Abstract
Background
This study determined whether transfer of the renin-gene from the Dahl salt resistant (Dahl R) strain into the Dahl salt-sensitive (SS) genetic background restores the relaxation of middle cerebral arteries (MCA) to different vasodilator stimuli in S/renRR renin congenic [SS.SR –(D13N1 and Syt2)/Mcwi] (RGRR) rats maintained on low salt (0.4% NaCl) diet.
Methods
Responses to vasodilator stimuli were evaluated in isolated MCA from SS (Dahl SS/Jr/Hsd/MCWi), RGRR rats, and Dahl salt-resistant (R) rats.
Results
MCA from SS rats failed to dilate in response to acetylcholine (10−6 mol/L), hypoxia (PO2 reduction to 40–45 mm Hg), and iloprost (10−11 g/ml). ACh- and hypoxia-induced dilations were present in Dahl R rats and restored in RGRR rats. MCA from RGRR and SS constricted in response to iloprost, while MCA from Dahl R rats dilated in response to iloprost. MCA from SS, RGRR, and Dahl R rats exhibited similar dilations in response to cholera toxin (10−9 g/ml) and dilated in response to the NO donor DEA-NONOate (10−5 mol/L).
Conclusions
1) restoration of normal regulation of the renin-angiotensin system restores dilations to acetylcholine and hypoxia that are impaired in SS rats, 2) prostacyclin signaling is impaired in SS and RGRR rats but intact in Dahl R rats, indicating that alleles other than the renin gene affect vascular relaxation in response to this agonist; and 3), vascular smooth muscle sensitivity to NO is preserved in SS and RGRR and is not responsible for impaired arterial relaxation in response to ACh in SS rats.
Keywords: Angiotensin II, Salt-Sensitive Hypertension, Renin, Physiological Genomics, Microcirculation
INTRODUCTION
Salt-sensitivity of blood pressure in normotensive humans is a cause for concern in light of long-term follow-up studies showing that salt-sensitive subjects are more likely to develop hypertension than salt-resistant individuals and also have a higher long-term mortality rate. 1 High salt diet itself can lead to impaired vascular relaxation, reduced NO availability, and elevated superoxide levels in blood vessels, independent of an elevated blood pressure.2–7 In arteries of Dahl salt-sensitive (SS) rats (a genetic model of human salt-sensitive hypertension),8–10 endothelial dysfunction, increased vascular oxidant stress, and impaired vascular relaxation are present even when the animals are normotensive and maintained on low salt diet.11
Similarities of phenotypic traits in SS rats and human patients with salt-sensitive hypertension include low renin, salt sensitivity, and early end stage renal disease. 8, 12–16 The presence of low plasma renin in SS rats and in salt-sensitive forms of human hypertension may have deeper pathophysiological significance than previously suspected. There is growing evidence that angiotensin II (ANG II) plays an important role in maintaining normal vascular relaxation mechanisms in resistance arteries of normotensive animals. 5–7 The loss of vascular relaxation, impaired endothelial cell [Ca2+] signaling 17, increased vascular oxidative stress 7, and reduced expression of Cu/Zn SOD in resistance arteries 18 of animals fed a high salt (HS) diet can all be prevented by chronic i.v. infusion of a subpressor dose of ANG II that prevents salt-induced ANG II suppression.
The chronically low plasma renin activity (PRA) in Dahl salt-sensitive (SS) rats maintained on LS diet is due to an impaired ability of the animals to regulate their renin-angiotensin system (RAS).8, 19, 20 In S/renRR congenic rats (RGRR), transfer of a region of chromosome 13 containing the renin gene from the Dahl salt-resistant (Dahl R) strain into the SS genetic background [SS.SR–(D13N1 and Syt2)/Mcwi] confers normal salt sensitivity. 19, 20 RGRR congenic rats also exhibit a significantly higher PRA than SS rats on LS diet due to the presence of the Dahl R renin allele. 19, 20
The cerebral circulation is not only exquisitely sensitive to vasoactive stimuli and crucially important for regulating blood flow to the brain, but is also a key target for stroke in hypertension and with elevated dietary salt intake.21, 22 Middle cerebral arteries of normotensive SS rats fed LS diet exhibit impaired relaxation to different vasodilator stimuli which can be restored by chronic i.v. infusion of a subpressor dose of ANG II (3 ng/kg/min).11 This protective effect of ANG II infusion to restore vasodilator responses is similar to that in Sprague-Dawley rats, where vascular relaxation mechanisms in salt-fed animals are restored by chronic low dose ANG II infusion to prevent salt-induced ANG II suppression. The goal of this study was to test the hypothesis that the low circulating ANG II levels in SS rats fed low salt diet contribute to impaired vascular relaxation. This hypothesis was tested by determining whether introgression of a smaller segment of chromosome 13 containing the renin allele from the Dahl R rat (and lacking potential protective alleles from the Brown Norway rat) restores normal vascular relaxation in middle cerebral arteries (MCA) of RGRR congenic rats.
MATERIALS AND METHODS
General Procedures
SS rats (Dahl SS/Jr/Hsd/MCWi) rats, RGRR congenic rats ([SS.SR –(D13N1 and Syt2)/Mcwi]) and Dahl salt-resistant (R) rats (10–12 weeks old at the time of the experiment, n=5–11 animals/experimental group) were maintained in an AAALAC-accredited animal facility and fed a LS diet (0.4% NaCl) (Dyets, Inc Bethlehem, PA) with tap water to drink ad libitum. The Medical College of Wisconsin IACUC approved all experimental procedures.
Rats were anesthetized with sodium pentobarbital (30–50 mg/kg, i.p.) (Abbot Laboratories, North Chicago, IL). The lower dose of pentobarbital was used to compensate for the enhanced sensitivity of SS rats to anesthesia. A femoral artery was cannulated for the direct measurement of arterial pressure. MCA were isolated and cannulated as described previously.4, 11, 23, 24
Intravascular pressure was maintained at 80 mm Hg and the arteries were perfused and superfused with physiological salt solution (PSS) equilibrated with a 21% O2-5% CO2-74% N2 gas mixture. The PSS used in these experiments had the following constituents (10−3 mol/L): 119 NaCl, 4.7 KCl, 1.17 MgSO4, 1.6 CaCl2, 1.18 NaH2PO4, 24 NaHCO3, 0.026 EDTA, and 5.5 dextrose. MCA that did not show active tone at rest, as confirmed by the lack of a large dilation in response to Ca2+ free PSS, were not used in the study.
After the equilibration period, vessel diameters were measured before and during simultaneous reduction of perfusate and superfusate O2 concentration by equilibrating the PSS with a 0% O2-5% CO2-95% N2 gas mixture, which lowers luminal and tissue bath PO2 to ~35–45 mmHg.25 Responses to the endothelium-dependent dilator acetylcholine (ACh; 10−6 mol/L), the stable prostacyclin analogue iloprost (10−11 g/ml) (2.8×10−10 mol/L), the Gsα-protein subunit activator cholera toxin (10−9 g/ml) (1.2×10−10 mol/L), and the NO donor DEA-NONOate (10−5 mol/L) were also tested by adding these agents to the vessel chamber. The roles of NO and cyclooxygenase metabolites in mediating the restored relaxation of the arteries in response to ACh and reduced PO2 in RGRR were evaluated by determining vessel responses to these vasodilator stimuli in the presence and absence of nitroG-monomethyl-L-arginine (L-NMMA) (10−4 mol/L) or indomethacin (10−6 mol/L), respectively.
Spontaneous active tone during resting conditions, which generally averages around 50%, was evaluated by measuring the diameter increase during maximal dilation with a Ca2+ free relaxing solution containing the following constituents (10−3 mol/L): 92.0 NaCl, 4.7 KCl, 1.17 MgSO4, 20.0 MgCl2, 1.18 NaH2PO4, 24.0 NaHCO3, 0.026 EDTA, 2.0 EGTA, and 5.5 dextrose. Active tone (%) was calculated as [(Dmax-Drest)/Dmax]X100, where Dmax and Drest are the maximum and resting diameters of the vessel, respectively.
Hypoxia-Induced Release of Cyclooxygenase Metabolites in RGRR Cerebral Arteries
To identify potential mediators of cyclooxygenase-dependent hypoxic dilation in RGRR congenic rats and to compare these responses to previously published findings in SS rats,23 prostacyclin, thromboxane A2, and prostaglandin E2 release were measured before and during exposure of RGRR cerebral arteries to reduced PO2 (5% O2-to match the perfusate and superfusate PO2 reached in the cannulated vessel studies 25) as described previously.4, 23 Metabolites were measured in the Physiology Department Biochemical Assay Core Facility, utilizing commercially available enzyme immunoassay kits (Cayman Chemical, Ann Arbor, MI) for PGE2, the stable prostacyclin metabolite 6-keto-prostaglandin F1α (PGF1α), and thromboxane B2 (TXB2), a stable metabolite of thromboxane A2.
Statistical Analysis
All data are summarized as mean ± SEM. Differences between two means within a group (control vs. response to stimulus) or between two individual groups were assessed using a paired or unpaired Student’s t-test, respectively. Differences among multiple groups in the response to reduced PO2 or agonist application were assessed by ANOVA, with a post hoc Student-Newman-Keuls test. A probability value of p<0.05 was considered to be statistically significant.
RESULTS
General Characteristics of Experimental Groups
Table 1 summarizes the internal resting and maximum diameters and spontaneous active tone (%) in MCA from the three groups. Resting internal diameter was significantly larger and active tone in MCA of Dahl R rats was significantly less than that in MCA of the SS and RGRR rats. Vessel diameters and active tone were similar in SS and RGRR rats.
Table 1.
Vessel Diameters and Active Tone in MCA of SS, RGRR and Dahl R Rats
| SS | RGRR | Dahl R | |
|---|---|---|---|
| Resting ID of MCA (μm) | 110 ± 5 (12) | 112 ± 8 (8) | 143±3 (17) * |
| Maximum ID of MCA (μm) | 222 ± 9 (12) | 228 ± 14 (8) | 201±4 (17) |
| Active Tone (%) | 50 ± 3 (12) | 51 ± 1 (8) | 29±2 (17) * |
ID-Inner Diameter; MCA-Middle Cerebral Artery; Active Tone: spontaneous resting tone (%) in the absence of any agonists. Data summarized as mean ± SEM; (n)—number of animals;
Significantly different from SS and RGRR rats, P <0.05.
Responses to Vasodilator Stimuli in MCA of SS and RGRR Rats
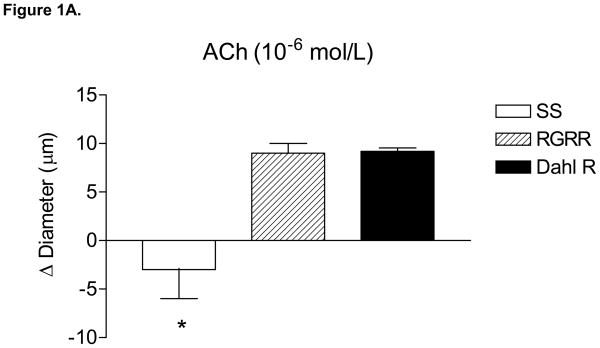
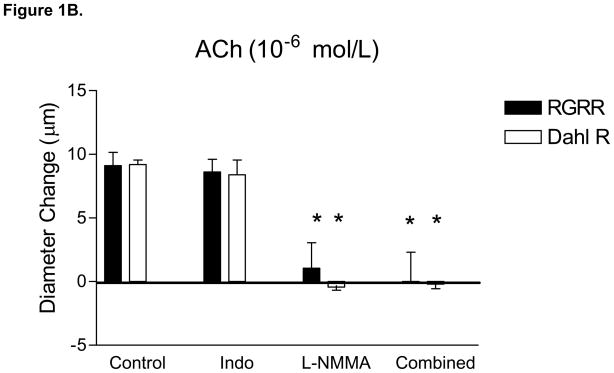
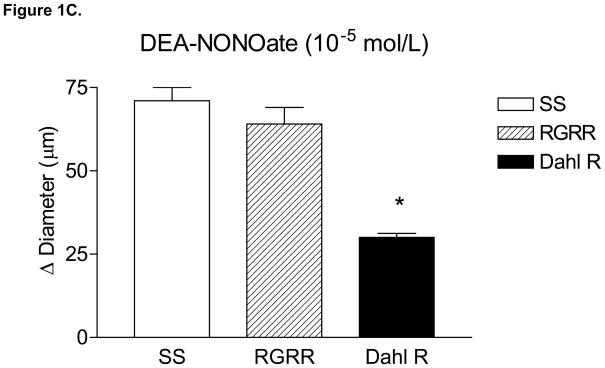
MCA of RGRR and Dahl R rats dilated in response to acetylcholine (ACh) while MCA from SS rats fed LS diet failed to relax in response to ACh (Figure 1A). ACh-induced dilation of MCA in RGRR and Dahl R rats was eliminated by L-NMMA and unaffected by indomethacin (Figure 1B), confirming that ACh-induced dilation is NO-dependent in the RGRR congenic rats and Dahl R rats. L-NMMA caused a significant constriction of MCA of RGRR (−10 ± 2.6 μm, n=5) and Dahl R rats (−22 ± 1.9 μm, n=6), but had no effect on resting diameter of MCA from SS rats (−2 ± 2.2 μm, n=6), consistent with the hypothesis that the reduced basal NO levels in SS rats fed LS diet are ameliorated by introgression of the Dahl R renin allele. Indomethacin had no effect on resting diameter of MCA in any of the groups (−1.6 ± 3.8 μm for SS, n=5; −1 ±4.8 μm or RGRR, n=6, and −5.8 ± 3.2 μm, n=5 for Dahl R). MCA from SS rats, RGRR rats, and Dahl R rats all dilated in response to DEA-NONOate (Figure 1C), demonstrating that loss of NO sensitivity is not responsible for the loss of dilation in response to ACh in SS rats. Vasodilatation in response to DEA-NONOate was significantly less in MCA of Dahl R compared to SS and RGRR rats, presumably due to the lower resting tone in vessels of the Dahl R rats.
Figure 1.
(A) Responses to 10−6 mol/L acetylcholine (ACh) in MCA from SS, RGRR, and Dahl R rats. Asterisk denotes a significant difference in the response in MCA from SS rats vs. RGRR and Dahl R rats. (B) Effect of the cyclooxygenase (COX) inhibitor indomethacin (Indo) (1O−6 mol/L), the NOS inhibitor L-NMMA (1O−4 mol/L) and combined COX and NOS inhibition with Indo +L-NMMA on response to ACh in MCA from RGRR rats (solid bars) and Dahl R rats (open bars) fed low salt diet. Asterisk denotes a significant difference from response in the absence of any inhibitor (P<0.05). (C) Responses to DEA-NONOate (10 μM) in MCA from SS, RGRR, and Dahl R rats fed low salt diet. Asterisk denotes a significant difference from the response in MCA from SS and RGRR rats. All data are presented as mean change (Δ) in diameter (μm) ± SEM from resting control diameter for 6–10 rats/group.
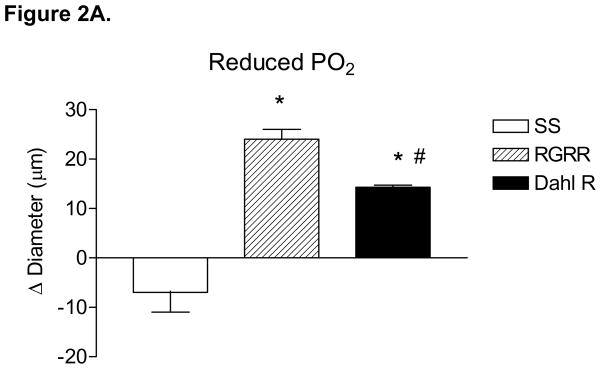
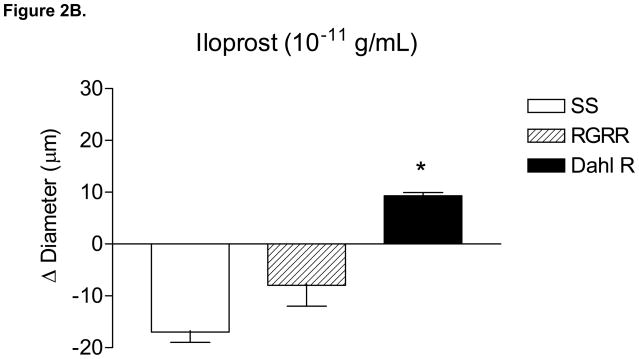
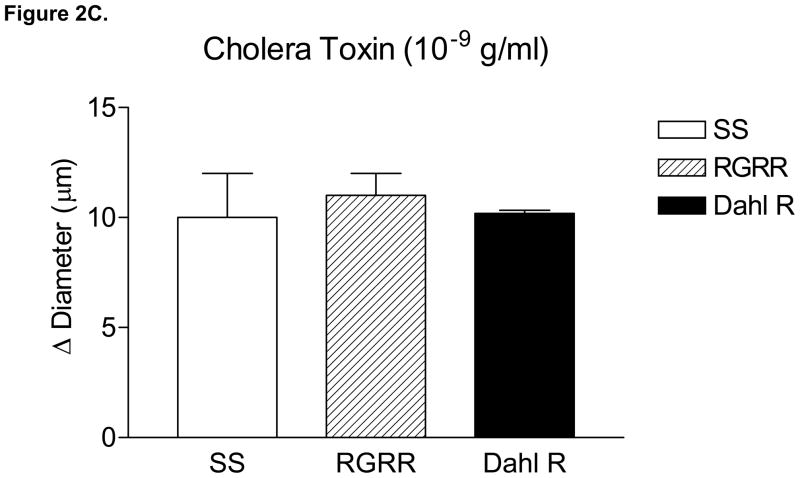
Figure 2 summarizes the responses of MCA to reduced PO2 (40–45 mm Hg) (A), iloprost (B), and cholera toxin (C) in SS, RGRR, and Dahl R rats. MCA from RGRR rats and Dahl R rats dilated in response to reduced PO2, in contrast to the lack of hypoxic dilation in SS rats. MCA from SS rats also exhibited a paradoxical constriction in response to iloprost, a stable analogue of prostacyclin, the primary mediator of hypoxic dilation in MCA of Sprague-Dawley rats.26 Interestingly iloprost-induced dilation, which was present in Dahl R rats, was not restored in MCA of RGRR. However, MCA from SS rats, RGRR rats, and Dahl R rats all relaxed in response to stimulation of the α subunit of the Gs protein with cholera toxin.
Figure 2.
Response of MCA to (A) reduced PO2 ; *-P<0.05 vs. SS; #-P<0.05 vs. RGRR; (B) iloprost, *-P<0.05 vs. SS; and (C) cholera toxin in MCA from SS and RGRR rats fed low salt diet. Data are presented as mean change (Δ) in diameter (μm) ± SEM for 6–9 rats/group.
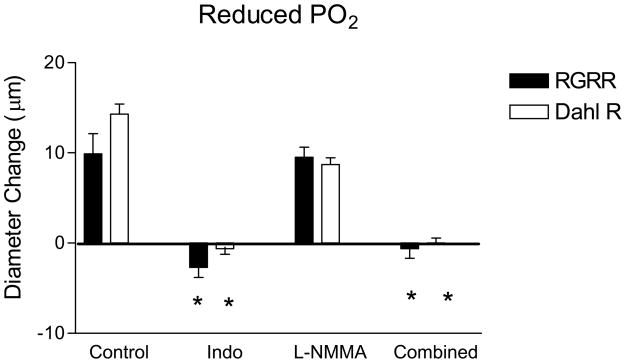
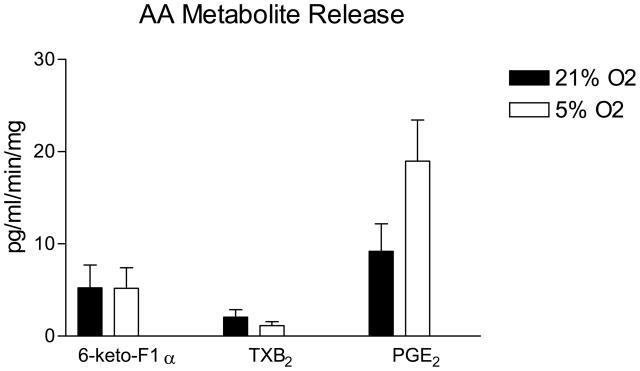
The dilation of MCA in response to reduced PO2 in RGRR and Dahl R rats was eliminated by indomethacin and unaffected by L-NMMA (Figure 3). The failure of RGRR MCA to dilate in response to iloprost suggests that a cyclooxygenase metabolite of arachidonic acid other than PGI2 mediates hypoxic dilation in RGRR. The latter conclusion is supported by the observation that PGE2 release doubled during exposure to reduced PO2 in cerebral arteries of RGRR while PGI2 release was unchanged and thromboxane release tended to decrease (Figure 4).
Figure 3.
Response to reduced PO2 in MCA from RGRR (solid bars) and Dahl R rats (open bars) on low salt diet in the presence or absence of the COX inhibitor indomethacin (Indo), the NOS inhibitor L-NMMA, or both inhibitors. Asterisk denotes a significant difference from control values measured in the absence of any inhibitor. Data are presented as mean change (Δ) in diameter (μm) ± SEM for 5–10 rats/group.
Figure 4.
Effect of reduced PO2 on prostacyclin (assessed as 6-keto PGF1α), thromboxane A2 (assessed as TXB2) and PGE2 production in cerebral arteries of RGRR rats. Data are expressed as mean % of the control (21% O2) value ± SEM following reduction of O2 concentration from 21% O2 to 5% O2 (3–6 measurements per group).
DISCUSSION
Previous studies have shown that the vasodilator responses to hypoxia and acetylcholine that are absent in SS rats fed LS diet are restored in consomic SS.13BN rats, in which the entire chromosome 13 from the Brown Norway rat containing the BN renin allele is introgressed into the SS genetic background.23, 24 RGRR rats have a smaller piece of chromosome 13 containing a functioning renin gene from the historical control strain for the Dahl SS rat (the Dahl salt-resistant rat) introgressed into the SS genetic background. This gene transfer not only narrows the chromosomal region that is transferred to the SS genetic background to the proximity of the Dahl R renin gene, but also eliminates any unique protective effects of BN alleles other than the renin gene that could contribute to the restoration of vascular relaxation mechanisms previously reported in the SS.13BN consomic rats. Thus, the restoration of vasodilator responses to ACh- (Figure 1) and reduced PO2 (Figure 2) in MCA of RGRR rats provides additional evidence supporting the hypothesis that restoring normal regulation of the renin-angiotensin system (RAS) preserves vascular relaxation in response to these vasodilator stimuli.
As previously reported, 11, 23, 24 MCA from SS rats fed LS diet exhibited a paradoxical constriction in response to iloprost (Figure 2), a stable prostacyclin analog that generally mediates relaxation of MCA by binding to the prostacyclin receptor (IP).26 Surprisingly, introgression of the Dahl R renin gene into the SS genetic background did not restore vasodilator responses to iloprost in MCA of RGRR, even though Dahl R MCA dilated in response to iloprost (Figure 2). This observation contrasts with findings in Sprague-Dawley rats fed HS diet, where low dose ANG II infusion restores vasodilator responses and cAMP generation in response to iloprost. 4–6 This finding was unexpected, because prostacyclin normally relaxes MCA and is the primary mediator of hypoxic dilation in MCA of Sprague-Dawley rats. 26 Our finding that prostacyclin release does not increase in response to reduced PO2 in cerebral arteries of RGRR contrasts with those of previous studies 23 showing that prostacyclin release increases in response to hypoxia in cerebral arteries of SS rats fed low salt diet, even though the vessels are apparently insensitive to the vasodilator effects of this metabolite. This difference in the arachidonic acid metabolite release in SS and RGRR rats during hypoxia presumably reflects strain differences between the SS and RGRR rats.
The present study shows that introgression of the area of chromosome 13 carrying the Dahl R renin allele is insufficient to restore iloprost-induced dilation in MCA from RGRR rats even though iloprost dilates MCA from Dahl R rats. This finding is consistent with previous studies demonstrating that MCA from Brown Norway rats dilate in response to iloprost, while those from SS.13BN consomic rats do not. Taken together, those findings suggest that factors in the SS genetic background other than the renin gene contribute to impaired vascular relaxation in response to iloprost in SS rats. In this regard, it is important to note that MCA of SS.13BN rats do relax in response to the stable prostaglandin E2 analogue butaprost 23, which is consistent with the restored ability of MCA from RGRR to dilate in response to reduced PO2 and the increase in PGE2 release by cerebral arteries of RGRR during exposure to reduced PO2 in our experiments.
The observation that MCA from SS and RGRR rats both dilate in response to Gsα protein activation with cholera toxin (Figure 2) indicates that G sα protein signaling is intact in SS rats. This is an important new finding, because the Gsα protein has an essential role in linking the binding of the PGI2 receptor to its downstream signaling events, including activation of adenylyl cyclase. However this finding was unexpected because vasodilator responses to cholera toxin are impaired in MCA of Sprague-Dawley rats fed high salt diet, but can be restored by chronic i.v. infusion of a low dose of ANG II to prevent salt-induced ANG II suppression 17, 27, 28 or by returning the salt-fed rats to a low salt diet. 18 In the present study, the lack of iloprost-induced dilation in both SS rats and RGRR rats in the face of intact Gsα signaling suggests that the structure, function or expression of the PGI2 receptor itself is altered in both strains of rats.
Given the lack of vascular relaxation in response to Iloprost in MCA of RGRR rats and the observation that alternate compensatory mechanisms of vasodilatation can emerge when the normal mechanisms of vascular relaxation are blocked or lost, 29–32 it was important to determine whether cyclooxygenase metabolites of arachidonic acid still contribute to hypoxic dilation in MCA from RGRR rats. The present study shows that hypoxic dilation of MCA from RGRR as well as Dahl R rats is eliminated by indomethacin and unaffected by L-NMMA (Figure 3), and that PGE2 release increases in response to reduced PO2 in cerebral arteries of RGRR rats (Figure 4). Taken together, these findings demonstrate that the restored vasodilation to reduced PO2 in MCA from RGRR is still mediated by a cyclooxygenase metabolite of arachidonic acid (most likely PGE2), rather than NO.
There is growing evidence that ANG II suppression and reduced interaction of ANG II with its AT1 receptor contribute to impaired vascular relaxation in Sprague-Dawley rats fed HS diet. 4–6, 33 A previous study showed that impaired vascular relaxation in SS rats fed LS diet can be restored by low dose ANG II infusion as well as restoration of normal RAS function by introgression of Brown Norway chromosome 13 (carrying the BN renin allele) into the SS genetic background11. The protective action of low dose ANG II infusion to restore vasodilator responses in salt-fed rats, 5 the ability of normal circulating ANG II levels to maintain vascular relaxation mechanisms in resistance arteries of Sprague-Dawley rats fed normal rodent chow,33 and the restoration of vascular relaxation in SS.13BN consomic rats by introgression of a normally functioning BN renin allele via chromosomal substitution11 can all be inhibited by chronic AT1 receptor blockade with losartan. Taken together, those findings suggest that tonic activation of the AT1 receptor by normal circulating levels of ANG II plays an important role in maintaining vascular relaxation mechanisms under normal physiological conditions. The present study showing that substitution of the Dahl R renin allele into the SS genetic background restores vasodilator responses in MCA of the RGRR congenic rats which have significantly higher PRA than SS rats fed low salt diet 19 provides further support for the hypothesis that the impaired vasodilator responses in SS rats fed LS diet are most likely related to their inability to regulate their RAS normally.
SS rats are a widely used genetic model of salt-sensitive hypertension in humans. As noted above, long-term follow-up studies have shown that salt-sensitive subjects not only have an increased chance of eventually developing hypertension compared to salt-resistant individuals, but also have higher long-term mortality rate, even if they do not develop hypertension.1 A recent review article by Widlansky et al.34 cited a number of clinical studies suggesting that endothelial dysfunction and increased oxidative stress may be predictive of future adverse cardiovascular events (including death) in patient populations. The present study and others suggest that marked endothelial dysfunction 11, 24 (Figure 1) and increased vascular oxidant stress related to a chronic reduction in PRA and low levels of circulating ANG II is present in normotensive SS rats in the absence of an elevated dietary salt intake.11 Taken together these findings suggest that the SS rat and congenic strains based on this strain may be a valuable genetic model to gain an increased understanding of vascular dysfunction in salt-sensitive individuals. If observations implicating chronic suppression of the RAS to vascular oxidant stress, and endothelial dysfunction in SS rats can be translated to humans, it raises the possibility of a previously overlooked problem in low renin forms of hypertension, namely the possible existence of impaired endothelial function and increased vascular oxidative stress even in the absence of elevated dietary salt intake or increases in arterial blood pressure.
Acknowledgments
This work supported by NIH grants #HL-29587; #HL65289, #HL72920; and #HL-092026. We thank Lynn Dondlinger for outstanding assistance, with manuscript preparation, Dani Didier for her assistance in obtaining RGRR rats, and Camille Torres for her help in the measurement of arachidonic acid metabolites.
Support: This work supported by NIH grants #HL-29587; #HL65289, #HL72920 and #HL-092026.
Footnotes
Disclosures: None
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–32. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 2.Lenda DM, Boegehold MA. Effect of a high salt diet on microvascular antioxidant enzymes. J Vasc Res. 2002;39:41–50. doi: 10.1159/000048992. [DOI] [PubMed] [Google Scholar]
- 3.Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol. 2000;279:H7–H14. doi: 10.1152/ajpheart.2000.279.1.H7. [DOI] [PubMed] [Google Scholar]
- 4.Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2003;284:H1124–H1133. doi: 10.1152/ajpheart.00835.2002. [DOI] [PubMed] [Google Scholar]
- 5.Weber DS, Lombard JH. Angiotensin II AT1 receptors preserve vasodilator reactivity in skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol. 2001;280:H2196–H2202. doi: 10.1152/ajpheart.2001.280.5.H2196. [DOI] [PubMed] [Google Scholar]
- 6.Weber DS, Lombard JH. Elevated salt intake impairs dilation of skeletal muscle resistance arteries via angiotensin II suppression. Am J Physiol. 2000;278:H500–H506. doi: 10.1152/ajpheart.2000.278.2.H500. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res. 2007;44:382–90. doi: 10.1159/000102955. [DOI] [PubMed] [Google Scholar]
- 8.Cowley AW, Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension. 2001;37:456–61. doi: 10.1161/01.hyp.37.2.456. [DOI] [PubMed] [Google Scholar]
- 9.Rapp JP. Dahl salt-susceptible and salt-resistant rats. Hypertension. 1982;4:753–63. doi: 10.1161/01.hyp.4.6.753. [DOI] [PubMed] [Google Scholar]
- 10.Rapp JP, Wang SM, Dene H. A genetic polymorphism in the renin gene of Dahl rats cosegregates with blood pressure. Science. 1989;243:542–4. doi: 10.1126/science.2563177. [DOI] [PubMed] [Google Scholar]
- 11.Drenjancevic-Peric I, Lombard JH. Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in Dahl salt-sensitive rats on low-salt diet. Hypertension. 2005;45:687–91. doi: 10.1161/01.HYP.0000154684.40599.03. [DOI] [PubMed] [Google Scholar]
- 12.Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications [clinical conference] Hypertension. 1994;23:531–50. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- 13.Grim CE, Wilson TW, Nicholson GD, Hassell TA, Fraser HS, Grim CM, Wilson DM. Blood pressure in blacks. Twin studies in Barbados. Hypertension. 1990;15:803–9. doi: 10.1161/01.hyp.15.6.803. [DOI] [PubMed] [Google Scholar]
- 14.Manning RD, Jr, Meng S, Tian N. Renal and vascular oxidative stress and salt-sensitivity of arterial pressure. Acta Physiol Scand. 2003;179:243–50. doi: 10.1046/j.0001-6772.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 15.Moreno C, Kaldunski ML, Wang T, Roman RJ, Greene AS, Lazar J, Jacob HJ, Cowley AW., Jr Multiple blood pressure loci on rat chromosome 13 attenuate development of hypertension in the Dahl S hypertensive rat. Physiol Genomics. 2007;31:228–35. doi: 10.1152/physiolgenomics.00280.2006. [DOI] [PubMed] [Google Scholar]
- 16.Tobian L, Lange J, Iwai J, Hiller K, Johnson MA, Goossens P. Prevention with thiazide of NaCl-induced hypertension in Dahl “S” rats. Evidence for a Na-retaining humoral agent in “S” rats. Hypertension. 1979;1:316–23. doi: 10.1161/01.hyp.1.3.316. [DOI] [PubMed] [Google Scholar]
- 17.Zhu J, Drenjancevic-Peric I, McEwen S, Friesema J, Schulta D, Yu M, Roman RJ, Lombard JH. Role of superoxide and angiotensin II suppression in salt-induced changes in endothelial Ca2+ signaling and NO production in rat aorta. Am J Physiol Heart Circ Physiol. 2006;291:H929–H938. doi: 10.1152/ajpheart.00692.2005. [DOI] [PubMed] [Google Scholar]
- 18.McEwen ST, Schmidt JR, Somberg L, de la Cruz L, Lombard JH. Time-course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin II infusion in rats fed a high-salt diet. Microcirc. 2009;16:220–34. doi: 10.1080/10739680802544177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang J, Stec DE, Drummond H, Simon JS, Koike G, Jacob HJ, Roman RJ. Transfer of a salt-resistant renin allele raises blood pressure in Dahl salt-sensitive rats. Hypertension. 1997;29:619–27. doi: 10.1161/01.hyp.29.2.619. [DOI] [PubMed] [Google Scholar]
- 20.Amaral SL, Roman RJ, Greene AS. Renin gene transfer restores angiogenesis and vascular endothelial growth factor expression in Dahl S rats. Hypertension. 2001;37:386–90. doi: 10.1161/01.hyp.37.2.386. [DOI] [PubMed] [Google Scholar]
- 21.Nagata C, Takatsuka N, Shimizu N, Shimizu H. Sodium intake and risk of death from stroke in Japanese men and women. Stroke. 2004;35:1543–7. doi: 10.1161/01.STR.0000130425.50441.b0. [DOI] [PubMed] [Google Scholar]
- 22.Karppanen H, Mervaala E. Sodium intake and hypertension. Prog Cardiovasc Dis. 2006;49:59–75. doi: 10.1016/j.pcad.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Drenjancevic-Peric I, Phillips SA, Falck JR, Lombard JH. Restoration of normal vascular relaxation mechanisms in cerebral arteries by chromosomal substitution in consomic SS.13BN rats. Am J Physiol Heart Circ Physiol. 2005;289:H188–H195. doi: 10.1152/ajpheart.00504.2004. [DOI] [PubMed] [Google Scholar]
- 24.Drenjancevic-Peric I, Lombard JH. Introgression of chromosome 13 in Dahl salt-sensitive genetic background restores cerebral vascular relaxation. Am J Physiol Heart Circ Physiol. 2004;287:H957–H962. doi: 10.1152/ajpheart.01087.2003. [DOI] [PubMed] [Google Scholar]
- 25.Fredricks KT, Liu Y, Lombard JH. Response of extraparenchymal resistance arteries of rat skeletal muscle to reduced PO2. Am J Physiol. 1994;267:H706–H715. doi: 10.1152/ajpheart.1994.267.2.H706. [DOI] [PubMed] [Google Scholar]
- 26.Lombard JH, Liu Y, Fredricks KT, Bizub DM, Roman RJ, Rusch NJ. Electrical and mechanical responses of rat middle cerebral arteries to reduced PO2 and prostacyclin. Am J Physiol. 1999;276:H509–H516. doi: 10.1152/ajpheart.1999.276.2.H509. [DOI] [PubMed] [Google Scholar]
- 27.Gross V, Kurth TM, Skelton MM, Mattson DL, Cowley AW., Jr Effects of daily sodium intake and ANG II on cortical and medullary renal blood flow in conscious rats. Am J Physiol. 1998;274:R1317–R1323. doi: 10.1152/ajpregu.1998.274.5.R1317. [DOI] [PubMed] [Google Scholar]
- 28.Petersen MC, Munzenmaier DH, Greene AS. Angiotensin II infusion restores stimulated angiogenesis in the skeletal muscle of rats on a high-salt diet. Am J Physiol Heart Circ Physiol. 2006;291:H114–H120. doi: 10.1152/ajpheart.01116.2005. [DOI] [PubMed] [Google Scholar]
- 29.Lamping KG, Nuno DW, Shesely EG, Maeda N, Faraci FM. Vasodilator mechanisms in the coronary circulation of endothelial nitric oxide synthase-deficient mice. Am J Physiol Heart Circ Physiol. 2000;279:H1906–H1912. doi: 10.1152/ajpheart.2000.279.4.H1906. [DOI] [PubMed] [Google Scholar]
- 30.Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, Koller A, Kaley G. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res. 1999;85:288–93. doi: 10.1161/01.res.85.3.288. [DOI] [PubMed] [Google Scholar]
- 31.Sofola OA, Knill A, Hainsworth R, Drinkhill M. Change in endothelial function in mesenteric arteries of Sprague-Dawley rats fed a high salt diet. J Physiol. 2002;543:255–60. doi: 10.1113/jphysiol.2002.022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frisbee JC, Roman RJ, Krishna UM, Falck JR, Lombard JH. Altered mechanisms underlying hypoxic dilation of skeletal muscle resistance arteries of hypertensive versus normotensive Dahl rats. Microcirculation. 2001;8:115–27. [PubMed] [Google Scholar]
- 33.Phillips SA, Lombard JH. Chronic AT1 receptor blockade alters the mechanisms mediating hypoxic dilation in middle cerebral arteries. J Cardiovasc Pharmacol. 2005;46:706–12. doi: 10.1097/01.fjc.0000184118.76188.8c. [DOI] [PubMed] [Google Scholar]
- 34.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]