therapeutic approach available to the clinician.
Figure 1. Treatment targets according to the pathogenesis mechanisms of NAFLD and NASH.
Pathogenesis mechanisms (dark blue) of NAFLD and NASH and various treatment options (yellow) are displayed. Treatment targets are indicated by the pink arrows. IL-6, interleukin-6; IR, insulin resistance; MCP-1, monocyte chemoattractant protein-1; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PDGF, platelet-derived growth factor; SAMe, S-adenosylmethionine; TGF, transforming growth factor; UDCA, ursodeoxycholic acid; VLDL, very low density lipoproteins.
Lifestyle modifications
Lifestyle modifications through a multidisciplinary approach, including dietary intervention, behavior therapy, and physical exercise, are the frontline therapy for this disease [8]. This is true not only in obese or overweight patients but also in normal-weight patients with cryptogenic NASH demonstrated by liver biopsy. Even though this approach could be ultimately cost-effective, it unfortunately is limited by the long-term compliance of the patients. Dietary intervention together with daily physical exercise is associated with a weight reduction and amelioration of IR and steatosis [9-12]. Both Suzuky and colleagues [12] in 2005 and more recently Harrison and Day [11] demonstrated that reducing weight by at least 5%, with subsequent weight control and regular exercise, improves or normalizes alanine aminotransferase (ALT) levels [12]; a reduction of body weight of more than 9% is associated with an improvement in hepatic histology [11,13]. Modifications of diet to improve NAFLD or avoid the progression to NASH have been attempted recently in pilot studies and included both the reduction of saturated fatty acids and simple carbohydrate intake and the increase of polyunsaturated fatty acids and slowly adsorbed carbohydrates [14,15]. Much-needed larger trials with appropriate histological follow-up are still lacking.
Pharmacotherapy
Oral hypoglycemic agents
Insulin-sensitizing agents, namely metformin and the thiazolidinediones (TZDs) (also called glitazones), have been investigated in controlled trials. Many studies demonstrated an improvement of aminotransferase levels, IR, and histology after metformin treatment [16]. More recently, metformin has been found to modulate the expression of the pro-inflammatory tumor necrosis factor-alpha (TNF-α) cytokines [17] and to improve liver function and ecographic steatosis more than dietary modifications alone [18]. Also, TZDs (pioglitazone and rosiglitazone) are proving promising in the treatment of NASH, and various randomized long-term controlled studies in limited series have demonstrated a significant reduction in serum ALT and liver fat content associated with an improvement of IR and liver histology [19]. However, the main limitations of TZD therapy are the short duration of the beneficial effects after the drug is stopped and its contraindication in patients with heart disease [20]. Further larger studies are required.
Weight-loss drugs
Contradictory data have been published on the treatment of NASH with orlistat, a lipase inhibitor reducing fat absorption. In randomized placebo-controlled trials, orlistat led to an improvement in serum liver enzymes, ultrasound findings, and hepatic inflammation and fibrosis, but only in patients who maintained a significant weight reduction [21]. In another pilot study, which compared orlistat and sibutramine (an enhancer of satiety), it was demonstrated that treatment with both drugs led to liver enzyme improvement and liver fat reduction at ultrasound [22]. These data suggest a possible role of orlistat for NASH treatment in facilitating significant weight loss.
The endocannabinoid (EC) system, involved in the regulation of food intake and body weight, represents a novel target for medical therapy of NASH (Figure 1). Rimonabant is a selective EC CB1 receptor antagonist decreasing hepatic lipogenesis and increasing satiety, adiponectin levels, and glucose uptake, thereby improving insulin levels and lipid profiles. In four large randomized placebo-controlled multicenter trials, rimonabant has been shown after 1 or 2 years of therapy to induce a greater significant average weight loss than placebo in obese patients, and more notably, a significant improvement in serum lipid profile, glycosylated hemoglobin, adiponectin, and C-reactive protein in patients with hyperlipidemia and diabetes. These impressive metabolic effects and preliminary animal studies, along with isolated case reports, suggested a direct effect of this medication on NASH and, in 2007, prompted two large multicenter trials. Unfortunately, both trials were interrupted by either American or European national medicine authorities (the US Food and Drug Administration and the European Medicine Agency, respectively) due to the development of significant psychiatric problems (severe depression and suicide) in some patients involved in the trial.
Statins
The role of statins in the treatment of NASH remains unclear. Recently, it was shown that patients treated with statins had a significant reduction in hepatic steatosis, liver enzymes, and TNF-α serum levels, and probably a low rate of fibrosis progression as well [23]. Although these early studies suggest that statins may be useful in hyperlipidemic NAFLD patients, controlled trials are necessary to validate these results.
Anti-oxidants, cytoprotective agents, and other drugs
Vitamin E, betaine, and ursodeoxycholic acid (UDCA) therapies have produced controversial results. Studies using vitamin E showed an improvement of liver function whereas others did not [24,25]. Despite the preliminary results of some pilot studies demonstrating the protective role of UDCA in NAFLD, a large multicenter trial with a sufficiently large number of patients treated with UDCA or placebo for 2 years showed histological improvement in both the UDCA group and the placebo group [26]. It is possible that UDCA used in combination with other medications, as in the case of vitamin E, may improve liver function in NAFLD-affected patients [27].
Betaine, a metabolite that increases levels of S-adenosylmethionine, was used in a small uncontrolled study demonstrating an improvement in aminotransferase levels, steatosis, inflammation, and fibrosis after 1 year of treatment [28]. In two small studies, treatment with the angiotensin II blocker, losartan, also led to improvements in liver histology [29]. However, the very limited number of patients involved in these studies renders the results questionable.
Bariatric surgery
Bariatric surgery is suitable in severely obese patients, often affected by NAFLD, who failed to lose weight with nutritional counseling. Different surgical approaches are possible, and in general, liver histology improves significantly after bariatric surgery [30].
Implications for clinical practice
The epidemiological explosion of obesity and diabetes in Western countries led to a parallel increase in the prevalence of NAFLD. Once the presence of fatty liver at ultrasound is recognized, it is important for the general practitioner and the hepatologist to consider NAFLD as a possible progressive liver disease, significantly associated with increased cardiovascular risks, which must be checked. Physicians have to exclude alcohol intake and to follow up NAFLD-affected patients to reduce the progression to NASH and other end-stage liver diseases. Despite its limitations, liver biopsy is still the gold standard for the diagnosis of NASH.
Once the diagnosis of NAFLD or NASH is reached, the main efforts must be focused on changing the lifestyle of the patient. Diet and physical exercise, which ideally should always be tailored to the individual, represent the only therapeutic approach available at present to treat NASH, by preventing cardiovascular risk and MS manifestations. This therapy needs a strong multidisciplinary counseling program to increase and maintain patient compliance. Enrolled patients should be referred to trained lifestyle therapists (dietitians, behavioral psychologists, physical activity supervisors, and case managers), and change in their social environment should be considered.
Other pharmacological or surgical treatments may be considered but only in patients with associated MS or severe obesity since no drugs or surgical procedures have been approved for the treatment of NAFLD or NASH. Larger multicenter clinical trials and validation studies are ongoing worldwide (Table 1), and the ideal treatment for NASH will hopefully be found soon. Meanwhile, lifestyle interventions and behavior therapy must be introduced in clinical practice, possibly supported by public health programs, to change the environment of our ‘fatty society’.
Table 1. Promising agents and ongoing clinical trials [31] to treat NASH.
| Phase II study | Phase III study | ||||
|---|---|---|---|---|---|
| Therapeutic target | Agent | Pilot | Multicenter | Single | Multicenter |
| Diet Macronutrients | Omega-3 fatty acids | × | |||
| Polyunsaturated fatty acids | × | ||||
| Weight loss | Orlistat | × | |||
| Rimonabant | × | ||||
| Antioxidants | Silymarin | × | |||
| Pentoxifilline | × | ||||
| Pentoxifilline versus pioglitazone | × | ||||
| SAMe | × | ||||
| Iron depletion | × | ||||
| Mitochondrial protection (TRO19662) | × | ||||
| Inflammation and fibrosis | PDE4 inhibitor | × | |||
| Reduction of insulin resistance | Metformin versus vitamin E versus placebo (TONIC) | × | |||
| Pioglitazone versus metformin versus placebo (PIVENS) | × | ||||
| Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan | × | ||||
NASH, non-alcoholic steatohepatitis; PDE4, phosphodiesterase-4; PIVENS, Pioglitazone versus Vitamin E versus Placebo for the Treatment of Non-diabetic Patients with Non-alcoholic Steatohepatitis; SAMe, S-adenosylmethionine; TONIC, Treatment of Non-alcoholic Fatty Liver Disease in Children.
Acknowledgments
The authors would like to acknowledge the help of Claudio Tiribelli in drafting and critically reading the manuscript.
Abbreviations
- ALT
alanine aminotransferase
- EC
endocannabinoid
- IR
insulin resistance
- MS
metabolic syndrome
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- TNF-α
tumor necrosis factor-alpha
- TZD
thiazolidinedione
- UDCA
ursodeoxycholic acid
Competing interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://F1000.com/Reports/Medicine/content/1/50
References
- 1.Zelman S. The liver in obesity. AMA Arch Intern Med. 1952;90:141–56. doi: 10.1001/archinte.1952.00240080007002. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. [PubMed] [Google Scholar]
- 3.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 4.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 5.Falck-Ytter Y, Younossi ZM, Marchesini G, McCullough AJ. Clinical features and natural history of nonalcoholic steatosis syndromes. Semin Liver Dis. 2001;21:17–26. doi: 10.1055/s-2001-12926. [DOI] [PubMed] [Google Scholar]
- 6.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, Okuda J, Ida K, Yoshikawa T. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–84. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–12. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Stefano Bellentani 08 Oct 2008
- 8.Bellentani S, Dalle GR, Suppini A, Marchesini G. Behavior therapy for nonalcoholic fatty liver disease: the need for a multidisciplinary approach. Hepatology. 2008;47:746–54. doi: 10.1002/hep.22009. [DOI] [PubMed] [Google Scholar]
- 9.de Luis DA, Aller R, Izaola O, Sagrado MG, Conde R, Gonzalez JM. Effect of a hypocaloric diet in transaminases in nonalcoholic fatty liver disease and obese patients, relation with insulin resistance. Diabetes Res Clin Pract. 2008;79:74–8. doi: 10.1016/j.diabres.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Krasnoff JB, Painter PL, Wallace JP, Bass NM, Merriman RB. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2008;47:1158–66. doi: 10.1002/hep.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison SA, Day CP. Benefits of lifestyle modification in NAFLD. Gut. 2007;56:1760–9. doi: 10.1136/gut.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuky A, Lindor K, Saver JS, Lymp J, Mendes F, Muto A, Angulo P. Effect of changes in body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol. 2005;43:1060–6. doi: 10.1016/j.jhep.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Rafiq N, Younossi ZM. Effects of weight loss on nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:427–33. doi: 10.1055/s-0028-1091986. [DOI] [PubMed] [Google Scholar]
- 14.Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007;86:285–300. doi: 10.1093/ajcn/86.2.285. [DOI] [PubMed] [Google Scholar]
- 15.Zhu FS, Liu S, Chen XM, Huang ZG, Zhang DW. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol. 2008;14:6395–400. doi: 10.3748/wjg.14.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–4. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 17.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 18.Angelico F, Burattin M, Alessandri C, Del Ben M, Lirussi F. Drugs improving insulin resistance for non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2007;1:CD005166. doi: 10.1002/14651858.CD005166.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E, Grimaldi A, Poynard T. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100–10. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 20.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–36. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Melvin Cheitlin 20 Dec 2007
- 21.Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology. 2009;49:80–6. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- 22.Sabuncu T, Nazligul Y, Karaoglanoglu M, Ucar E, Kilic FB. The effects of sibutramine and orlistat on the ultrasonographic findings, insulin resistance and liver enzyme levels in obese patients with non-alcoholic steatohepatitis. Rom J Gastroenterol. 2003;12:189–92. [PubMed] [Google Scholar]
- 23.Ekstedt M, Franzen LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol. 2007;47:135–41. doi: 10.1016/j.jhep.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667–72. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 25.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–9. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 26.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–8. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 27.Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, Zala JF, Helbling B, Steuerwald M, Zimmermann A. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–43. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 28.Abdelmalek MF, Angulo P, Jorgensen RA, Sylvestre PB, Lindor KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol. 2001;96:2711–7. doi: 10.1111/j.1572-0241.2001.04129.x. [DOI] [PubMed] [Google Scholar]
- 29.Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, Hasegawa T, Tokusashi Y, Miyokawa N, Nakamura K. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004;40:1222–5. doi: 10.1002/hep.20420. [DOI] [PubMed] [Google Scholar]
- 30.Verna EC, Berk PD. Role of fatty acids in the pathogenesis of obesity and fatty liver: impact of bariatric surgery. Semin Liver Dis. 2008;28:407–26. doi: 10.1055/s-0028-1091985. [DOI] [PubMed] [Google Scholar]
- 31.Clinicaltrials.gov homepage. [ http://www.clinicaltrials.gov/]