Abstract
Charged-particle microbeams, developed to provide targeted irradiation of individual cells, and then of sub-cellular components, and then of 3-D tissues and now organisms, have been instrumental in challenging and changing long accepted paradigms of radiation action. However the potential of these valuable tools can be enhanced by integrating additional components with the direct ability to measure biological responses in real time, or to manipulate the cell, tissue or organism of interest under conditions where information gained can be optimized. The RARAF microbeam has recently undergone an accelerator upgrade, and been modified to allow for multiple microbeam irradiation laboratories. Researchers with divergent interests have expressed desires for particular modalities to be made available and ongoing developments reflect these desires. The focus of this review is on the design, incorporation and use of multiphoton and other imaging, micro-manipulation and single cell biosensor capabilities at RARAF. Additionally, an update on the status of the other biology oriented microbeams in the Americas is provided.
Keywords: Microbeam, Particle Accelerator, Cell Irradiation, Radiation Biology
INTRODUCTION
In order to study the effects of ionizing radiation on single cells, it is practical to have a device that can locate single-cell and sub-cellular targets and deliver a prescribed specific dose to each target.(1) A minimum delivery of exactly one ion per cell nuclei demonstrates the precision of a single-particle single-cell ion microbeam and is particularly applicable to studying effects from Radon exposure.(2) Particle accelerators with specialized ion optics or collimators are capable of forming charged-particle microbeams with sub-cellular resolution, where the beam size is smaller than a cell nucleus. Coupled to an endstation with imaging, location, and positioning techniques, a microbeam is a powerful tool for controlled, cell-irradiation experiments.
Whereas single-cell microbeam irradiation studies date back to developments in the 1950s at the University of Chicago,(3) additional pioneers in the field of single-cell ion microbeam irradiation include groups at the Pacific Northwest National Laboratory,(4) the Gray Cancer Institute,(5, 6) and Columbia University.(7) Since that time, the number of planned and operational microbeam facilities worldwide has increased significantly. To provide a cohesive example of a microbeam facility, this review article will first concentrate on the development of light ion microbeam technology at the Radiological Research Accelerator Facility (RARAF), Columbia University and subsequently provide technical summaries of microbeam facilities in the Americas.
RARAF – A MICROBEAM FACILITY
A single-cell-single particle microbeam is a device for depositing ionizing energy in cells or subcellular sites, with micron or submicron precision. As depicted in Fig. 1, a microbeam irradiator generally functions in the following sequence, 1) a target cell is moved in front of the microbeam, 2) the shutter is opened, 3) one (or more) particles traverse the cell (and are detected by the radiation detector), 4) the shutter is closed, 5) the next cell is moved in front of the microbeam.
FIGURE 1.
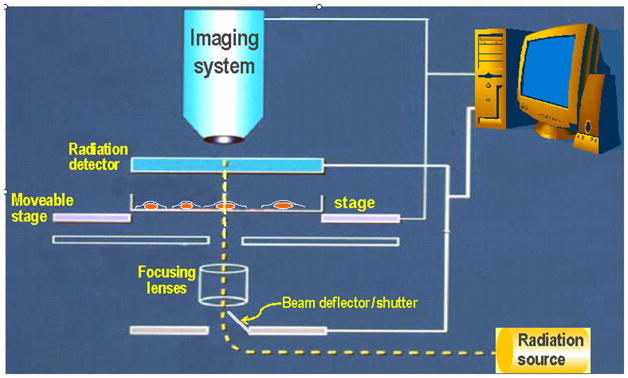
General schematic of microbeam components at RARAF.
Microbeam technologies at RARAF are geared towards cell-irradiation experiments, such as, the bystander effect, genomic instability, and adaptive response. For biology experiments, such as mutation and oncogenic transformation studies,(2, 8–12) available high throughput of about 11,000 cells per hour was vital for obtaining statistically relevant numbers of irradiated samples. Researchers at RARAF have two particle microbeam systems at their disposal: Microbeam II and the Permanent Magnetic Microbeam (PMM). In these systems at RARAF, several design standards have been conserved for successful operation. For one, a vertical ion beam for irradiation has a practical advantage when working with plated cells covered by a very thin, nominally uniform, layer of medium. Also, cell-dish transfer between the biologist and the microbeam operator is expedited by having a biology workbench and incubator in the same room and adjacent to the irradiator.
Ion beams for both microbeam systems start from the 5-MV Singletron (HVE, Netherlands) electrostatic particle accelerator that was installed at RARAF during summer/fall of 2005. Additionally, protons from this accelerator will be utilized to induce soft x rays to be zone-plate focused on cellular targets on a microscope-based workstation.(13)
Microbeam II
RARAF’s current irradiation system, Microbeam II, includes a focused ion beam on a dedicated beamline with a double-focusing magnet. With electrostatic ion optics in place to focus the beam, the current system offers a smaller beam compared to that from its predecessor, Microbeam I, which featured a collimated beam. The Microbeam II endstation incorporates a Nikon Eclipse 600FN microscope that rests in a kinetic mount and is also attached to a pivot arm for on-line and off-line positioning. Sample illumination is regulated with an adjustable, light-guide coupled, shuttered UV lamp. Ion beam scattering at the vacuum exit window is minimized by using a 100-nm thick SiN exit window. A precision xyz-stage incorporated into the endstation of Microbeam II comprises two components: a custom coarse xy-stage, designed and constructed in-house, coupled to a 3-axis, piezoelectric-actuated, fine motion stage, the LP-200 low-profile nano-positioner from Mad City Labs (Madison, WI).(14)
A microbeam formed with focusing elements brought a promise of a smaller beam spot size, which matched the biological interests of targeting sub-cellular components. Electric and magnetic quadrupole fields are the preferred choices of ion optics to focus ion beams, because these fields are reasonable to produce and they can easily be modeled with transfer matrices.(15) For the Columbia University Microbeam II system, a compound lens consisting of two electrostatic quadrupole triplets with “Russian symmetry” was developed.(16, 17) The compound triplet lens was installed in the Microbeam II beamline for biology experiments that began in October 2007 and has achieved a sub-micron beam diameter. For technical details, refer to the extended abstract in this issue by Gerhard Randers-Pehrson.
Ion beam profiles are measured by stepping xy-crossed, 3-μm thick, Havar knife edges through the beam at the desired focal plane, while monitoring the ion energies with a solid-state detector. Threshold settings on a single-channel analyzer enable the distinction between the unobstructed ion counts from those that penetrate the knife edges. The ion beam size is determined using a χ2-monitored linear fit to the fraction of obstructed beam between approximately 0.2–0.8. Assuming a largely uniform ion beam profile, the reciprocal of the fitted slope is the full width of the beam distribution. An auto-focusing routine that uses the downhill simplex method successively measures ion beam sizes for combinations of electrostatic lens voltages to optimize the lens settings. When the ion beam focus is determined, it’s xy-location reference is found for the imaging system by scanning a 4-μm diameter fluorescent bead from a position roughly over the ion beam focus in an outward rectangular spiral pattern, using the same energy-loss technique that was used during the auto-focus routine. When the fluorescent bead position is determined to be at the center of the ion beam, the solid state detector is removed and an image of the bead is acquired, registering the actual beam position in the imaging software.
Accurate cell imaging and targeting is facilitated through a program written for real-time image analysis and stage positioning. Developed in Visual Basic, using the Matrox imaging library, under the Windows NT operating system, the irradiation algorithm is essentially: 1) with a low-magnification objective lens, acquire a series of adjacent images for coarse cellular location, 2) under high magnification, re-image each region that contains at least one cell in the coarse image, then locate, position, and irradiate cells. For each cell to be irradiated, a mechanical stage moves the cell target to the previously determined coordinates of the ion beam.
Each microbeam experiment includes an irradiation protocol with 1) a prescribed number of particles (0, 1, 2, …) per targeted cell, 2) a percentage of cells to be targeted, and 3) targeting modes: uniform targeting, sub-cellular, site-specific targeting (nucleus or cytoplasm), and patterned targeting, such as stripes on tissue.(18) This protocol is realized by shuttering the beam with an upstream electric-field deflector once the prescribed number of particles has been detected by an ion counter.
Ions exit the vacuum beamline through a 100-nm thick SiN exit window and traverse a 100-μm-thick air gap prior to irradiating cells plated on a 3.8-μm-thick polypropylene membrane. After penetrating the cells, these ions have sufficient range to then enter an ion counter filled with P10 gas (90% argon and 10% methane).(7) This particle detector design includes an optically transparent, 2.5-μm-thick mica entrance window to allow in-line mounting at the end of the high-magnification objective. As well, a side passage in the detector transfers humidified air with 5% CO2 over the cells to prevent dehydration. The ion detection signal propagates through a preamplifier and amplifier combination and on through a single-channel analyzer and scaler. The gate signal of the scaler is sent to the input of a model 609E-6 high-voltage amplifier (Trek, Inc., Medina, NY), where the output produces an electrostatic deflection across the upstream shutter electrodes.
Permanent Magnetic Microbeam (PMM)
In addition to Microbeam II, a permanent magnetic microbeam (PMM), based on permanent magnetic quadrupole lenses, was developed on a dedicated beam line at RARAF. The permanent quadrupole lenses (STI Optronics, Inc., Bellevue, WA) form a double triplet Russian quadrupole arrangement designed to have the same pole strengths and configuration as the electrostatic lens system on Microbeam II. Fine tuning of the lens strengths is achieved by physically moving rare-earth permanent magnets in a shaped yoke – modifying field strength.(19) Similar to an electrostatic lens, a permanent magnet lens also benefits from a small pole gap (due to the lack of large coils) and high stability. The PMM can be operated with particles from either a particle accelerator or from an isotopic source.(20, 21) The present spot size diameter is 4 μm.
The PMM provides a useful secondary microbeam facility at RARAF for biology to be performed simultaneously with system developments on the electrostatic microbeam. Much of the cell imaging and targeting infrastructure on the PMM parallels that which is described in the section above on Microbeam II; cell positioning is achieved using a stepping-motor driven xy stage.
Laser Ion Source
With the interest to increase the range of available linear energy transfer (LET) values from the RARAF microbeam, a laser ion source (LIS) has been designed and built as an ion source option for the 5 MV High Voltage Engineering (HVE) Singletron particle accelerator.(22) This LIS is expected to provide ions from hydrogen to iron that, after acceleration, will have sufficient energy to irradiate mammalian cells on a thin membrane at atmospheric pressure with an LET range of about 10 to 4,500 keV/μm. The LIS scheme at Columbia University creates ions through laser ablation, using high-power pulses from a Nd:YAG laser (Spectra Physics) that are focused onto a solid target. The LIS is pictured in a table-top configuration in Fig. 2.
FIGURE 2.
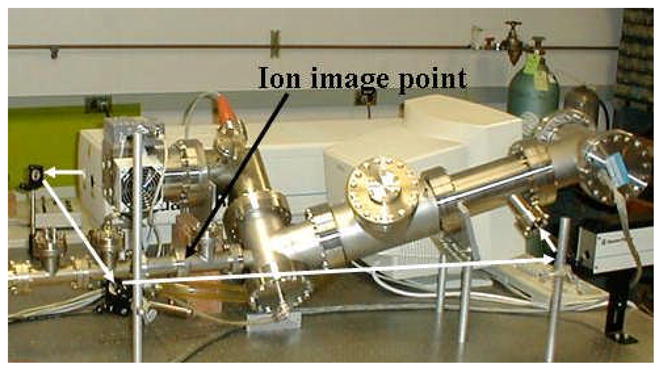
Picture of the Columbia University laser ion source (LIS) on the work bench. The optical path of the Nd:YAG laser has been drawn with white arrows to show where it enters the vacuum system. Except for the pump manifold, the vacuum components on the right, up to the ion image point, are the essential LIS parts for installation at the accelerator terminal.
A spherical electrostatic analyzer (ESA) selects ions with a particular energy per charge and focuses them to a point coinciding with the 3.18-mm entrance aperture of the accelerator tube. It is crucial to have a match between the ion source emittance and acceptance of the Singletron accelerator tube. Hence, in collaboration with HVE, appropriate modifications were made to the primary accelerator tube sections to optimize the transmission of the LIS-produced ions and also conserve the function of the duoplasmatron, the primary ion source incorporated on the Singletron.
Single-Cell Biosensors
There is increasing evidence that bystander signals of damage between cells may originate with a diffusible mediator.(23–25) These notions can be directly tested through measurements of production of NO, H2O2 or O2- or oxygen consumption, on single cells immediately after microbeam exposure. At RARAF, an online microsensor facility is under development for continuous measurement of ion fluxes from distinct regions around single cells, during and after microbeam irradiation. These tools can be applied to questions relating to the biodiversity of transporter structure/function as cells adapt to radiation damage, boundary layer conditions and function.
This project is in collaboration with Dr. Peter Smith at the Biocurrents Research Center (BRC), Woods Hole, who has had extensive experience with self-referencing polarographic (Serp) microsensor technology, for the detection of a variety of ion fluxes.(26, 27) Several new microsensor designs have been developed by Smith, et. al., notably a unique array of self-referencing sensor designs based on potentiometric and amperometric detection. The latter is particularly suitable for the detection of radical mediators, such as nitric oxide.(27, 28)
These self referencing polarographic microsensors allow sensitive and non-invasive measurement in single cells of the flux of oxygen (see Fig. 3), or nitric oxide, superoxide or hydrogen peroxide, or calcium; this is achieved through the translational movement of the Serp at a known frequency through an ion gradient, between known points. Specifically, the microsensor tip (diameter 2–3 μm) moves through the extracellular gradient at a known frequency and between points a known distance apart. By signal averaging the current obtained at each position, then referencing the signals together, a differential current is obtained that can be converted into a directional measurement of flux. Referencing the signals in this manner has the advantage that sources of interference caused by random drift and noise are effectively filtered from the signal and fluxes can be monitored in real time. The Serp microsensors operate on platforms very similar to standard electrophysiological set-ups – critical issues, which will be elaborated on in a forthcoming publication, are electrode access, vibration and electrical noise. A picture of an oxygen microsensor in use is shown in Fig. 4.
FIGURE 3.
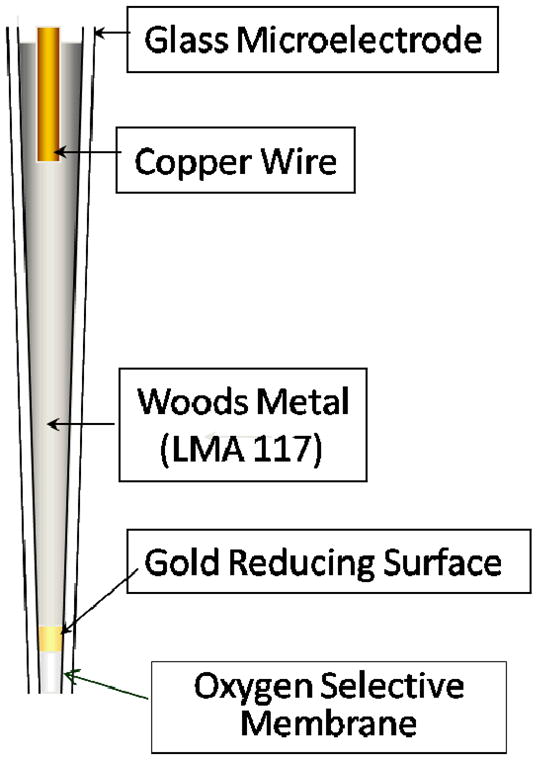
Structure of an oxygen microsensor. The aperture is typically 1 μm in diameter.
FIGURE 4.

Oxygen microsensor in position in a microbeam irradiation dish.
Imaging In 3D – Multiphoton Microscopy
While results from microbeam irradiation experiments on cell-cultures have contributed to studies on low-dose radiation effects, such as the bystander effect, radiation studies using tissue samples have the potential to represent cellular response within organisms.(18) For observing post-irradiation cellular dynamics within tissue, multiphoton microscopy offers optical sectioning compatibility with live samples and can image at depths of several hundred micrometers. Multiphoton excitation of fluorochromes can occur at high photon densities, such as, at the focal point of a tunable, mode-locked Titanium Sapphire (Ti:S) laser source.
Unique in the microbeam community, a microbeam-integrated multiphoton imaging system is now operational at RARAF.(29) This multiphoton microscope was custom-built and conforms to the particular geometrical constraints of the irradiation endstation, which has a Nikon Eclipse 600FN microscope at the end of a vertical ion beam. A tunable Chameleon Ultra II (Coherent, Inc., Santa Clara, CA) Ti:S laser source provides wavelengths in the range: 680–1080 nm. For two photon excitation, the available wavelength effectively doubles down to 340–540nm to access fluorophores with absorption spectra in that range.
Multiphoton microscopy on the RARAF microbeam II endstation has become a routine technique for visualizing samples before, during and after radiation. For instance, with time-lapse imaging, it has been utilized in tracking particle-induced focus formation in single cells and, through collecting z-stacks, it has captured 3D image information of bulk samples, such as, tissues grown from human umbilical vein endothelial cells (HUVEC).(29)
Along with imaging modes that involve cellular stains and fluorophores, multiphoton microscopy integrates two non-stain imaging modes: autofluorescence (AF) and second harmonic generation (SHG). These two modes were used to image the head of a wild-type C. elegans, shown in Fig. 5. Anatomical features in the head section of this wild-type specimen responded differently to these two types of imaging, allowing visualization of potential targets for radiation experiments. The AF and SHG images were acquired simultaneously, with the incident wavelength set at 780 nm. AF and SHG signals were both detected in a backscattered mode (back through the objective) using the following optics: DI410LP (dichroic), 390BP10 (emission filter for SHG) and 530ASP (emission filter for AF) (Omega Optical Inc., Brattleboro, VT).
FIGURE 5.
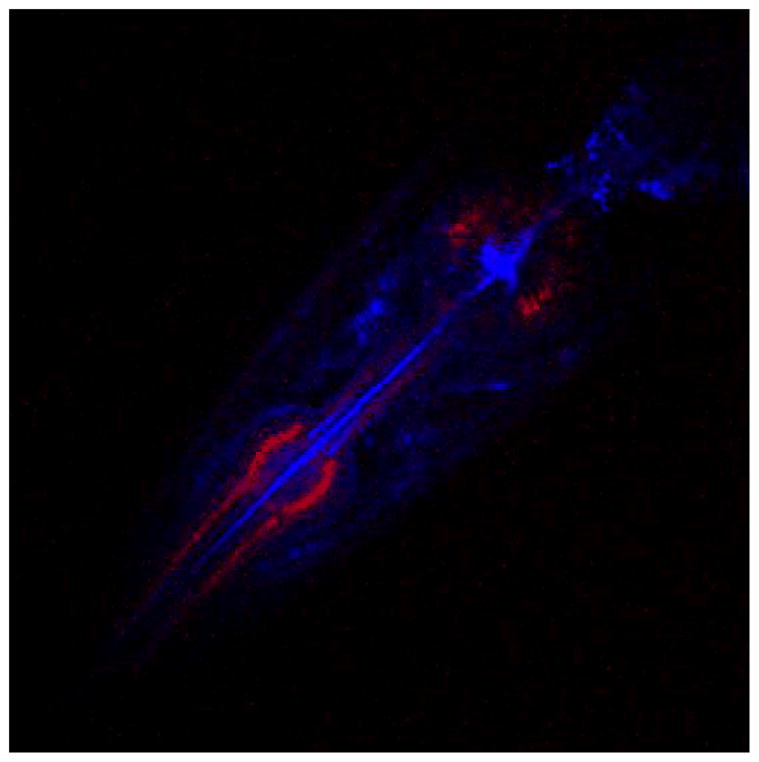
Head section of a live wild-type C. elegans specimen imaged using SHG (red) and AF (blue).
With a sub-micron ion beam focus available with RARAF’s Microbeam II irradiator, there is a capacity to irradiate sub-cellular organelles, such as mitochondria. Fig. 6 is a multiphoton image that shows small airway epithelial cell mitochondria which were tagged with GFP. Nuclei are imaged using Hoechst stain and are shown as a reference. The red cross-hairs positioned on mitochondria image indicate the middle of the image and correspond to the location of the sub-micron ion beam focus. Mitochondria radiation experimental results are emerging in the form of time-lapse multiphoton imaging, to be disseminated in the future.
FIGURE 6.
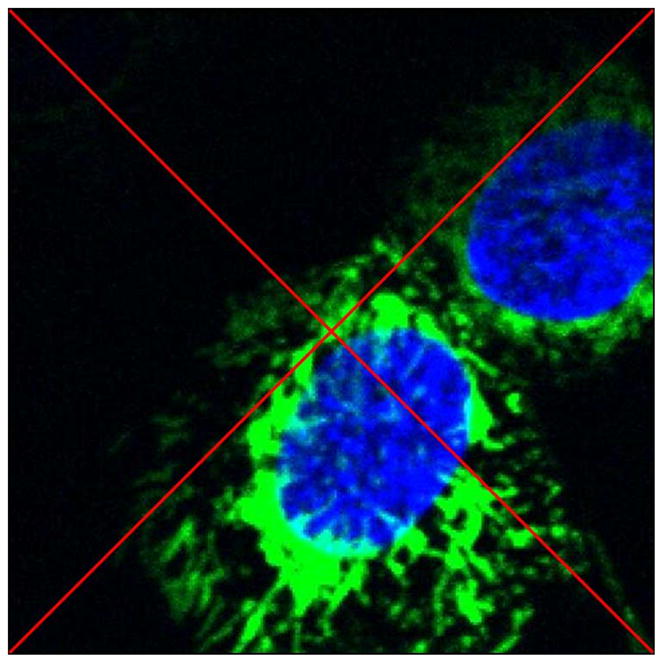
Multiphoton image of small airway epithelial cells with mitochondria tagged with GFP and nuclei stained with Hoechst. The red diagonal cross-hairs indicate target locations for mitochondria irradiation.
UV Microspot
Traditional UV laser microbeam systems involve an elongated laser path of exposure. At RARAF, a modification to the conventional design is being developed to use multiphoton excitation to produce a micro-volume of effective UV radiation - the defining characteristic of the photon-irradiation term: UV Microspot. Integrating a UV Microspot within the Microbeam II charged-particle cell-irradiation platform will provide a cocktail of photon and particle irradiations within one system.
The Ti:S laser for the RARAF multiphoton microscope, a Chameleon Ultra II (Coherent, Inc., Santa Clara, CA), covers a wavelength range of 680–1080 nm. These long/infrared wavelengths are minimally damaging to a tissue sample. However, at the laser’s focal point, the two-photon process and the three-photon process effectively provide (340–540 nm) and (227–360 nm), respectively. These wavelengths and their energies are shown in Table 1. With xyz-manipulation, the laser-induced damage can be projected anywhere in a tissue sample accessible by the photons (several hundred microns penetration) while leaving adjacent cells un-irradiated. The microspot of UV radiation is contained within a volume defined by the point spread function of the microscope objective, or approximately a 0.65 micron × 2.8 micron ellipsoid.
TABLE 1.
Photon availability from Chameleon-Ultra II
| Multiphoton Mode | Wavelength Range | Energy Range | Abbreviation |
|---|---|---|---|
| Single-photon | 680–1080 nm | 1.15–1.82 eV | Vis/IR |
| Two-photon | 340–540 nm | 2.30–3.65 eV | UVA |
| Three-photon | 227–360 nm | 3.45–5.47 eV | UVA, B&C |
The UV Microspot under development at RARAF has been used in preliminary experiments to induce single- and double-strand DNA breaks in single cells. For single strand break response, HT1080 Fibro-Sarcoma cells with GFP-tagged XRCC1 protein were exposed to UV Microspot irradiation (from 976 nm incident light), sufficient to induce SSB repair foci. Fig. 7 shows a time series of multiphoton microscopy images of a cell that was UV Microspot-irradiated with (976 nm) at two points. The first image of the series was taken prior to irradiation, which took 30 seconds per point; the lower part of the cell was irradiated first, followed by irradiating the top portion of the same nucleus. The 1 minute frame was taken 30 seconds after the lower point was irradiated and immediately after the upper point was irradiated. In that frame, the lower focus has reached maximally-captured brightness and the upper focus is still forming. At 2 minutes, the lower focus is fading (indicating rapid SSB repair) and the top focus is still forming. And at 5 minutes, both foci are greatly diminished.
FIGURE 7.
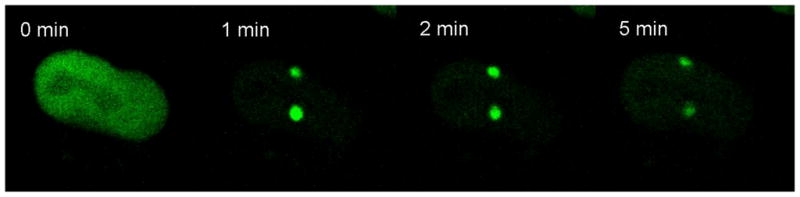
Multiphoton time-lapse imaging following UV-Microspot-induced SSB foci formation in Fibro-Sarcoma HT1080 cells. The lower portion of the cell nucleus was irradiated first, followed immediately by the upper portion. Incident light of 976 nm acts like 488 nm (two-photon excitation) and 325 nm (three-photon excitation).
OTHER CHARGED-PARTICLE MICROBEAM FACILITIES IN THE AMERICAS
Pacific Northwest National Laboratory
The Pacific Northwest National Laboratory (PNNL) currently houses a variable-energy, electron microbeam designed to examine the effects of low-LET radiation on cells and tissues.(30) The electron microbeam uses energetic electrons to mimic radiation damage from γ- and X-ray sources while depositing energy in a pre-selected subset of cells for which the un-irradiated neighbors can be easily identified. The device consists of a pulsed electron beam capable of operating at energies from ~5 keV to 80 keV and producing individual cell exposure ranging from a few to thousands of electrons. The current can be adjusted over a wide range (~1 pA to ~10 μA). The total dose delivered to the target is set by the dose rate and the beam pulse duration (~20 ns resolution) and can be varied over many orders of magnitude.
Spatial resolution on the micron scale is achieved by passing the electron beam through a very high aspect ratio hole in a collimator/interface plate. A variety of apertures with diameters ranging from ~3 μm up to 25 μm can be used depending on the requirements of the experiments. The microbeam is built as an integral part of an epifluorescence imaging system to allow for in situ monitoring of the irradiated area. Cells are cultured on specially designed re-locatable sample dishes (1.5 μm Mylar growth surface). Samples can be targeted, irradiated, and removed for study at a later time while still retaining information on the precise location of the targeted dose. This allows for the possibility of combining the microbeam with other imaging analysis techniques and conducting fractioned dose experiments. The cell irradiation area is maintained at 37°C.
Texas A&M University
At Texas A&M University, the Special Microbeam Utilization Research Facility (SMURF) provides specialized irradiation capabilities needed to implement radiation biology experiments to understand the cellular and molecular mechanisms controlling the risk of long term health effects related to low doses of ionizing radiation. Radiation sources include an electron microbeam, where electrons are produced by a tungsten filament. This system uses a high voltage, low current power supply, accelerator tube, and cathode to produce an electron beam with energy up to 100 keV and currents of a few nanoamps per square centimeter.(31) This electron source was equipped with a collimator for irradiation of selected areas within a growing cell population, to address if the bystander effect is significant for low LET radiation.
McMaster University
The first-generation, collimated microbeam at RARAF, now decommissioned and referred to as Microbeam I, was initially used for biology experiments starting in February 1994. Apertures for beam collimation consisted of a 2-mm diameter aperture located 1.3 m before a final collimating pair of apertures. The final consecutive collimating apertures were 5-μm and 6-μm diameter laser-drilled holes in 12.5-μm thick stainless steel sheets, separated by 300 μm. The 6-μm diameter aperture acted as an anti-scatter element, to limit the particle irradiation to an area smaller than the nucleus of a human cell. Nevertheless, a small fraction of the particles (8%) arrived outside of the prescribed target region. This is a general characteristic of all collimated microbeams and is overcome by using a focusing system. Two actuators were used to adjust the alignment of the final collimator, which was mounted in a spherical gimbal. This decommissioned collimator assembly is currently located at the McMaster Accelerator Laboratory (McMaster University, Hamilton, Ontario, Canada) for microbeam development in that facility.(32)
Supplementary Material
Acknowledgments
This work was supported in part by the National Institute of Biomedical Imaging and Bioengineering under Grant: NIBIB 5 P41 EB002033-12 and by the National Cancer Institute under Grant: 5P01-CA049062-16. We thank Aroumougame Asaithamby from David Chen’s group, University of Texas Southwestern, Dallas, TX, for providing HT1080 cells with GFP-tagged XRCC1. We also thank Antonella Bertucci for providing C. elegans, Helen Turner for assistance with cell culture preparation for UV Microspot irradiations, and Hongning Zhou for providing small airway epithelial cells for microbeam irradiation of mitochondria.
References
- 1.Randers-Pehrson G. Microbeams, microdosimetry and specific dose. Radiation Protection Dosimetry. 2002;99:471–472. doi: 10.1093/oxfordjournals.rpd.a006835. [DOI] [PubMed] [Google Scholar]
- 2.Miller RC, Randers-Pehrson G, Geard CR, Hall EJ, Brenner DJ. The oncogenic transforming potential of the passage of single alpha particles through mammalian cell nuclei. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:19–22. doi: 10.1073/pnas.96.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zirkle RE, Bloom W. Irradiation of parts of individual cells. Science. 1953;117:487–493. doi: 10.1126/science.117.3045.487. [DOI] [PubMed] [Google Scholar]
- 4.Nelson JM, Brooks AL, Metting NF, Khan MA, Buschbom RL, Duncan A, Miick R, Braby LA. Clastogenic Effects of Defined Numbers of 3.2 MeV Alpha Particles on Individual CHO-K1 Cells. Radiation Research. 1996;145:568–574. [PubMed] [Google Scholar]
- 5.Folkard M, Vojnovic B, Prise KM, Bowely AG, Locke RJ, Schettino G, Michael BD. A charged-particle microbeam. I. Development of an experimental system for targeting cells individually with counted particles. International Journal of Radiation Biology. 1997;72:375–385. doi: 10.1080/095530097143158. [DOI] [PubMed] [Google Scholar]
- 6.Folkard M, Vojnovic B, Hollis KJ, Bowey AG, Watts SJ, Schettino G, Prise KM, Michael BD. A charged-particle microbeam: II. A single-particle micro-collimation and detection system. International Journal of Radiation Biology. 1997;72:387–395. doi: 10.1080/095530097143167. [DOI] [PubMed] [Google Scholar]
- 7.Randers-Pehrson G, Geard CR, Johnson G, Elliston CD, Brenner DJ. The Columbia University single-ion microbeam. Radiation Research. 2001;156:210–214. doi: 10.1667/0033-7587(2001)156[0210:tcusim]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Ponnaiya B, Jenkins-Baker G, Brenner DJ, Hall EJ, Randers-Pehrson G, Geard CR. Biological responses in known bystander cells relative to known microbeam-irradiated cells. Radiation Research. 2004;162:426–432. doi: 10.1667/rr3236. [DOI] [PubMed] [Google Scholar]
- 9.Zhou HN, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2099–2104. doi: 10.1073/pnas.030420797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawant SG, Randers-Pehrson G, Geard CR, Brenner DJ, Hall EJ. The bystander effect in radiation oncogenesis: I. Transformation in C3H 10T(1)/(2) cells in vitro can be initiated in the unirradiated neighbors of irradiated cells. Radiation Research. 2001;155:397–401. doi: 10.1667/0033-7587(2001)155[0397:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Zhou HN, Suzuki M, Randers-Pehrson G, Vannais D, Chen G, Trosko JE, Waldren CA, Hei TK. Radiation risk to low fluences of alpha particles may be greater than we thought. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14410–14415. doi: 10.1073/pnas.251524798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu LJ, Randers-Pehrson G, Xu A, Waldren CA, Geard CR, Yu ZL, Hei TK. Targeted cytoplasmic irradiation with alpha particles induces mutations in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4959–4964. doi: 10.1073/pnas.96.9.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harken A, Randers-Pehrson G, Brenner D. Development of a Proton-Induced X-Ray Microbeam at Columbia University. Journal of Radiation Research current 2009 [Google Scholar]
- 14.Bigelow AW, Ross GJ, Randers-Pehrson G, Brenner DJ. The Columbia University microbeam II endstation for cell imaging and irradiation. Nuclear Instruments & Methods in Physics Research Section B-Beam Interactions with Materials and Atoms. 2005;231:202–206. doi: 10.1016/j.nimb.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wollnik H. Optics of Charged Particles. San Diego: 1987. [Google Scholar]
- 16.Dymnikov AD, Brenner DJ, Johnson G, Randers-Pehrson G. Theoretical study of short electrostatic lens for the Columbia ion microprobe. Review of Scientific Instruments. 2000;71:1646–1650. [Google Scholar]
- 17.Bigelow AW, Brenner DJ, Garty G, Randers-Pehrson G. Single-particle/single-cell ion microbeams as probes of biological mechanisms. IEEE Transactions on Plasma Science. 2008;36:1424–1431. [Google Scholar]
- 18.Belyakov OV, Mitchell SA, Parikh D, Randers-Pehrson G, Marino SA, Amundson SA, Geard CR, Brenner DJ. Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14203–14208. doi: 10.1073/pnas.0505020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottschalk SC, Dowell DH, Quimby DC. Permanent magnet systems for free-electron lasers. Nucl Instr & Meth A. 2003;507:181–185. [Google Scholar]
- 20.Garty G, Ross GJ, Bigelow A, Randers-Pehrson G, Brenner DJ. A microbeam irradiator without an accelerator. Nuclear Instruments & Methods in Physics Research Section B-Beam Interactions with Materials and Atoms. 2005;241:392–396. [Google Scholar]
- 21.Ross GJ, Garty G, Randers-Pehrson G, Brenner DJ. A single-particle/single-cell microbeam based on an isotopic alpha source. Nuclear Instruments & Methods in Physics Research Section B-Beam Interactions with Materials and Atoms. 2005;231:207–211. [Google Scholar]
- 22.Bigelow AW, Randers-Pehrson G, Kelly RP, Brenner DJ. Laser ion source for Columbia University’s microbeam. Nuclear Instruments & Methods in Physics Research Section B-Beam Interactions with Materials and Atoms. 2005;241:874–879. doi: 10.1016/j.nimb.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao C, Furusawa Y, Aoki M, Matsumoto H, Ando K. Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumour cells. International Journal of Radiation Biology. 2002;78:837–844. doi: 10.1080/09553000210149786. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto H, Hayashi S, Hatashita M, Ohnishi K, Shioura H, Ohtsubo T, Kitai R, Ohnishi T, Kano E. Induction of radioresistance by a nitric oxide-mediated bystander effect. Radiation Research. 2001;155:387–396. doi: 10.1667/0033-7587(2001)155[0387:iorban]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Azzam EI, de Toledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Research. 2002;62:5436–5442. [PubMed] [Google Scholar]
- 26.Land SC, Porterfield DM, Sanger RH, Smith PJS. The self-referencing oxygen-selective microelectrode: Detection of transmembrane oxygen flux from single cells. Journal of Experimental Biology. 1999;202:211–218. doi: 10.1242/jeb.202.2.211. [DOI] [PubMed] [Google Scholar]
- 27.Porterfield DM, Laskin JD, Jung SK, Malchow RP, Billack B, Smith PJS, Heck DE. Proteins and lipids define the diffusional field of nitric oxide. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2001;281:L904–L912. doi: 10.1152/ajplung.2001.281.4.L904. [DOI] [PubMed] [Google Scholar]
- 28.Kumar SM, Porterfield DM, Muller KJ, Smith PJS, Sahley CL. Nerve injury induces a rapid efflux of nitric oxide (NO) detected with a novel NO microsensor. Journal of Neuroscience. 2001;21:215–220. doi: 10.1523/JNEUROSCI.21-01-00215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bigelow AW, Geard CR, Randers-Pehrson G, Brenner DJ. Microbeam-integrated multiphoton imaging system. Review of Scientific Instruments. 2008;79:123707. doi: 10.1063/1.3043439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan WF, Goetz W, Sowa MB. No Bystander Effect After Irradiation of Mammalian Cells with a Variable Energy Electron Microbeam. Journal of Radiation Research current 2009 [Google Scholar]
- 31.Braby L, Ford J, Botting T. Electron microbeam for investigation of bystander cell effects. Poster Session 30 abstract, Radiation Research Society & North American Hyperthermia Society Annual Meeting 2002 [Google Scholar]
- 32.Thompson J, Labonte F, McMaster S, Boreham D. Development and current status of the single-ion biological microprobe at McMaster University. Journal of Radiation Research current 2009 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


