Abstract
Background:
The effects of skeletal maturity on functional ligament healing are unknown. Prior studies have suggested that ligament injuries in skeletally mature animals heal with improved mechanical properties. In this study, we hypothesized that skeletally immature animals have improved functional healing compared with skeletally mature animals.
Methods:
Twenty-one Yucatan minipigs (eight juvenile, eight adolescent, and five adult animals) underwent bilateral anterior cruciate ligament transection. On one side, the ligament injury was left untreated to determine the intrinsic healing response as a function of age. On the contralateral side, an enhanced suture repair incorporating a collagen-platelet composite was performed. Biomechanical properties of the repairs were measured after fifteen weeks of healing, and histologic analysis was performed.
Results:
Anterior cruciate ligaments from skeletally immature animals had significantly improved structural properties over those of adult animals at three months after transection in both the untreated and repair groups. Use of the enhanced suture technique provided the most improvement in the adolescent group, in which an increase of 85% in maximum load was noted with repair. The repair tissue in the adult tissue had the highest degree of hypercellularity at the fifteen-week time point.
Conclusions:
Functional ligament healing depends on the level of skeletal maturity of the animal, with immature animals having a more productive healing response than mature animals.
Clinical Relevance:
As future investigations assess new techniques of ligament healing in animal models, skeletal maturity should be considered in the design and the interpretation of those experiments.
Anterior cruciate ligament injuries present a challenge to surgeons and patients alike. The prevalence of this injury is high, especially in active, adolescent athletes1. These injuries are commonly treated with surgical reconstruction, in which the torn ligament is replaced with a tendon graft. Although anterior cruciate ligament reconstruction restores short-term function, the premature onset of osteoarthritis continues to affect many patients2. Thus, there has been recent interest in engineering a means to stimulate anterior cruciate ligament healing or regeneration. If such efforts are successful, a less invasive procedure with improved long-term outcomes may be possible. Comparisons of healing of the anterior cruciate ligament with that of the medial collateral ligament, which spontaneously heals, have led to the hypothesis that the deficiency in anterior cruciate ligament healing is due to incomplete scaffold formation within the injury site3,4. As a result, an enhanced anterior cruciate ligament repair technique, in which a scaffold is surgically placed in the gap between the torn ends of the ligament to provide a provisional structure on which new tissue can develop, has shown promise in preliminary animal studies5-7. A collagen-platelet composite has recently been shown to provide a suitable provisional scaffold that stimulates functional healing of the anterior cruciate ligament in large animal models5-7.
The influence of age and skeletal maturity on the ability of the anterior cruciate ligament to heal has not been thoroughly characterized. In a study comparing anterior cruciate ligament healing in immature and mature rabbits, ligaments that were completely sectioned did not heal in either group8. However, the stiffness of partially sectioned ligaments returned to normal by three months in both groups, and the partially torn ligaments had regained a higher percentage of strength in the mature group compared with the immature group after one year8.
Building on the previous rabbit study8, the current study was designed to evaluate the effects of skeletal maturity on the functional healing of the transected anterior cruciate ligament in a large animal model when left untreated or treated with enhanced suture repair with use of a collagen-platelet composite. We hypothesized that the structural properties of the torn anterior cruciate ligament of juvenile animals (open physes) would be greater than those of adolescent (open physes nearing closure) or adult animals (physes closed) after fifteen weeks of healing, and that those treated with enhanced repair would produce superior structural properties than those not treated. Similarly, we hypothesized that the anteroposterior laxity of the knee in juvenile animals would be less than that of the adolescent and adult animals and that the knees treated with enhanced repair would be less lax than those left untreated. A separate group of knees with intact anterior cruciate ligaments from age-matched controls was also evaluated for comparison.
Materials and Methods
Experimental Design
Institutional Animal Care and Use Committee approvals were obtained prior to initiating this study. Twenty-one Yucatan minipigs representing three different age groups were obtained: eight were juvenile (six to nine months of age), eight were adolescent (twelve to thirteen months of age), and five were adult (thirty-six to sixty months of age). Each animal underwent bilateral surgical transection of the anterior cruciate ligament. One side was left unrepaired, while the other side was immediately treated with suture repair enhanced with the collagen-platelet composite. The knees were allowed to heal for fifteen weeks after which the animals were killed. Both knees were harvested and subjected to tensile testing so that the structural properties between treatments (no treatment or enhanced repair) and maturity level could be compared. An additional group of knees with intact anterior cruciate ligaments in age-matched animals (six juvenile, six adolescent, and six adult) were also evaluated. Histologic analysis of the repair tissue was performed after mechanical testing.
Surgical Procedure
After the induction of general anesthesia, the animals underwent range of flexion-extension motion and Lachman measurements of both knees. The lower limbs were then prepared and sterilely draped. The animals were randomly selected for surgery, and alternate knees were used for the anterior cruciate ligament-deficient and enhanced anterior cruciate ligament repair procedures. For both knees, a medial arthrotomy was made to expose the anterior cruciate ligament. The anterior cruciate ligament was cut at the proximal third with use of a scalpel, and the knee was irrigated. A Lachman test was performed to verify functional loss of the anterior cruciate ligament.
For the knee undergoing enhanced anterior cruciate ligament repair, a Kessler suture of number-1 Vicryl (polyglactin; Ethicon, Somerville, New Jersey) was placed in the tibial stump with its ends exiting through the proximal cut of the anterior cruciate ligament (Fig. 1). The knee was then hyperflexed, and a guide-pin was drilled through the femoral anterior cruciate ligament footprint and out through the lateral cortex of the femur. The pin was overdrilled with an EndoButton drill, and an EndoButton (Smith and Nephew, Andover, Massachusetts) loaded with three number-1 Vicryl sutures was passed through the femoral tunnel and flipped on the lateral cortex to provide femoral fixation of the sutures. A 2.4-mm drill-pin was then used to make a tunnel in the tibia with use of a drill guide (Acufex Director Drill Guide; Smith and Nephew) to ensure that the entrance into the joint was just medial to the anterior cruciate ligament footprint. The tibial tunnel would later serve to secure the repair sutures directly to the tibia as previously described9,10. The resorbable sutures bridging the femur and tibia (as shown in purple in Figure 1) served as a temporary stent within the knee. Once both suture tunnels had been drilled, a collagen scaffold (described below) was threaded onto four of the Vicryl suture ends from the EndoButton. The remaining suture ends were used to directly tie to the anterior cruciate ligament stump. The scaffold was then passed up into the notch until femoral contact was verified visually. The sutures were passed through the tibial button and tied over the front of the tibia with the knee in 30° of flexion (which is the maximum extension angle for the porcine knee). The scaffold was then saturated with 3 mL of platelet-rich plasma at five times the baseline systemic platelet concentration. The variable depth suture that had been previously placed in the tibial anterior cruciate ligament stump was then tied to the remaining sutures exiting the femoral tunnel.
Fig. 1.
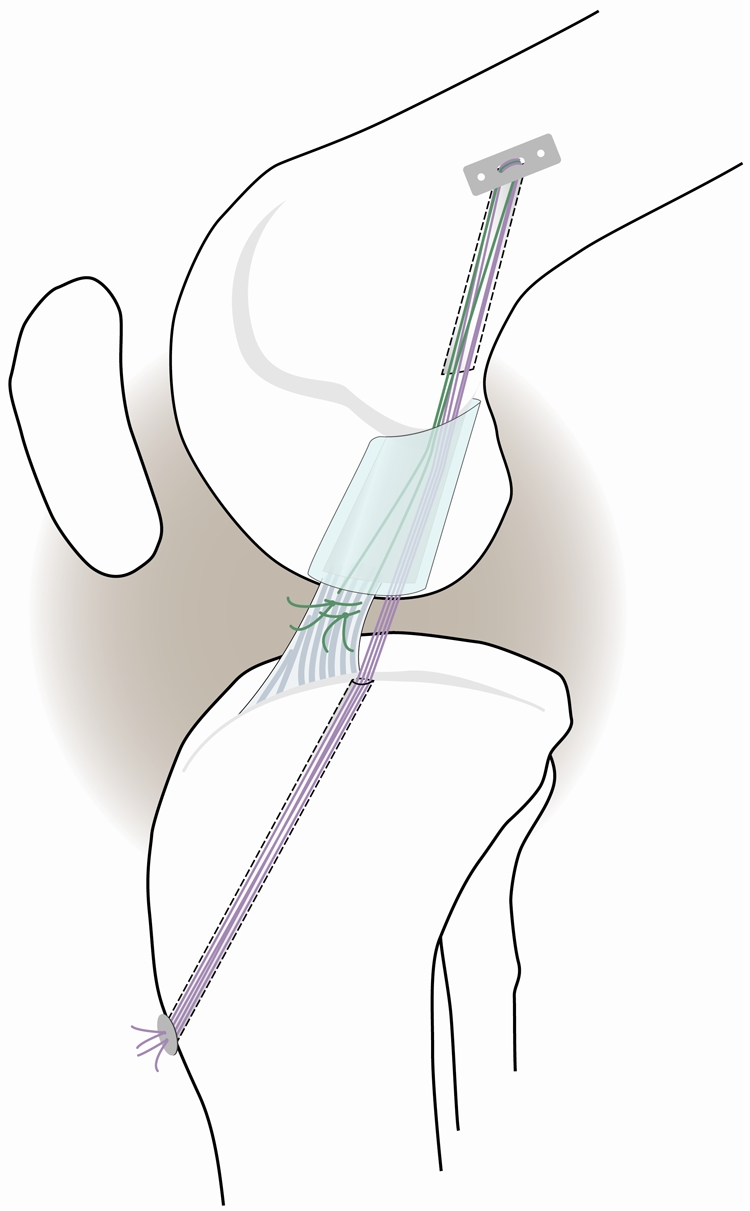
Schematic diagram depicting the primary suture repair with the collagen-platelet composite scaffold in place. Sutures were fixed proximally with an EndoButton device. The sponge was threaded onto four of the trailing suture ends (purple), which were then passed through the tibial tunnel and tied over a button to provide initial knee stability. The remaining two suture ends (green) were tied to the sutures in the tibial stump of the anterior cruciate ligament.
The contralateral anterior cruciate ligament was cut, the knee was irrigated, and then the arthrotomy was closed. The limbs were not restrained postoperatively. The animals were allowed ad libitum activity during the fifteen-week postoperative period.
Knees with intact anterior cruciate ligaments were obtained from age-matched animals (six juvenile, six adolescent, and six adult). They underwent identical procurement, storage, and mechanical testing protocols.
Harvest
The knees were harvested fifteen weeks after surgery. Following the induction of anesthesia, the clinical measurements were repeated. After the animals were killed, the limbs were harvested at the hip and stored at –20°C until testing.
Collagen-Platelet Composite
At the time of surgery, 60 mL of whole blood was drawn from each animal with use of a large-bore needle (≤18 gauge) and placed into 15-mL tubes containing sodium citrate. The blood was centrifuged (Beckman GS-6 Centrifuge with GH-3.8 Rotor; Beckman Coulter, Fullerton, California) to isolate a platelet pellet. The platelet pellet was resuspended in a specified amount of platelet-poor plasma to create a platelet concentrate with an enrichment of approximately five times the systemic platelet level. Initial and final platelet concentrations were determined with use of a VetScan HM5 Analyzer (Abaxis, Union City, California). The platelet concentrate was maintained at room temperature until use (less than thirty minutes).
Scaffold
The collagen scaffold was made by solubilizing bovine tissue in an acidic solution. The resulting collagen solution was frozen, lyophilized, and rehydrated. It was then neutralized, with use of HEPES buffer (Mediatech, Manassas, Virginia), sodium hydroxide (Fisher Scientific, Fair Lawn, New Jersey), phosphate-buffered saline solution (HyClone, Logan, Utah), and calcium chloride (Sigma-Aldrich, St. Louis, Missouri), and relyophilized. The resulting scaffolds were stored frozen and under vacuum until use.
Mechanical Testing
The frozen knees were thawed overnight. The soft tissues surrounding the tibia and femur were removed, leaving the capsule intact, and the knees were potted as previously described so that they could be secured within a materials testing system11-13. All mechanical testing evaluators were blinded as to treatment group and age during the testing process.
Anteroposterior laxity values for the three study groups were measured, as previously described9,14, with the knee flexion angle locked at 30°, 60°, and 90° with use of a custom-designed fixture. Anterior and posterior-directed shear loads of ±30 N were applied to the femur, with respect to the tibia, with use of a materials testing system (MTS 810; MTS, Eden Prairie, Minnesota), while the anteroposterior displacements were measured. After completion of the anteroposterior laxity tests, all soft tissue between the femur and tibia except the anterior cruciate ligament scar mass was resected and tensile testing was performed with the knee in 30° of flexion as previously described11,15. The femur-graft-tibia complexes were loaded in tension to failure at a rate of 20 mm per minute6,16. From the load-displacement curves, the following structural properties were established: yield load, failure load, linear stiffness, and displacement to 5 N (low-load displacement). Yield, displacement, and maximum displacement were determined as previously described12. Identical testing was performed for the knees with intact anterior cruciate ligaments in each age group.
Histologic Analysis
A total of twelve anterior cruciate ligaments were analyzed for cellular proliferation. Four ligaments from each age group were harvested at fifteen weeks after the injury, and histologic analysis was performed with use of hematoxylin and eosin staining. Each ligament was divided into five sections: femoral insertion, proximal anterior cruciate ligament, central anterior cruciate ligament (the wound site), distal anterior cruciate ligament, and tibial insertion. Cellular proliferation was quantitatively and qualitatively determined at the central anterior cruciate ligament wound as previously reported17.
The cell nuclear size and shape were calculated by measuring the length of the major and minor axes of the cell nucleus for fifteen fibroblasts at the wound site of all ligaments. The area of the nucleus was calculated as the length of the major axis multiplied by that of the minor axis multiplied by pi and divided by four.
Statistical Analysis
Continuous variables were tested for normality with use of the Shapiro-Wilk test and were found to conform to a normal Gaussian-shaped distribution18. Anteroposterior knee laxity results, clinical examination parameters, and the structural properties after fifteen weeks of healing were compared among the three maturity groups by analysis of variance, while treatment (no treatment compared with enhanced repair) was considered as a within-subject variable to account for multiple knees from the same animal, with the F test used to assess age-group differences for untreated anterior cruciate ligaments, intact anterior cruciate ligaments, and anterior cruciate ligaments treated with sutures and a collagen-platelet composite19. In addition, differences were also evaluated with use of analysis of variance among untreated, intact, and collagen-platelet composite-treated anterior cruciate ligaments. Data are presented in terms of mean values and standard errors. Two-tailed values of p < 0.05, with a Bonferroni post hoc adjustment to account for multiple group comparisons, were considered significant.
A power analysis indicated that a minimum of five knees for each treatment and age group would provide 80% power (α = 0.05, β = 0.20) to detect 30% to 40% differences in variables including displacements, yield load, maximum load, linear stiffness, and anteroposterior laxity between the age groups, assuming a variability of 20% with use of analysis of variance20.
Source of Funding
This project was funded by the National Institutes of Health through the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Results
Hematology
Preoperatively, the whole blood of the animals in each age group had similar red blood-cell counts (p = 0.84), white blood-cell counts (p = 0.42), and platelet counts (p = 0.21). In addition, there was no significant difference in the platelet count in the platelet-rich plasma for any of the age groups (mean [and standard error of the mean], 1914 ± 140 × 103/μL, 1779 ± 140 × 103/μL, and 1443 ± 177 × 103/μL for juvenile, adolescent, and adult groups, respectively; p = 0.138). There were significantly more white blood cells in the juvenile and adolescent platelet-rich plasma (mean, 16.5 ± 7.5 × 103/μL, 14.5 ± 7.4 × 103/μL, and 4.4 ± 2.6 × 103/μL for juvenile, adolescent, and adult groups, respectively; p = 0.013).
Clinical Assessment
The knees in the adult group had approximately 20° more extension and 10° less flexion than those in the juvenile and adolescent groups preoperatively (p < 0.006 for all comparisons) (Table I). The preoperative Lachman examination revealed approximately 50% less laxity in the knees in the adult group than in the knees in the juvenile or adolescent groups (p < 0.015 for all comparisons) (Table I).
TABLE I.
Range of Motion and Lachman Test Among Age Groups for Untreated Animals and Animals Treated with Collagen-Platelet Composite
| Variable | Juvenile* | Adolescent* | Adult* | P Value |
| Fat pad resected (g) | ||||
| No treatment | 3.3 ± 4.8 | 5.1 ± 3.1 | 5.6 ± 2.1† | 0.038‡ |
| Collagen-platelet composite | 2.8 ± 2.9 | 5.0 ± 2.8 | 6.0 ± 1.7§ | 0.004‡ |
| Concentration of platelets in platelet-rich plasma (×103/μL) | ||||
| Collagen-platelet composite | 1914 ± 140 | 1779 ± 140 | 1443 ± 177 | 0.138 |
| Preop. flexion (deg) | ||||
| No treatment | 136.9 ± 1.7 | 135.0 ± 1.7 | 128.0 ± 2.2# | 0.011‡ |
| Collagen-platelet composite | 135.0 ± 1.8 | 136.9 ± 1.7 | 125.0 ± 2.1# | <0.001‡ |
| Preop. extension (deg) | ||||
| No treatment | 33.1 ± 3.2 | 34.4 ± 3.2 | 15.0 ± 4.0# | 0.003‡ |
| Collagen-platelet composite | 31.3 ± 3.2 | 32.5 ± 3.2 | 15.0 ± 4.0# | 0.006‡ |
| Change in flexion (deg) | ||||
| No treatment | –9.3 ± 3.8 | –5.0 ± 3.5 | 17.0 ± 4.5# | <0.001‡ |
| Collagen-platelet composite | –7.1 ± 4.0 | –6.9 ± 3.6 | 24.0 ± 4.4# | <0.001‡ |
| Change in extension (deg) | ||||
| No treatment | –2.1 ± 3.8 | –1.9 ± 3.5 | 42.0 ± 4.5# | <0.001‡ |
| Collagen-platelet composite | 1.4 ± 3.7 | –1.8 ± 3.5 | 39.0 ± 4.5# | <0.001‡ |
| Preop. Lachman (mm) | ||||
| No treatment | 2.8 ± 0.4 | 3.0 ± 0.4 | 1.2 ± 0.5# | 0.022‡ |
| Collagen-platelet composite | 2.9 ± 0.4 | 2.8 ± 0.4 | 1.0 ± 0.5# | 0.014‡ |
| Lachman at 15 wks (mm) | ||||
| No treatment | 6.4 ± 0.8 | 6.3 ± 0.7 | 1.8 ± 0.9 | <0.001‡ |
| Collagen-platelet composite | 2.3 ± 0.7** | 2.8 ± 0.7** | 2.0 ± 0.9 | 0.797 |
The data are given as the mean and the standard error of the mean.
Bonferroni post hoc analysis indicated p < 0.05 compared with juvenile group.
Overall group differences based on repeated-measures analysis of variance with knee as a within-animal factor
Bonferroni post hoc analysis indicated p < 0.01 compared with juvenile group.
Bonferroni post hoc analysis indicated p < 0.01 compared with juvenile group and p < 0.01 compared with adolescent group.
Compared with no treatment, the collagen-platelet-composite effect was significant (p < 0.01).
There was no significant difference in the amount of fat pad resected in the treated and untreated knees in any age group (Table I). In addition, there was no significant difference in the absolute number of platelets added to the repairs in any of the age groups (p > 0.10) (Table I). The animals recovered well and were bearing full weight within twenty-four hours of surgery. No animal presented with surgical complications. All of the animals appeared to be walking normally at the time that they were killed.
During the healing period, there was a significantly greater loss of extension and flexion of the knees in the adult group than in the juvenile or adolescent groups (p < 0.001 for all comparisons) (Table I). No significant differences were found between the treated and untreated knees in any age group. The Lachman examination after the healing period was also an average of 4.5 mm tighter in the untreated knees in the adult group than in the untreated knees in the juvenile or the adolescent group; however, no effect of age was noted for the knees treated with the collagen-platelet composite (p > 0.75, Table I).
Gross Dissection
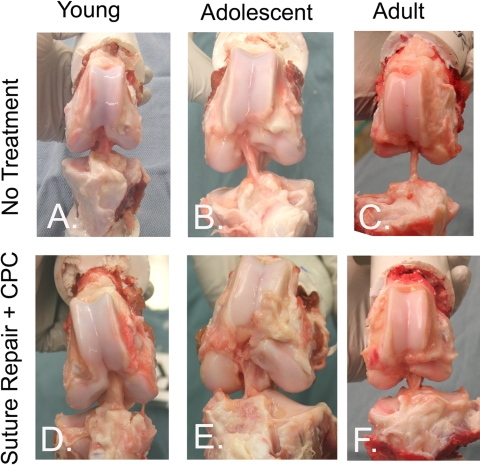
At retrieval, all knees had visible anterior cruciate ligament repair tissue in the notch (Fig. 2). In many of the knees left untreated, the repair tissue was minimal and lax with little collagenous structure. This finding was noted in one of eight untreated anterior cruciate ligament-deficient knees in the juvenile group, in three of eight untreated anterior cruciate ligament-deficient knees in the adolescent group, and in three of five untreated anterior cruciate ligament-deficient knees in the adult group. For the collagen-platelet composite-treated knees, the repair tissue had little collagenous structure in only one of five knees in the adult group. Organized fibrous scar was visible in the region of the anterior cruciate ligament for all knees that had enhanced repair of the anterior cruciate ligament in the adolescent and juvenile groups. There was no evidence of infection in any of the knees. There was no evidence of the suture material at the time of retrieval.
Fig. 2.
Gross appearance of the healing anterior cruciate ligament in the juvenile, adolescent and adult age groups in both the untreated knees (top row) and the knees treated with suture repair augmented with a collagen-platelet composite (CPC) (bottom row). For the knees treated with the collagen-platelet composite, organized collagenous tissue was visible in the region of the anterior cruciate ligament and appeared more robust and less lax than that in the untreated contralateral knees.
Biomechanical Outcomes
Intact Anterior Cruciate Ligaments
The intact anterior cruciate ligaments in the adolescent and adult groups had 60% and 80% higher maximum load than the anterior cruciate ligaments in the juvenile group (p < 0.01) (Table II). There was no significant difference in maximum load between the adolescent and adult groups (p = 0.43). There was no significant difference in stiffness (p > 0.775), anteroposterior laxity (p > 0.125), or displacement parameters (p > 0.053) among any of the groups.
TABLE II.
Comparison of Knee Laxity and Structural Properties Among Age Groups for Untreated Animals, Animals with Intact Anterior Cruciate Ligaments, and Animals with Anterior Cruciate Ligaments Treated with Sutures and Collagen-Platelet Composite
| Variable | Juvenile* | Adolescent* | Adult* | P Value |
| Load at yield (N) | ||||
| No treatment | 237 ± 45.7 | 185 ± 43.8 | 121 ± 35.6 | 0.126 |
| Collagen-platelet composite | 300 ± 27.4 | 369 ± 39.1† | 220 ± 29.3‡ | 0.007§ |
| Intact | 952 ± 36.2 | 1414 ± 107.9 | 1721 ± 103.6# | 0.008§ |
| Load at max (N) | ||||
| No treatment | 243 ± 46.6 | 200 ± 47.8 | 137 ± 41.9 | 0.231 |
| Collagen-platelet composite | 315 ± 25.7† | 374 ± 40.0† | 225 ± 28.7‡# | 0.004§ |
| Intact | 1023 ± 56.4 | 1616 ± 117.8# | 1866 ± 65.2# | 0.005§ |
| Linear stiffness (N/mm) | ||||
| No treatment | 51 ± 8.0 | 43 ± 10.0 | 31 ± 8.6 | 0.232 |
| Collagen-platelet composite | 71 ± 5.9† | 79 ± 7.5† | 47 ± 6.4‡# | 0.003§ |
| Intact | 136 ± 3.0 | 150 ± 13.8 | 154 ± 27.3 | 0.775 |
| Displacement to 5 N (mm) | ||||
| No treatment | 4.6 ± 0.86 | 4.1 ± 0.96 | 6.0 ± 3.77 | 0.846 |
| Collagen-platelet composite | 2.4 ± 0.34† | 2.9 ± 0.79 | 3.8 ± 1.21 | 0.479 |
| Intact | 1.8 ± 0.17 | 1.7 ± 0.30 | 1.3 ± 0.50 | 0.573 |
| Displacement to yield (mm) | ||||
| No treatment | 10.8 ± 0.86 | 11.8 ± 1.88 | 11.0 ± 3.90 | 0.888 |
| Collagen-platelet composite | 10.2 ± 0.72 | 10.8 ± 1.00 | 9.4 ± 1.17 | 0.645 |
| Intact | 13.6 ± 0.92 | 15.9 ± 0.63 | 13.7 ± 1.25 | 0.208 |
| Displacement to max (mm) | ||||
| No treatment | 11.3 ± 1.06 | 12.3 ± 1.91 | 11.6 ± 3.80 | 0.901 |
| Collagen-platelet composite | 11.0 ± 1.01 | 10.9 ± 1.01 | 9.6 ± 1.20 | 0.638 |
| Intact | 14.7 ± 1.04 | 19.1 ± 1.07 | 14.6 ± 0.97 | 0.053 |
| Anteroposterior laxity at 30° (mm) | ||||
| No treatment | 4.5 ± 0.44 | 4.6 ± 1.00 | 2.4 ± 0.49# | 0.004§ |
| Collagen-platelet composite | 4.8 ± 0.47 | 3.9 ± 0.84 | 3.1 ± 0.85 | 0.176 |
| Intact | 2.2 ± 0.11 | 1.8 ± 0.09 | 2.3 ± 0.68 | 0.516 |
| Anteroposterior laxity at 60° (mm) | ||||
| No treatment | 7.7 ± 0.52 | 8.3 ± 0.60 | 7.0 ± 0.81 | 0.404 |
| Collagen-platelet composite | 7.3 ± 0.42 | 6.8 ± 0.56† | 7.0 ± 0.93 | 0.763 |
| Intact | 1.9 ± 0.11 | 2.2 ± 0.37 | 2.8 ± 0.80 | 0.349 |
| Anteroposterior laxity at 90° (mm) | ||||
| No treatment | 6.2 ± 0.76 | 6.2 ± 0.48 | 6.5 ± 0.74 | 0.914 |
| Collagen-platelet composite | 5.5 ± 0.32 | 5.6 ± 0.39 | 8.9 ± 0.93‡# | 0.002§ |
| Intact | 2.0 ± 0.14 | 1.5 ± 0.10 | 2.3 ± 0.65 | 0.125 |
The data are given as the mean and the standard error of the mean. The juvenile and adolescent groups had eight knees in the no-treatment group, eight knees in the collagen-platelet composite group, and six knees in the intact group. The adult group had five knees in the no-treatment group, five in the collagen-platelet composite group, and six in the intact group.
Compared with no treatment, the collagen-platelet composite effect was significant (p < 0.01).
Bonferroni post hoc analysis indicated p < 0.01 compared with adolescent group.
Overall group differences with use of repeated-measures analysis of variance with knee as a within-animal factor and treatment and age group as between-group factors.
Bonferroni post hoc analysis indicated p < 0.01 compared with juvenile group.
Untreated Anterior Cruciate Ligament After Transection (Intrinsic Healing Potential of the Anterior Cruciate Ligament)
There was no significant difference in the absolute measures of maximum load, yield load, or stiffness among the age groups for the untreated transections (Table II). However, when the biomechanical outcomes were normalized by the intact values for each age group, the differences became significant (Table III). While the anterior cruciate ligaments in the juvenile group reached 25% of the intact yield load at three months after transection, the anterior cruciate ligaments in the adult group reached only 7% at the same time point (p < 0.01) (Table III). Similar results were found for the maximum load to failure. In addition, the linear stiffness of the healing anterior cruciate ligament in the juvenile group was double that found for the adult group, although the difference only approached significance (p = 0.09). Interestingly, the anteroposterior laxity at 60° was approximately 25% lower in the adult group than in either the adolescent or juvenile groups (p < 0.01 for both comparisons).
TABLE III.
Normalized Tensile Property and Knee Laxity Results Among Age Groups
| Variable | Juvenile* | Adolescent* | Adult* | P Value |
| Yield load/intact (%) | ||||
| No treatment | 25 ± 4.8 | 13 ± 3.1 | 7 ± 2.1† | 0.002‡ |
| Collagen-platelet composite | 32 ± 2.9 | 26 ± 2.8§ | 13 ± 1.7†# | <0.001‡ |
| Max load/intact (%) | ||||
| No treatment | 24 ± 4.6 | 12 ± 3.0 | 8 ± 2.3† | 0.006‡ |
| Collagen-platelet composite | 31 ± 2.5 | 23 ± 2.5§ | 12 ± 1.5†# | <0.001‡ |
| Stiffness/intact (%) | ||||
| No treatment | 37 ± 5.8 | 29 ± 6.7 | 20 ± 5.4 | 0.086 |
| Collagen-platelet composite | 52 ± 4.4 | 52 ± 5.0§ | 30 ± 4.1†# | <0.001‡ |
| Anteroposterior laxity at 60° – intact (mm) | ||||
| No treatment | 4.1 ± 0.27 | 3.8 ± 0.27 | 2.6 ± 0.30†# | <0.001‡ |
| Collagen-platelet composite | 3.9 ± 0.22 | 3.1 ± 0.25§ | 2.6 ± 0.34† | 0.002‡ |
The data are given as the mean and the standard error of the mean.
Bonferroni post hoc analysis indicated p < 0.01 compared with juvenile group.
Overall group differences based on repeated-measures analysis of variance with knee as a within-animal factor.
Compared with no treatment, the collagen-platelet composite effect was significant (p < 0.01).
Bonferroni post hoc analysis indicated p < 0.01 compared with the adolescent group.
Transected Anterior Cruciate Ligament Treated with Enhanced Suture Repair
The repaired ligaments also had biomechanical properties that were dependent on animal age. The yield load in the adolescent animals was 68% greater than that of the adult animals (p < 0.01) (Table II). The maximum load of both the adolescent and the juvenile animals after repair was 66% and 40% greater, respectively, than that of the adult animals (p < 0.01 for both comparisons) (Table II). The anteroposterior laxity at 90° of flexion was 37% and 38% lower in the adolescent and juvenile groups, respectively, than in the adult group (p < 0.01). Finally, the stiffness of the repair tissue in the adolescent and juvenile animals was 68% and 51% greater, respectively, than the stiffness of the repair tissue in the adult animals (p < 0.01).
The Response to Enhanced Anterior Cruciate Ligament Repair in Each Age Group
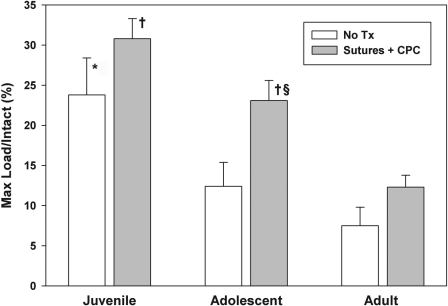
In the juvenile animals, surgical repair with use of absorbable sutures and the collagen-platelet composite resulted in a 50% reduction in the low load displacement, a 30% improvement in the maximum load of the ligament, and a 40% improvement in the linear stiffness of the repair tissue over the untreated transections (p < 0.01 for all comparisons) (Table II). In the adolescent animals, surgical repair with use of absorbable sutures and the collagen-platelet composite resulted in a 100% improvement in the yield load, an 85% improvement in the maximum load of the ligament (Fig. 3), an 18% decrease in the anteroposterior laxity of the knee at 60°, and an 83% improvement in the linear stiffness of the repair tissue compared with the untreated transections (p < 0.01 for all comparisons) (Table II). In the adult animals, there were no significant effects of surgical repair with a collagen-platelet composite compared with the untreated transection group.
Fig. 3.
The maximum load in each age group as normalized by the maximum load of the intact anterior cruciate ligament for that age group. *The juvenile animals had a threefold greater normalized maximum load than the adult animals in the untreated (no Tx) group (p < 0.01). †For ligaments treated with collagen-platelet composite (CPC), both the juvenile and adolescent animals had higher normalized maximum loads than the adult group (p < 0.01 for both comparisons). §The addition of the collagen-platelet composite resulted in an 85% increase in maximum load in the adolescent group (p < 0.01).
Histologic Results
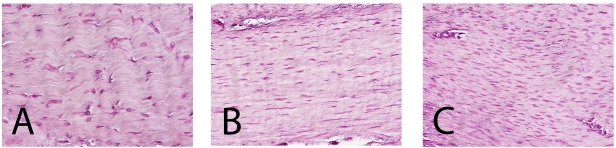
At fifteen weeks following surgery, fibroblasts were oriented along the axis of the ligament. There was a significantly greater density of fibroblasts in the adult and adolescent animals compared with that in the juvenile animals (p < 0.01 and p < 0.001). Adult animals had the greatest amount of fibroblasts (mean [and standard deviation], 1537 ± 163 cells/mm2) followed by the adolescent and juvenile animals (mean, 1340 ± 90 and 823 ± 102 cells/mm2, respectively). There were significant differences in nuclear area between the adult and adolescent animals (7 ± 2 μM2 compared with 20 ± 4 μM2; p < 0.001) and the adult and juvenile animals (7 ± 2 μM2 compared with 18 ± 11 μM2; p < 0.01) at the fifteen-week time point (Fig. 4).
Fig. 4.
Histologic sections of representative areas of the repair tissue in the juvenile (A), adolescent (B), and adult (C) groups after fifteen weeks of healing. Note the increased cell density in the adolescent and adult specimens and the larger nuclei of the cells in the juvenile repair tissue (hematoxylin and eosin, ×400).
Discussion
The results of this study demonstrate that both age and treatment affect healing of an injury of the anterior cruciate ligament in the porcine knee. The preoperative and fifteen-week Lachman test values were lower in the adult animals than in younger pigs. Thus, while the adult pigs had little tensile strength in the healing repair tissue, they did not exhibit increased laxity by physical examination (Lachman testing) or by ex vivo anteroposterior laxity testing compared with the younger animals. While the formation of anterior cruciate ligament tissue in the adult knees was less, they did have a thicker capsular reaction and greater loss of range of motion than the other knees. This suggests the possibility of a different biologic response of the joint as a whole in the adult animals after the surgical intervention of anterior cruciate ligament transection, with or without enhanced repair. Possible contributors to this effect include synovial hypertrophy and capsular thickening, hypertrophy of other secondary joint restraints, muscular atrophy, and joint adhesions. Further studies are needed to define the biologic mechanism behind this specific finding. While there was greater loss of motion in the adult animals over time, there was no difference in these parameters between the knees with an untreated transection of the anterior cruciate ligament and those treated with collagen-platelet composite and suture repair.
In addition, the results of this study support the hypothesis that the capacity for functional healing of a ligament after injury and enhanced repair is dependent on the level of skeletal maturity of the animal. Juvenile animals had a more productive repair response to anterior cruciate ligament transection without treatment than the older animals, with the repair tissue having a higher maximum load and stiffness. The adolescent animals derived the most benefit from suture repair with use of a collagen-platelet composite, with a doubling of the yield load in this group with treatment. The adult animals had the least functional intrinsic repair response (no treatment of the transected anterior cruciate ligament), and the use of the collagen-platelet composite had little effect on ligament healing in the adult animals.
An important confounder to consider is the different activity levels among the animals, as the adult animals were less active than the younger groups. This is a substantial limitation, since it is known that mechanical load has an important effect on connective tissue biology. The diminished activity level in the adult animals may also have contributed to the increased stiffness noted in that group. Future studies to address the effect of rehabilitation parameters in animal models in which rehabilitation can be better controlled are needed to assess the impact of this important confounder.
Characteristics of patients who may be good candidates for stimulating healing of the ligament have not yet been defined. Conventional orthopaedic wisdom is that “fractures in children heal faster than those in adults,” and basic science studies in animals and clinical studies in humans appear to support this clinical experience21,22. However, basic science studies of partial transection of the anterior cruciate ligament and patellar tendon healing have suggested that the scar formed in skeletally mature animals is actually stronger than that formed in skeletally immature animals8,23. The results in the present study show that the functional healing of the skeletally immature animals was better than that of the adult animals. Potential reasons for the difference in this study from prior studies include the use of a model involving complete transection of the anterior cruciate ligament (the prior studies evaluated only partial tears or defects) or the use of a large animal model compared with smaller animals (rabbits) as has been previously done.
The molecular mechanisms that explain these results require further study. However, in vitro studies of the effect of age on anterior cruciate ligament fibroblast function have determined that anterior cruciate ligament cells from skeletally immature animals are capable of greater cellular proliferation and migration than those cells from adolescent or adult animals24, which may be due to age-related changes in growth factor receptor expression25. A follow-up in vivo study evaluating the effect of age on the histologic response to anterior cruciate ligament injury and enhanced suture repair in the porcine model found that, in all three age groups, the anterior cruciate ligament wound site had been completely populated with fibroblasts by one week17. However, the juvenile animals had a significantly greater cellular density than the adults at two and four weeks after surgery. This finding suggests an increased proliferative capacity of the cells in the juvenile animals. It is possible that the increased migratory and proliferative potential of the cells from the juvenile animals may be playing a role in the improved intrinsic functional healing response noted in the present study. Whether the impaired healing response of adult animals can be improved by stimulating additional migratory or proliferative function remains to be proven but certainly poses an important and interesting line of inquiry.
At fifteen weeks, adult animals showed greater cellularity than juvenile or adolescent animals; however, increased cell density, particularly at time points when tissue-remodeling should be well under way, does not mean an increased capacity for ligament healing17. Previous studies have shown that the rapid proliferation of cells can deter regenerative scar formation, creating a nonuniform and abnormally high distribution of cells within the matrix, thus disrupting a crucial component of proper wound-healing17,26. Prior studies in this animal model have shown that cellular density typically increases early in successful wound-healing, peaking at four to six weeks, and then declines. Only after the cell density begins to decrease are increases in tissue strength seen7. Thus, a delayed hypercellularity in the adult tissues continuing at fifteen weeks may represent a delayed healing response in that group and may have been contributing to the inferior mechanical properties noted in this study.
The paired analysis afforded by the use of two groups in a bilateral model greatly increases the power of the study to detect treatment effects, but it limits the number of groups that can be studied to two. In this study, an additional group of age-matched knees with intact anterior cruciate ligaments was also tested to improve our ability to compare the results of this study with those in other studies of anterior cruciate ligament treatment in large animal models. For example, the graft strength at three months in a Yorkshire pig model was previously reported at 28%27. This suggests the values obtained in the present study for enhanced suture repair in the juvenile and adolescent groups compare favorably in a similar model. In addition, the only repair type attempted in this study was suture repair with a collagen-platelet composite to enhance the repair. In prior studies, we looked at using suture repair alone for anterior cruciate ligament healing in the adolescent Yucatan porcine model27.
The porcine model has a few limitations common to large animal studies in quadrupeds, primarily because these animals bear weight on four limbs. While the pig model was selected because of its anatomical and biomechanical similarities to the human knee, there may be differences in gait and rehabilitation that cannot be reliably controlled. Furthermore, there may be subtle differences in the wound-healing cascade that are not yet appreciated. In addition, the effect of aging on the human model may not be the same as that observed with porcine aging. In addition, we studied only one time point. Prior studies have demonstrated a nadir of strength of the healing tissue between six and nine weeks after repair of porcine anterior cruciate ligament7. The time point of fifteen weeks was selected for this study as it has been found to be beyond that low point of strength in the healing process and to be a time when successfully healing ligaments are increasing in strength. Future studies evaluating long-term outcomes are needed. Finally, while this study may serve as a stimulus for future clinical studies, it cannot be used to conclude any effect for human treatment, given the nature of the model and study.
Despite the limitations of large animal models in general, the results remain exciting. This work demonstrates that partial healing of the anterior cruciate ligament with an enhanced suture repair is beneficial in this porcine model and may provide a new treatment alternative—to stimulate healing of this important ligament rather than developing new ways to replace or reconstruct it. While this study may serve as a foundation for understanding the effects of age on anterior cruciate ligament healing in large animals, further studies are clearly needed before the effect of age on ligament healing in humans can be defined.
Footnotes
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (R01 AR054099). In addition, one or more of the authors or a member of his or her immediate family received, in any one year, payments or other benefits in excess of $10,000 or a commitment or agreement to provide such benefits from a commercial entity (Connective Orthopedics).
References
- 1.Myer GD, Ford KR, Hewett TE. Rationale and clinical techniques for anterior cruciate ligament injury prevention among female athletes. J Athl Train. 2004;39:352-64 [PMC free article] [PubMed] [Google Scholar]
- 2.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray MM, Martin SD, Martin TL, Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82:1387-97 [DOI] [PubMed] [Google Scholar]
- 4.Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007-17 [DOI] [PubMed] [Google Scholar]
- 5.Murray MM, Spindler KP, Devin C, Snyder BS, Muller J, Takahashi M, Ballard P, Nanney LB, Zurakowski D. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820-30 [DOI] [PubMed] [Google Scholar]
- 6.Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, Kelly M, Frino J, Zurakowski D, Valenza M, Snyder BD, Connolly SA. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81-91 [DOI] [PubMed] [Google Scholar]
- 7.Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37:2401-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hefti FL, Kress A, Fasel J, Morscher EW. Healing of the transected anterior cruciate ligament in the rabbit. J Bone Joint Surg Am. 1991;73:373-83 [PubMed] [Google Scholar]
- 9.Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26:1500-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray MM, Magarian E, Zurakowski D, Fleming BC. Bone-to-bone fixation enhances functional healing of the porcine anterior cruciate ligament. Arthroscopy. 2010 Sep 12[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spindler KP, Murray MM, Carey JL, Zurakowski D, Fleming BC. The use of platelets to affect functional healing of an anterior cruciate ligament (ACL) autograft in a caprine ACL reconstruction model. J Orthop Res. 2009;27:631-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray MM, Palmer M, Abreu E, Spindler KP, Zurakowski D, Fleming BC. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res. 2009;27:639-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37:1554-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming BC, Abate JA, Peura GD, Beynnon BD. The relationship between graft tensioning and the anterior-posterior laxity in the anterior cruciate ligament reconstructed goat knee. J Orthop Res. 2001;19:841-4 [DOI] [PubMed] [Google Scholar]
- 15.Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19:217-25 [DOI] [PubMed] [Google Scholar]
- 16.Sakai T, Yasuda K, Tohyama H, Azuma H, Nagumo A, Majima T, Frank CB. Effects of combined administration of transforming growth factor-beta1 and epidermal growth factor on properties of the in situ frozen anterior cruciate ligament in rabbits. J Orthop Res. 2002;20:1345-51 [DOI] [PubMed] [Google Scholar]
- 17.Mastrangelo AN, Haus BM, Vavken P, Palmer MP, Machan JT, Murray MM. Immature animals have higher cellular density in the healing anterior cruciate ligament than adolescent or adult animals. J Orthop Res. 2010;28:1100-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika. 1965;52:591-611 [Google Scholar]
- 19.Crowder MJ, Hand DJ. Analysis of repeated measures. Monographs on statistics and applied probability 41. New York: Chapman & Hall; 1990 [Google Scholar]
- 20.Bausell RB, Li Y- F. Power analysis for experimental research. A practical guide for the biological, medical and social sciences. New York: Cambridge University Press; 2006 [Google Scholar]
- 21.Bak B, Andreassen TT. The effect of aging on fracture healing in the rat. Calcif Tissue Int. 1989;45:292-7 [DOI] [PubMed] [Google Scholar]
- 22.Skak SV, Jensen TT. Femoral shaft fracture in 265 children. Log-normal correlation with age of speed of healing. Acta Orthop Scand. 1988;59:704-7 [DOI] [PubMed] [Google Scholar]
- 23.Dressler MR, Butler DL, Boivin GP. Effects of age on the repair ability of mesenchymal stem cells in rabbit tendon. J Orthop Res. 2005;23:287-93 [DOI] [PubMed] [Google Scholar]
- 24.Mastrangelo AN, Magarian EM, Palmer MP, Vavken P, Murray MM. The effect of skeletal maturity on the regenerative function of intrinsic ACL cells. J Orthop Res. 2010;28:644-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vavken P, Saad FA, Murray MM. Age dependence of expression of growth factor receptors in porcine ACL fibroblasts. J Orthop Res. 2010;28:1107-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res. 2008;466:622-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleming BC, Magarian EM, Harrison SL, Paller DJ, Murray MM. Collagen scaffold supplementation does not improve the functional properties of the repaired anterior cruciate ligament. J Orthop Res. 2010;28:703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]