Abstract
Background
Kaempferol is one of the most important constituents in ginkgo flavonoids. Recent studies indicate kaempferol may have anti-tumor activities. The objective in this study was to determine the effect and mechanisms of kaempferol on pancreatic cancer cell proliferation and apoptosis.
Materials and Methods
Pancreatic cancer cell lines MIA PaCa-2 and Panc-1 were treated with Kampferol, and the inhibitory effects of kaempferol on pancreatic cancer cell proliferation were examined by direct cell counting, 3H-thymidine incorporation and MTS assay. Lactate dehydrogenase (LDH) release from cells was determined as an index of cytotoxicity. Apoptosis was analyzed by TUNEL assay.
Results
Upon the treatment with 70 μM kaempferol for 4 days, MIA PaCa-2 cell proliferation was significantly inhibited by 79% and 45.7% as determined by direct cell counting and MTS assay, respectively, compared with control cells (P<0.05). Similarly, the treatment with kaempferol significantly inhibited Panc-1 cell proliferation. Kaempferol treatment also significantly reduced 3H-thymidine incorporation in both MIA PaCa-2 and Panc-1 cells. Combination treatment of low concentrations of kaempferol and 5-fluorouracil (5-FU) showed an additive effect on the inhibition of MIA PaCa-2 cell proliferation. Furthermore, kaempferol had a significantly less cytotoxicity than 5-FU in normal human pancreatic ductal epithelial cells (P=0.029). In both MIA PaCa-2 and Panc-1 cells, apoptotic cell population was increased when treated with kaempferol in a concentration-dependent manner.
Conclusions
Ginkgo biloba extract kaempferol effectively inhibits pancreatic cancer cell proliferation and induces cancer cell apoptosis, which may sensitize pancreatic tumor cells to chemotherapy. Kaempferol may have clinical applications as adjuvant therapy in the treatment of pancreatic cancer.
Keywords: Kaempferol, Pancreatic cancer, Cell proliferation, Apoptosis
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States, with approximately 33,730 new cases diagnosed in 2006 and 32,300 deaths [1]. Overall, pancreatic cancer has the worst prognosis of all cancers, in which the 5-year survival rate is less than 5%. The poor survival is due to the lack of early diagnosis and no effective therapies once the metastasis has occurred. Therefore, more effective diagnostic and treatment strategies are needed [2]. Pancreatic cancer has a significant growth advantage through autocrine and paracrine mechanisms of many growth factors including epidermal growth factor (EGF) and transforming growth factor (TGF). The high rate of cell proliferation reflects the aggressiveness of pancreatic cancer. Pancreatic cancer patients with moderate or high vascular endothelial growth factor (VEGF) levels have significantly shorter survival rates than patients with low VEGF levels [3-5].
Ginkgo biloba extract has been well known for its antioxidant and anti-inflammatory activities. The major components in Ginkgo biloba are flavonoids such as quercetin, kaempferol, rutin and robinin [6,7]. Intake of beverages or food containing flavonoids has been frequently associated with reduced risk for developing various cancers including prostate, lung, stomach, and breast cancer [8-10]. It has been shown that Ginkgo biloba extract ginkgetin or isoginkgetin inhibits lymphocyte proliferation induced by concanavalin A (ConA) or lipopolysaccharides (LPS) [11]. Ginkgetin selectively inhibited the proliferation of human ovarian carcinoma cells OVCAR-3 via the induction of apoptosis in a dose dependent manner [12]. The function of flavonoids such as kaempferol and quercetin is mediated by interaction with type II estrogen receptors [13]. Kaempferol can be an estrogen agonist or growth inhibitor depending on its concentrations used. At concentrations (20–90 mM), kaempferol inhibits DNA synthesis and growth of human breast adenocarcinoma cell line MCF-7 cells [14]. Kaempferol also induces nuclear DNA degradation concurrent with lipid peroxidation [15]. However, it is not clear whether kaempferol could have any impact on pancreatic cancer cells.
In the current study, we hypothesized that kaempferol might inhibit pancreatic cancer cell growth and induce cell apoptosis. To test this hypothesis, we treated two pancreatic cancer cell lines MIA PaCa-2 and Panc-1 with different concentrations of kaempferol. Cell proliferation and apoptosis were studied with different methods. This study suggests that kaempferol may have therapeutic applications for pancreatic cancer.
MATERIALS AND METHODS
Chemicals and Reagents
Kaempferol was obtained from LKT Laboratories (St. Paul, MN). [3H]-Thymidine was purchased from Perkin Elmer (Boston, MA). CellTiter 96® Cell Proliferation Assay (MTS) Kit was purchased from Promega (Madison, WI). Apoptosis detection Kit (APO-Direct) was purchased from BD Biosciences (Franklin Lakes, NJ). Lactate dehydrogenase (LDH) release from cells was determined by using an LDH detection kit (Promega, Madison, WI).
Cell Cultures
Human pancreatic cancer cell lines (MIA PaCa-2 and Panc-1) were obtained from the American Type Culture Collection (ATCC, Rockville, MD). MIA PaCa-2 cells were cultured in DMEM with 10% fetal bovine serum (FBS) and 2.5% horse serum at 37°C with 5% CO2. Panc-1 cells were cultured in DMEM with 10% FBS at 37°C with 5% CO2. The human pancreatic ductal epithelium (HPDE) cells were provided as a generous gift from Dr. Ming-Sound Tsao from the University of Toronto, Canada. HPDE cells were cultured in keratinocyte serum-free (KSF) medium supplied with 5 ng/mL EGF and 50 g/mL bovine pituitary extract (Invitrogen, Carlsbad, CA).
Cell Proliferation Assay
Cell proliferation was assayed with three different methods including direct cell counting, [3H]-thymidine incorporation and MTS test. MIA PaCa-2 and Panc-1 cells were seeded in 96-well plates (2×103 cells/well) or 48-well plates (4×103 cells/well), and serum-starved (0% FBS) for 24 h before adding 5-fluorouracil (5-FU) (10 μg/ml) as a positive control, kaempferol (17.5 μM, 35 μM, and 70 μM) or DMSO (1:2500 dilution) as a negative control. For the drug combination treatment, we used 5-FU (1 μg/ml), kaempferol (35 μM), or 5-FU (1 μg/ml) plus kaempferol (35 μM) to treat MIA PaCa-2 cells for the MTS assay. For direct cell counting, the cells in 48-well plates were trypsinized and resuspended in 1 ml growth medium and stained with Trypan blue. Cell numbers (cells/ ml) were counted under microscope from day 0 to day 4. For [3H]-thymidine incorporation, 1 μCi/ml [3H]-thymidine was added to each well for 6 h, and the cells were incubated further for another 18 h, and then [3H]-thymidine incorporation was measured in scintillation solution using a microplate scintillation & luminescence counter (Topcounter, Packard Biosciences, Meriden, CT). For MTS assay, cell growth was assessed at 0 and 4 days after 5-FU or kaempferol treatment. Twenty μl MTS reagent mixed with 100 μl growth medium was added to each well, and incubated in 37°C for 2 h. Absorbance was recorded at 490 nm using an EL-800 universal microplate reader.
TUNEL Assay
Cell apoptosis was assessed at three days after starvation with 5-FU or kaempferol treatment. Cells were trypsinized and fixed in 1% (w/v) paraformaldehyde in PBS (pH 7.4) at the concentration of 1-2 × 106 cells/ml. After washed with PBS and stored in 70% (v/v) ice cold ethanol at −20 °C for 12h, the cells were resuspended with 1.0 ml of Wash Buffer (6548AZ), and centrifuged. The supernatant was removed by aspiration, and cell pellets were resuspended in 50 μl of the staining solution and incubated for 60 min at 37 °C. The APO-DIRECT cell samples were analyzed by flow cytometry. Two dyes were used including propidium iodide (PI) staining total DNA and FITC-dUTP labeling apoptotic cells. PI fluoresces were detected at 623 nm and FITC at 520 nm. The gating display was set at the standard dual parameter DNA doublet with the DNA area signal on the Y-axis and the DNA width on the X-axis.
LDH Release (Cytotoxicity)
LDH release from cells was determined by using an LDH detection kit (Promega) as an index of cytotoxicity. Normal HPDE were seeded 3000 cells/well one day before and cultured for 24 h with kaempferol (35 μM) or 5-FU (35 μM) in 96-well plates. DMSO was used as a control. The assay procedure was performed according to the instruction included in the kit.
Statistical Analysis
Data from direct cell counting, [3H]-thymidine incorporation, MTS and TUNEL assays were presented as mean ± SEM. Student’s t-test was used for the comparison between two groups. Inter-group differences were analyzed using one-way ANOVA for the comparison of three or more groups. A P value < 0.05 was regarded as significant.
RESULTS
Kaempferol Inhibits Cell Growth in Pancreatic Cancer In Vitro
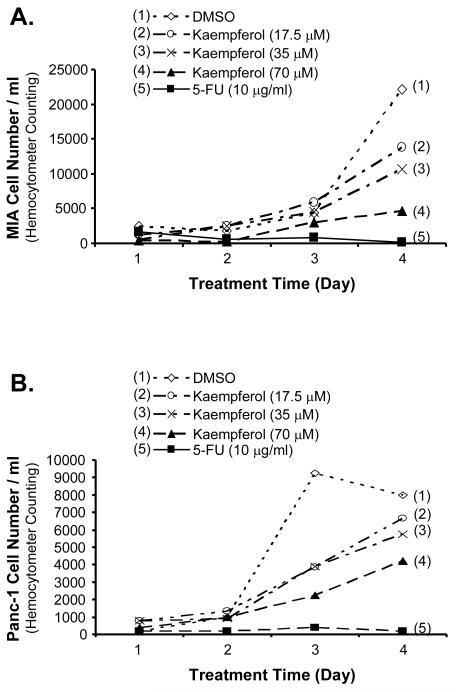
To determine the effect of kaempferol on pancreatic cancer cell growth, MIA PaCa-2 and Panc-1 cells were treated with kaempferol at 17.5 μM, 35 μM, and 70 μM for 4 days and cell numbers were directly counted. As shown in Fig. 1A and B, kaempferol significantly inhibited MIA PaCa-2 and Panc-1 cell growth. At day 4, low concentration of kaempferol (17.5 μM) decreased MIA PaCa-2 and Panc-1 growth to 62% and 83%, respectively, compared with DMSO negative controls (100%). With the addition of 35 μM of kaempferol, the MIA PaCa-2 and Panc-1 cell growth was decreased to 48% and 72%, respectively, compared with controls (P < 0.05). MIA PaCa-2 and Panc-1 cell growth in the presence of 70 μM of kaempferol was decreased to 21% and 53%, respectively, compared with the control cells (P < 0.01). As a positive control, 5-FU (10 μg/ml) treatment significantly reduced cell numbers of MIA PaCa-2 and Panc-1 cells to below 10% compared with the DMSO negative control (P < 0.01).
FIG. 1.
Effects of kaempferol on cell growth of MIA CaPa-2 and Panc-1 pancreatic cancer cells. MIA PaCa-2 and Panc-1 cells were seeded in 48-well plates (4×103 cells/well) and serum-free DMEM starved for 24 h before treated with 5-FU (10 μg/ml) as a positive control, kaempferol (17.5 μM, 35 μM, and 70 μM) or DMSO (1:2500 dilution) as a negative control. The cells in each well were trypsinized, resuspended in 1 ml growth medium, stained with Trypan blue, and directly counted under a phase contract microscope. Kaempferol inhibited MIA PaCa-2 (A) and Panc-1 (B) cell growth in a concentration-dependent manner from day 0 to day 4.
Kaempferol Decreases [3H]-Thymidine Incorporation in Pancreatic Cancer Cells
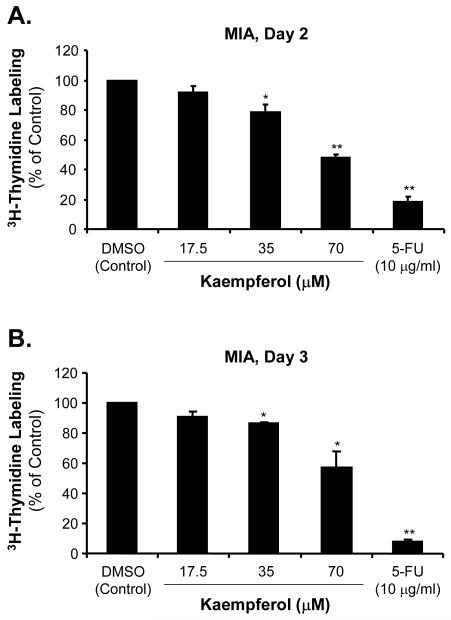
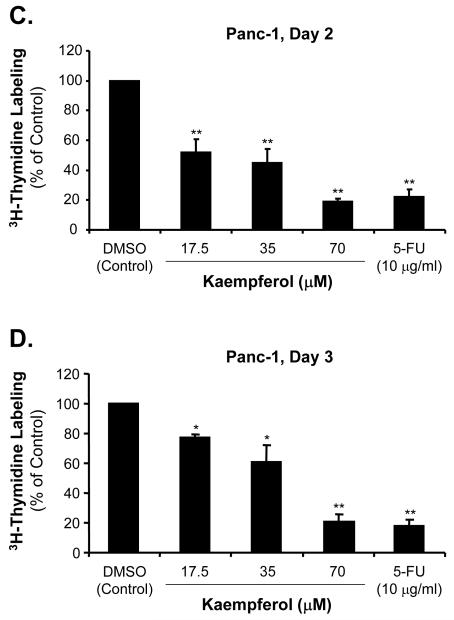
The effects of kaempferol on cell proliferation in pancreatic cancer were also detected by [3H]-thymidine incorporation assay. MIA PaCa-2 and Panc-1 cells were incubated with kaempferol at various concentrations for 2 and 3 days before adding [3H]-thymidine. As shown in Fig. 2A and C, at day 2, low concentration of kaempferol (17.5 μM) caused a decrease of MIA PaCa-2 and Panc-1 [3H]-thymidine incorporation to 91% and 52%, respectively, compared with DMSO treated control cells (100%). With the addition of 35 μM of kaempferol, the MIA PaCa-2 and Panc-1 [3H]-thymidine incorporation was decreased to 79% and 45%, respectively, compared with controls (P < 0.05). The [3H]-thymidine incorporation in the presence of 70 μM of kaempferol was significantly decreased to 48% and 19% in MIA PaCa-2 and Panc-1 cells, respectively, compared with the control cells (P < 0.01).
FIG. 2.
Effects of kaempferol on [3H]-thymidine incorporation in MIA CaPa-2 and Panc-1 pancreatic cancer cells. MIA CaPa-2 and Panc-1 cells were serum-starved and then treated with the indicated concentrations of kaempferol, 5-FU or DMSO for 2 and 3 days, respectively, and labeled with [3H]-thymidine at 1 μCi/ml for 6 h. [3H]-thymidine incorporation was measured in scintillation solution. Data are expressed as mean±SEM of triplicate values from two separate experiments. *P < 0.05 and **<0.01 as compared with DMSO controls. [3H]-thymidine incorporation assay was performed for MIA CaPa-2 cells at day 2 (A) and day 3 (B), and for Panc-1 cells at day 2 (C) and day 3 (D) after different concentrations of kaempferol treatment.
When MIA PaCa-2 and Panc-1 cells were treated with different concentrations of kaempferol for 3 days, [3H]-thymidine incorporation was determined as shown in Fig. 2B and D. Low concentration of kaempferol (17.5 μM) caused a decrease of MIA PaCa-2 and Panc-1 [3H]-thymidine incorporation to 91% and 80%, respectively, compared with control DMSO treated cells (100%). The middle concentration of kaempferol (35 μM) caused the MIA PaCa-2 and Panc-1 [3H]-thymidine incorporation decreasing to 90% and 61%, respectively, compared with controls (P < 0.05). In the presence of 70 μM kaempferol, MIA PaCa-2 and Panc-1 [3H]-thymidine incorporation was significantly decreased to 60% and 21%, respectively, compared with the control cells (P < 0.01).
Kaempferol Decreases Pancreatic Cancer Cell Proliferation as Detected by MTS Assay
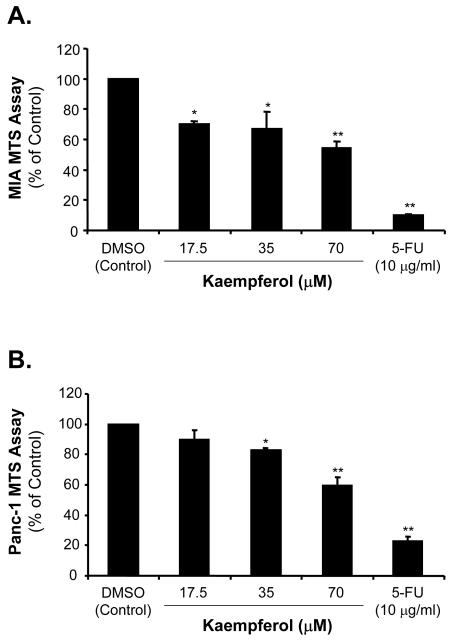
We confirmed the cell proliferation by MTS assay, which detects mitochondrial enzyme activity correlating with the numbers of living cells. We found that in the presence of 17.5 μM of kaempferol, cell proliferation of MIA PaCa-2 and Panc-1 was decreased to 70% and 90%, respectively, compared with the control cells (Fig. 3A and B). With the addition of 35 μM of kaempferol, the MIA PaCa-2 and Panc-1 cell proliferation was decreased to 67% and 83%, respectively, compared with controls (P < 0.05). MIA PaCa-2 and Panc-1 cell proliferation in the presence of 70 μM of kaempferol was significantly decreased to 54% and 60%, respectively, compared with the control cells (P < 0.01). These data are consistent with [3H]-thymidine incorporation assay, and indicate that kaempferol significantly inhibits MIA PaCa-2 and Panc-1 cell proliferation.
FIG. 3.
Effects of kaempferol on MTS in MIA PaCa-2 and Panc-1 pancreatic cancer cells. After starvation and 5-FU or kaempferol treatment, MIA PaCa-2 and Panc-1 cell proliferation was assessed in 96-well plate at day 0 and day 4 by MTS assay. Twenty μl MTS reagent (Promega) mixed with 100 μl growth medium was added to each well, and incubated in 37 °C for 2 h. Absorbance was recorded at 490 nm using an EL-800 universal microplate reader. The effects of the kaempferol on MIA PaCa-2 (A) and Panc-1 (B) cell proliferation were shown.
Kaempferol Increases Apoptosis of Pancreatic Cancer Cells
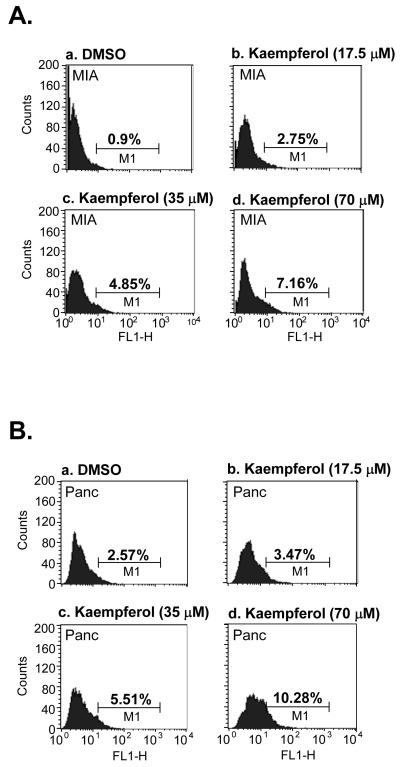
To further study the action of growth inhibition by kaempferol in pancreatic cancer cells, we examined the apoptosis population in MIA PaCa-2 and Panc-1 cells upon kaempferol treatment. MIA PaCa-2 and Panc-1 cells were incubated with increasing concentrations of kaempferol (17.5, 35 and 70 μM) in growth medium for 3 days. Apoptotic cells were determined by TUNEL assay as shown in Fig. 4A and B. In MIA PaCa-2 cells, 0.9%, 2.75%, 4.85% and 7.16% of apoptotic cell populations were observed in DMSO, 17.5, 35 and 70 μM kaempferol treated groups, respectively (P < 0.05, Fig. 4A). In Panc-1 cells, 2.57%, 3.47%, 5.51% and 10.28% of apoptotic cell populations were found in DMSO, 17.5, 35 and 70 μM kaempferol treated groups, respectively (P < 0.05, Fig. 4B). These data demonstrate that kaempferol is able to increase the apoptosis in pancreatic cancer cells in vitro.
FIG. 4.
Effects of kaempferol on apoptosis of MIA CaPa-2 and Panc-1 pancreatic cancer cells. Cell apoptosis was assessed by using TUNEL assay at 3 days after 5-FU or kaempferol treatment. Cells were fixed in 1% (w/v) paraformaldehyde and stained with FITC-dUTP for 60 min at 37 °C. A. Kaempferol increased MIA CaPa-2 (A) and Panc-1 (B) cell apoptosis.
Combination treatment of Kaempferol with 5-FU enhances their inhibitory effects on Pancreatic Cancer Cell Prolfieration
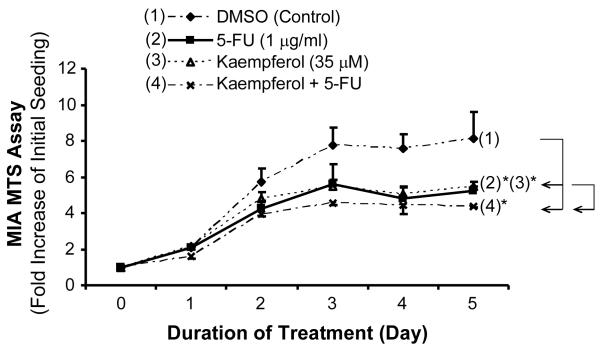
We further determined the effect of combination treatment of kaempferol with 5-FU on MIA PaCa-2 cell proliferation by MTS assay. We found that with the addition 1 μg/ml (7.7 μM) of 5-FU or 35 μM of kaempferol, cell proliferation of MIA PaCa-2 was decreased to 64.6% and 67.5%, respectively, by 5 days, compared with the control cells (Fig. 5). In the presence of combination of low concentrations of 5-FU (1 μg/ml = 7.7 μM) with kaempferol (35 μM), the MIA PaCa-2 cell proliferation was decreased to 54.1% compared with controls (P < 0.05). These data indicate that the combination treatment of kaempferol with low concentration of 5-FU has an additive effect on the inhibition of MIA PaCa-2 cell proliferation.
FIG. 5.
Effects of combination treatment of kaepferol and 5-FU on MTS in MIA PaCa-2 pancreatic cancer cells. After starvation, MIA PaCA-2 cells were treated with 5-fluorouracil (5-FU) (1 μg/ml), kaempferol (35 μM), or 5-FU (1 μg/ml) plus kaempferol (35 μM), and cell proliferation was assessed in 96-well plate from day 0 and day 5 by MTS assay. Twenty μl MTS reagent (Promega) mixed with 100 μl growth medium was added to each well, and incubated in 37 °C for 1.5 h. Absorbance was recorded at 490 nm using an EL-800 universal microplate reader. The effects of the 5-FU (2), kaempferol (3) and Kaempferol plus 5-FU (4) on MIA PaCa-2 cell proliferation were shown. Data are expressed as mean±SEM of triplicate values. *P < 0.05.
Kaempferol has a lower cytotoxicity than 5-FU in HPDE
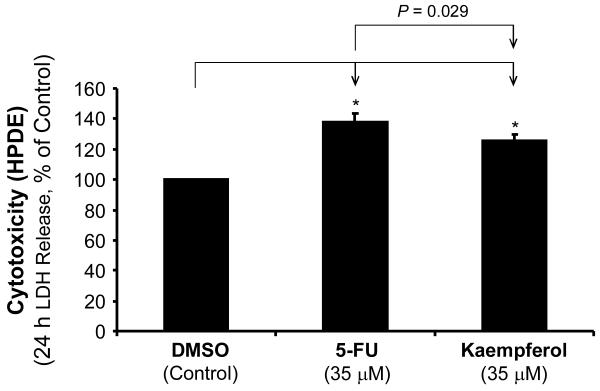
To compare the cytocoxicity of kaempferol with 5-FU to normal cells, we measured LDH release from HPDE cells treated with the same molar concentration (35 μM) of 5-FU and Kaempferol for 24 h by using an LDH detection kit (Promega) as an index of cytotoxicity. Kaempferol (35 μM) showed a significantly less cytotoxicity than 5-FU in HPDE (P = 0.029) (Fig. 6). These data confirmed that kaempferol was less toxic to normal cells compared with the traditional chemotherapy agent 5-FU.
FIG. 6.
Effects of kaempferol with 5-FU on LDH release (cytotoxicity) from HPDE cells. The cytotoxicity of kaempferol and 5-FU in normal human pancreatic ductal epithelial cells (HPDE) was evaluated by LDH release assay. HPDE cells were cultured for 24 h with kaempferol (35 μM) or 5-FU (35 μM). The cytotoxicity index in kaempferol-treated cells was significantly lower than that in 5-FU-treated cells. Data are expressed as mean±SEM of triplicate values. *P < 0.05.
DISCUSSION
In the current study, we demonstrate that kaempferol effectively inhibits pancreatic cancer cell growth and induces cell apoptosis in vitro in a concentration-dependent manner. Kaempferol also showed an additive effect with the most commonly used chemiotherapy agent 5-FU and has significantly less cytotoxicity than 5-FU. These findings are important because kaempferol could have great potential to become a new therapeutic agent for pancreatic cancer.
Pancreatic adenocarcinoma is characterized by poor prognosis, late diagnosis and lack of response to conventional therapies. New chemotherapeutic agents would be extremely beneficial for the control of unresectable cancer and metastatic lesions of pancreatic cancer [16]. Ginkgo biloba extract kaempferol has been shown to provide antiproliferative effects in different systems based on its striking inhibition of diverse cellular events associated with tumor pathogenesis [17]. The inhibitory effect of kaempferol on cell proliferation may be attributed by its antioxidation capacity and cytotoxic action. Furthermore, kaempferol inhibits the activity of several enzymes involved in cell growth and signal transduction pathway including cAMP-phosphodiesterase and tyrosine kinase [18], cdc25 phosphatase [17], DNA topoisomerase II, topoisomerase I catalyzed DNA religation [19], proline-directed protein kinase fatty acid in human prostate carcinoma cells, and myosin light chain kinase [20]. Several studies on kaempferol have been reported including the activation of human estrogen receptors and unique signaling pathways. One of the mechanisms proposed to explain the activity of kaempferol to lower the risk of breast cancer is its ability to bind and activate human estrogen receptor alpha (ERalpha) and human estrogen receptor beta (ERbeta). Harris DM et al. [21] tested nine phytoestrogens including kaempferol for their ability to transactivate ERalpha or ERbeta in mammary adenocarcinoma (MCF-7) cells. Kaempferol showed transactivation of ERalpha- and ERbeta-induced transcription, with a stronger activation of ERbeta. Brusselmans K et al. [22] found five flavonoids, luteolin, quercetin, kaempferol, apigenin and taxifolin, markedly inhibited cancer cell lipogenesis with their ability to inhibit fatty acid synthase (FAS, a key lipogenic enzyme overexpressed in many human cancers). In both prostate and breast cancer cells, a remarkable dose-dependent response was observed between flavonoid-induced inhibition of fatty acid synthesis, inhibition of cell growth, and induction of apoptosis. The results indicated that the potential of flavonoids to induce apoptosis in cancer cells is strongly associated with their FAS inhibitory properties, by which the flavonoid compounds may apply their cancer-preventive and anti-tumor effects. In addition, Nguyen TT et al. [20] investigated kaempferol effects on cellular growth and signal transduction pathways in human lung cancer cell line A549. Kaempferol treatment resulted in a dose- and time-dependent reduction in cell viability and DNA synthesis. It led to an increase in Bax and Bad pathway. Expression of Bcl-2 and Bcl-xL was inhibited in a dose-dependent manner, and Akt-1 and phosphorylated Akt-1 were also inhibited, while mitogen-activated protein kinase (MAPK) was activated. They demonstrated that the inactivation of Akt-1 and alteration of Bcl-2 family of proteins are not sufficient for kaempferol to induce apoptosis. Activation of MEK-MAPK is required for kaempferol-induced cell death machinery in A549 cells. Furthermore, Gopalakrishnan A et al. [23] demonstrated that most flavonoids inhibited AP-1 activity at higher concentrations and the ERK pathway which played an important role in kaempferol-induced AP-1 activity in human prostate cancer cells (PC3). However, there are no reports about the effects of kaempferol on pancreatic cancer cells. In the current study, we, for the first time, demonstrated kaempferol is a potent inhibitor of pancreatic cancer cells with relatively low toxicity to normal cells. Since kaempferol has different mechanisms of action and lower toxicity to normal cells as compared with other clinically used chemotherapy agents such as 5-FU, it could be used as adjuvant therapy in combination with 5-FU or gemcitabine in pancreatic cancer patients.
In the current study, the effects of kaempferol on MIA CaPa-2 and Panc-1 cell viability and proliferation were determined by using three different methods including direct cell counting, [3H]-thymidine incorporation and MTS assay. For cell counting, trypan blue was used to stain dead or dying cells, but not live cells. The number of live cells was directly counted. Tritiated thymidine method was used to quatitate the incorporation of [3H]-thymidine into DNA during cell growth as an index of DNA synthesis. MTS test was used to measure the reduction mitochondrial metabolism rate of the tetrazolium salt compound MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) to formazan. MTS assay is also correlated well with the number of live cells [24]. Kaempferol significantly inhibited cell proliferation of these cell lines with a peak inhibition at 70 μM in four days. While kaempferol showed more potent growth inhibition in MIA PaCa-2 cells than in Panc-1 cells. Recently, Ginkgo biloba extract has been reported to affect gene expression related to cell growth [25]. Alexandrakis M et al. found that certain flavonoids with a flavone structure inhibit human leukemic mast cells (HMC-1) proliferation using cell number counting method and lead to accumulation of secretary granules with variable electron granular content [26]. Importantly, we demonstated that the combination of low concentration of 5-FU and kaempferol had an additive effect on inhibition of MIA PaCa-2 pancreatic cancer cell proliferation, and kaempferol had a significantly less cytotoxicity than 5-FU, thereby providing a rationale for the clinical use of kaempferol as adjuvant therapy for pancreatic cancer.
Normal cell growth is maintained by the balance between cell proliferation and cell death. Apoptosis or programmed cell death is a central regulator of tissue homeostasis [27]. Because chemotherapy and radiotherapy act primarily by inducing apoptosis, defects in the apoptotic pathway can cause cancer cell resistance [28]. Nguyen et al. demonstrated similar anti-proliferation and apoptosis induction effects in A549 lung cancer cells treated with kaempferol at concentrations similar to those used in the current study. They found that 17.5, 35, 52.5 and 70 μM kaempferol treatment caused a time- and dose-dependent reduction in DNA synthesis and cell proliferation [20]. Our current study also clearly demonstrated that apoptosis may account, at least in part, for the anti-tumor activity of kaempferol. We found that kaempferol at concentrations of 17.5, 35 and 70 μM induced 3-, 5- and 8-fold increase in MIA PaCa-2, and 1.4-, 2- and 4-fold increase in Panc-1 cells of the cell apoptosis compared with the DMSO treated control cells.
In conclusion, we demonstrate that kaempferol inhibits pancreatic cancer cell growth and induces apoptosis. These results are of significance because kaempferol, a natural compound, may have clinical applications in the treatment of pancreatic cancer in both direct inhibitory effects and enhancing effects of other chemotherapy or radiotherapy with a low toxicity. Combination therapy of kaempferol with chemotherapy agents such as 5-FU or gemcitabine may provide a new and more effective strategy for the treatment of pancreatic cancer.
ACKNOWLEDGEMENTS
This work is partially supported by research grants from the National Institutes of Health (NIH) Grants HL065916, HL072716, EB002436, HL083471 (C. Chen), RO1 DE15543, and R21 AT003094 (Q. Yao), and from American Cancer Society Grant #IRG-93-034-09 (M. Li).
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Cohenuram M, Saif MW. Epidermal growth factor receptor inhibition strategies in pancreatic cancer: past, present and the future. JOP. 8:4–15. 20079. [PubMed] [Google Scholar]
- 3.Ikeda N, Nakajima Y, Sho M, Adachi M, Huang CL, Iki K, Kanehiro H, Hisanaga M, Nakano H, Miyake M. The association of K-ras gene mutation and vascular endothelial growth factor gene expression in pancreatic carcinoma. Cancer. 2001;92:488–499. doi: 10.1002/1097-0142(20010801)92:3<488::aid-cncr1347>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M, Nakano H, Miyake M. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79:1553–1563. doi: 10.1038/sj.bjc.6690248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knoll MR, Rudnitzki D, Sturm J, Manegold BC, Post S, Jaeger TM. Correlation of postoperative survival and angiogenic growth factors in pancreatic carcinoma. Hepatogastroenterology. 2001;48:1162–1165. [PubMed] [Google Scholar]
- 6.Anton R. Flavonoids and traditional medicine. Prog Clin Biol Res. 1988;280:423–439. [PubMed] [Google Scholar]
- 7.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 8.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 9.Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 10.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr. Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Choi JH, Son KH, Chang HW, Kang SS, Kim HP. Suppression of mouse lymphocyte proliferation in vitro by naturally-occurring biflavonoids. Life Sci. 1995;57:551–558. doi: 10.1016/0024-3205(95)00305-p. [DOI] [PubMed] [Google Scholar]
- 12.Su Y, Sun CM, Chuang HH, Chang PT. Studies on the cytotoxic mechanisms of ginkgetin in a human ovarian adenocarcinoma cell line. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:82–90. doi: 10.1007/s002100000240. [DOI] [PubMed] [Google Scholar]
- 13.Ranelletti FO, Ricci R, Larocca LM, Maggiano N, Capelli A, Scambia G, Benedetti-Panici P, Mancuso S, Rumi C, Piantelli M. Growth-inhibitory effect of quercetin and presence of type-II estrogen-binding sites in human colon-cancer cell lines and primary colorectal tumors. Int J Cancer. 1992;50:486–492. doi: 10.1002/ijc.2910500326. [DOI] [PubMed] [Google Scholar]
- 14.Sathyamoorthy N, Wang TT, Phang JM. Stimulation of pS2 expression by diet-derived compounds. Cancer Res. 1994;54:957–961. [PubMed] [Google Scholar]
- 15.Sahu SC, Gray GC. Kaempferol-induced nuclear DNA damage and lipid peroxidation. Cancer Lett. 1994;85:159–164. doi: 10.1016/0304-3835(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 16.Ding XZ, Tong WG, Adrian TE. Cyclooxygenases and lipoxygenases as potential targets for treatment of pancreatic cancer. Pancreatology. 2001;1:291–299. doi: 10.1159/000055827. [DOI] [PubMed] [Google Scholar]
- 17.Aligiannis N, Mitaku S, Mitrocotsa D, Leclerc S. Flavonoids as cycline-dependent kinase inhibitors: inhibition of cdc 25 phosphatase activity by flavonoids belonging to the quercetin and kaempferol series. Planta Med. 2001;67:468–470. doi: 10.1055/s-2001-15807. [DOI] [PubMed] [Google Scholar]
- 18.Landolfi R, Mower RL, Steiner M. Modification of platelet function and arachidonic acid metabolism by bioflavonoids. Structure-activity relations. Biochem Pharmacol. 1984;33:1525–1530. doi: 10.1016/0006-2952(84)90423-4. [DOI] [PubMed] [Google Scholar]
- 19.Boege F, Straub T, Kehr A, Boesenberg C, Christiansen K, Andersen A, Jakob F, Köhrle J. Selected novel flavones inhibit the DNA binding or the DNA religation step of eukaryotic topoisomerase I. J Biol Chem. 1996;271:2262–2270. doi: 10.1074/jbc.271.4.2262. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen TT, Tran E, Ong CK, Lee SK, Do PT, Huynh TT, Nguyen TH, Lee JJ, Tan Y, Ong CS, Huynh H. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J Cell Physiol. 2003;197:110–121. doi: 10.1002/jcp.10340. [DOI] [PubMed] [Google Scholar]
- 21.Harris DM, Besselink E, Henning SM, Go VL, Heber D. Phytoestrogens induce differential estrogen receptor alpha- or Beta-mediated responses in transfected breast cancer cells. Exp Biol Med (Maywood) 2005;230:558–568. doi: 10.1177/153537020523000807. [DOI] [PubMed] [Google Scholar]
- 22.Brusselmans K, Vrolix R, Verhoeven G, Swinnen JV. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem. 2005;280:5636–5645. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- 23.Gopalakrishnan A, Xu CJ, Nair SS, Chen C, Hebbar V, Kong AN. Modulation of activator protein-1 (AP-1) and MAPK pathway by flavonoids in human prostate cancer PC3 cells. Arch Pharm Res. 2006;29:633–644. doi: 10.1007/BF02968247. [DOI] [PubMed] [Google Scholar]
- 24.Gieni RS, Li Y, HayGlass KT. Comparison of [3H]thymidine incorporation with MTT- and MTS-based bioassays for human and murine IL-2 and IL-4 analysis. Tetrazolium assays provide markedly enhanced sensitivity. J Immunol Methods. 1995;187:85–93. doi: 10.1016/0022-1759(95)00170-f. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Pretner E, Shen L, Drieu K, Papadopoulos V. Common gene targets of Ginkgo biloba extract (EGb 761) in human tumor cells: relation to cell growth. Cell Mol Biol. 2002;48:655–662. [PubMed] [Google Scholar]
- 26.Alexandrakis M, Letourneau R, Kempuraj D, Kandere-Grzybowska K, Huang M, Christodoulou S, Boucher W, Seretakis D, Theoharides TC. Flavones inhibit proliferation and increase mediator content in human leukemic mast cells (HMC-1) Eur J Haematol. 2003;71:448–454. doi: 10.1046/j.0902-4441.2003.00167.x. [DOI] [PubMed] [Google Scholar]
- 27.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 28.Westphal S, Kalthoff H. Apoptosis: targets in pancreatic cancer. Mol Cancer. 2003;2:6. doi: 10.1186/1476-4598-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]