Abstract
Obesity, smoking, and conduct problems have all been associated with decrements in brain function. However, their additive and interactive effects have rarely been examined. To address the deficiency, we studied P300a and P300b electroencephalographic potentials in 218 women grouped by the presence versus absence of: (1) a BMI ≥30 kg/m2; (2) recent smoking; and (3) ≥2 childhood conduct problems. Analyses revealed smaller P300a and P300b amplitudes over the posterior scalp among recent smokers versus nonsmokers. No corresponding group differences were found in P300 latencies or frontal scalp amplitudes. The most interesting analysis result was an interaction between conduct problems and obesity limited to the frontally-generated P300a component: its latency was significantly greater in women with both attributes than in those with either or neither attribute. An exploratory ANOVA, substituting the genotype of a GABRA2 SNP for conduct problems, also demonstrated the interaction. It is hypothesized that conduct problems, and a conduct-problem-associated GABRA2 genotype, decrease the age-of-onset and/or increase the lifetime duration of obesity. As a result, they may potentiate the adverse effects of obesity on frontal white matter and thereby increase P300a latency. Smoking may affect brain function by a different mechanism to reduce posterior scalp P300a and P300b amplitudes while preserving frontal scalp P300a latency and amplitude.
Keywords: GABRA2, gene, smoking, obesity, Conduct Disorder
Introduction
The adverse effects of cigarette smoking and obesity on brain structure (1–3) and function (4) have been discussed in the literature for many years. Both disorders predict stroke (5, 6) and other forms of cerebrovascular disease (7, 8). They are also associated with neurophysiological (9, 10) and cognitive impairments (11, 12) among subjects with no overt signs or symptoms.
The present study is unique in focusing on a childhood behavior control disorder, Conduct Disorder (CD), known to increase risk for both smoking (13, 14) and obesity (15). It is especially unique in examining the degree to which CD may enhance the adverse neurovascular (5, 8, 16) and neurophysiological (2, 3, 9, 17–19) effects of these disorders. Several models explain how the enhancement may occur:
In the first model, the contribution of CD to brain dysfunction is hypothesized to be independent of, and add to, the effects of the other disorders. Indeed, among subjects with no history of smoking or weight problems, CD is associated with decrements in white and grey matter integrity (20, 21) and cognitive components (e.g., P300) of the evoked electroencephalographic response (22, 23). Various localization methods, employing either region of interest (21, 24) or other (22) techniques, suggest greater structural and functional decrements in the frontal brain. Obesity and smoking may affect these as well as other brain regions (17, 25).
In the second model, the disorders are hypothesized to interact. Support for this model derives from demonstrations of an earlier age-of-onset of smoking (14, 26) or weight control problems (27) as well as greater resistance to behavior change (28) among subjects with a childhood history of CD. As a result, the duration and severity of smoking or obesity may increase with a resulting increase in adverse vascular and neural effects.
In the third model, an interaction is also hypothesized. However, within this model, CD, smoking, and obesity are viewed as overlapping expressions of a common behavioral phenotype (e.g., impulsivity) with common neurophysiological and genetic origins. This model is consistent with recent findings from linkage or candidate gene analyses wherein common genetic etiologies for smoking and CD (29, 30), obesity and substance dependence (31), and substance dependence and electroencephalographic (EEG) differences (32–34), have been suggested.
One goal of the present study was to discern which of these three models best fit the data.
A second goal was to determine if the decrement in brain function associated with CD problems was also associated with a variant of the GABRA2 gene previously shown to promote risk for CD (35). More specifically, we asked whether CD problems and GABRA2 genotype would similarly enhance the changes associated with smoking or obesity. To accomplish the goal, an exploratory analysis was conducted in which the presence versus absence of 2 or more problems was replaced by presence versus absence of the genotype previously associated with conduct problems.
To assess brain function, we employed two highly reliable neurophysiological indices that can, unlike functional magnetic resonance imaging, be practically employed to study subjects whose body weights (>300 lbs) or trunk diameters (> 60 cm) exceed the physical limits of many research scanners. The indices were the P300a and P300b components of the event related electroencephalographic potential (ERP). The latencies of these components are inversely correlated with the integrity of white matter pathways connecting their generators (36) whereas their amplitudes are more closely related to the gray matter volumes of the generators themselves (37, 38). P300a is generated frontally. In contrast, the P300b has a diffuse distribution of frontal and non-frontal generators (39–41).
The P300a and P300b analyses were conducted in a large sample (N=218) of subjects who already possessed a risk factor for vascular disease and neurophysiological impairment -- a previous or current diagnosis of alcohol dependence — and therefore at greater risk than non-alcoholics for achieving a clinically significant level of impairment. The focus on females was inspired by their higher risk for obesity [i.e., 33.2% women vs. 27.6% men: (42)], especially in samples with adequate racial/ethnic minority representation, as well as the female bias in the importance of conduct problems in promoting obesity (43). We acknowledge that limiting the analysis in this manner limits the generality of the findings. However, it does focus the interpretation of the results and provides supporting data for future, larger studies in which many additional grouping factors could be examined.
Method
Subject Recruitment and Screening
The subjects were sampled from a larger data set generated by the multi-site Collaborative Study on the Genetics of Alcoholism [COGA (44)]. They resided within 150 miles of one of COGA’s six participating sites (Indiana University School of Medicine; SUNY Health Science Center, Brooklyn; University of California at San Diego; University of Connecticut Health Center; University of Iowa Health Center; and Washington University School of Medicine). All provided written, informed consent prior to participation. They were financially compensated for their time and effort.
Subjects were recruited and evaluated identically across the participating sites (45). At each site, male and female alcohol-dependent probands and their family members were recruited, as well as members of control families. The present analyses were limited to a subset of the former sample: women from families densely-affected by alcoholism who themselves met DSM-IIIR criteria for a lifetime diagnosis of alcohol dependence. The Semi-Structured Assessment for the Genetics of Alcoholism [SSAGA (46, 47)] was used to establish this diagnosis as well as the presence of CD problems and a smoking history. The SSAGA and other instruments were also used to identify and exclude individuals with psychiatric (i.e., mania, psychosis) or medical conditions (i.e., acute intoxication, seizures, stroke, meningitis, heart or liver disease, past year pregnancy) that would confound the interpretation of the results.
The subjects included in the initial analysis, examining the independent and interactive effects of smoking, obesity, and childhood conduct problems, were 218 women. They were 18–66 years of age, of mixed race and ethnicity, and drawn from separate families. For the secondary analysis of the effects of the GABRA2 genotype and obesity, 110 women, 19–65 years of age, from separate families were included. The secondary analysis was limited to European Americans to eliminate admixture artifact.
P300 Measurement
The subject was seated in a sound-attenuated chamber and instructed to focus attention on the center of a computer screen. She wore a fitted electrode cap (Electro-Cap International, Eaton, OH) containing 21 leads arranged in the montage of the International 10–20 System. The reference electrode was placed on the tip of the nose. A forehead electrode served as ground. Vertical and horizontal eye movements were monitored using electrodes situated above and below the left eye.
EEG activity was recorded during a task involving a minimum of 200 visual stimuli presented at a rate of 1 every 2.2 seconds. Each stimulus subtended a visual angle of 2.5°. Stimulus duration was 60 ms.
Three types of visual stimuli were presented during the task: the letter “X”, a square, and colored geometric figures that changed with each presentation. Their respective probabilities of occurrence were 0.125, 0.75, and 0.125. The subject was instructed to press a response key when she detected the “X” (target stimulus), and ignore the square (frequent nontarget) and geometric figures (novel nontarget). Response speed was emphasized, but not at the cost of accuracy. The task terminated automatically after a minimum of 25 target, 150 frequent nontarget, and 25 novel nontarget artifact-free trials had been acquired. Trials with response times greater than 1000 ms were rejected.
EEG activity was amplified by a factor of 10 K (Sensorium EPA-2 Electrophysiology Amplifiers), filtered to pass frequencies between 0.02 and 50 Hz, and sampled at a rate of 256 Hz. EEG epochs from 187 ms preceding to 750 ms following the onset of each stimulus were retained by the computer and processed offline through a 32-Hz low-pass digital filter. Epochs with eye movement or EEG voltage deviations > 73.3 microvolts (peak-to-peak) were excluded from analysis. Remaining epochs were formed into time-point averages sorted by stimulus type.
P300 ERP components elicited by target (P300b) and novel nontarget (P300a) stimuli were identified within each averaged epoch. P300 was defined at each electrode as the highest positive peak within a time range of 275–575 ms after stimulus onset. P300 amplitude was measured as the voltage difference between the peak and the average voltage during the prestimulus period. P300 latency was the millisecond difference between stimulus onset and the peak.
Genetic Analyses
Detailed information about marker locations selected by COGA, and marker genotype frequencies, is presented in recent publications (34, 35). The single nucleotide polymorphism at rs279871 was specifically chosen for analysis because it has been previously associated with risk for EEG differences, conduct problems, and substance dependence.
Subjects were assigned to low or high risk genotype groups. The low risk genotype groups possessed less than 2 copies of the “A” allele previously associated with risk. The high risk genotype groups possessed 2 copies of this “high” risk allele.
Statistical Analyses
Four sets of analyses were performed. The first set examined the effects of obesity (OB-: BMI < 30 kg/m2, OB+: BMI ≥30), childhood Conduct Disorder problems (48, 49) (CP− : < 2 problems, CP+: ≥2 problems), and smoking (9, 50) (Smoking+: never or former, Smoking+: daily smoking during past year) in a simple 2 × 2 × 2 factorial design. The 8 groups of subjects were initially compared on background characteristics using Pearson’s χ2 Test to evaluate group equivalence on categorical variables and a three- factor ANOVA for continuous variables.
The next step within this analysis set involved an examination of differences among the groups in P300a and P300b amplitudes and latencies using a three factor MANCOVA. The analyses were not performed separately for each electrode site. Instead, a data reduction method was employed to reduce Type 1 error. A factor analysis was performed on P300 amplitude and latency across the sites to find topographic regions of homogeneity which could be reduced to a single score. A principal components analysis followed by varimax rotation yielded two factors (22) which explained most of the variance across the sites. The anterior sites exhibited greater loadings on the first factor. Sites around and posterior to the central sulcus exhibited greater loadings on the second factor. Highly similar factor structures were obtained for P300a and P300b. We therefore chose to calculate average scores representing P300 amplitudes and latencies within anterior (F3, F4, F7, F8, Fz, C3, C4, and Cz) and posterior (P3, P4, Pz, P7, P8, O1, and O2) regions. These regional values were incorporated into the MANCOVA as separate variates and individually tested with univariate ANCOVAs. Age and the total lifetime number of alcohol, cocaine, and opiate dependence were included as covariates in all analyses because they are known to affect P300.
The second analysis set was designed to validate the significant results revealed by the prior set. More specifically, it discarded the dichotomous variable, obesity, and the MANCOVA/ANCOVA design. The analysis instead tested partial correlations between BMI expressed as a continuous variable and selected P300 measures. Separate correlations were computed for the CP− and CP+ groups. Age and the total lifetime number of alcohol, cocaine, and opiate dependence were again included as covariates.
The third set of analyses was limited to European-American, alcohol-dependent women. These analyses asked whether CD problems and GABRA2 genotype similarly enhanced the association of obesity with P300. Two separate MANCOVAs were performed on P300a and P300b amplitudes and latencies within anterior and posterior regions. The first MANCOVA examined the effects of CP and OB in a 2 × 2 factorial with age, the total number of alcohol and drug dependence symptoms, and the duration of cigarette smoking as covariates. The remaining MANCOVA substituted GABRA2 genotype for the CP risk factor. Significant effects revealed by the MANCOVAs were further evaluated with univariate ANCOVAs.
The final analysis set employed path analysis methods to illuminate and clarify the interactive relationships revealed in analysis set #3 between conduct problems, obesity, and frontal P300a latency. Using Mplus™(51), we constructed and tested two alternative models. The first model hypothesized a causal chain in which the number of conduct problems predicts BMI which, in turn, predicts P300a latency. The second model viewed P300a latency as a phenotypic marker indicating risk for obesity rather than, as in Model #1, a physiological index of the neural damage caused by it. Its causal chain began with frontal P300a latency predicting conduct problems, which then predicts BMI. Conventional measures of model fit, i.e., discrepancy χ2, Root Mean Square Error of Approximation (RMSEA), and Comparative Fit Index (CFI), as well as standardized path coefficients, were computed.
Results
Analysis Set #1: Obesity × Conduct Problems × Smoking
Background Characteristics
Table 1 summarizes the demographic, psychological, and medical characteristics of each subject group. Formal comparisons of the groups revealed few significant differences. The only unexpected difference was a small but statistically significant 4.2 year age difference between the OB+ and OB− groups.
Table 1.
Background Characteristics x̄ (SE) or %
| N | Yrs Agea | Yrs Educ | # MDD symptoms endorsed | BMI in kg/m2a | # CD symptoms endorsedb | % any alcohol past week | % Cocaine or Opiate Dependent(lifetime)b | |
|---|---|---|---|---|---|---|---|---|
| CP− OB− Smoking− |
55 | 34.3(1.3) | 13.3(.2) | 3.5(.4) | 23.6(.4) | .8(.1) | 50.9 | 14.5 |
| CP+ OB− Smoking− |
12 | 30.4(2.9) | 13.3(.5) | 5.6(.9) | 23.4(1.0) | 4.3(.2) | 25 | 16.6 |
| CP− OB+ Smoking− |
14 | 36.9(2.7) | 11.3(.5) | 4.2(.8) | 36.2(.9) | 1.0(.2) | 28.6 | 14.3 |
| CP+ OB+ Smoking− |
20 | 41.5(5.1) | 13.2(.9) | 4.5(.4) | 36.9(1.7) | 3.5(.4) | 35 | 15 |
| CP- OB- Smoking+ |
57 | 35.0(1.2) | 12.7(.2) | 3.0(.3) | 23.4(.4) | .9(.1) | 31.5 | 21.0 |
| CP+ OB− Smoking+ |
30 | 31.1(1.8) | 12.1(.4) | 4.1(.5) | 22.8(.6) | 4.1(.1) | 40 | 30 |
| CP− OB+ Smoking+ |
18 | 38.1(2.4) | 11.1(.4) | 4.1(.7) | 33.9(.8) | .7(.2) | 33.3 | 22.2 |
| CP+ OB+ Smoking+ |
12 | 31.3(2.9) | 11.0(.6) | 4.2(.8) | 34.8(1.0) | 3.3(.2) | 41.7 | 30.3 |
Subscript definitions:
Obesity, p<.05;
Conduct Problems, p<.05;
Smoking, p<.05.
All of the other significant differences between the groups were expected from the manner in which they were defined: (1) the OB+ and OB− groups differed significantly in BMI (F1,210 = 315, p < .001); (2) the CP+ and CP− groups differed in the number of conduct disorder symptoms endorsed (F1,210 = 276, p < .001); and (3) the Smoking+ and Smoking− groups differed in the percent reporting other drug (cocaine and opiate) dependence (χ2 = 9.9, p < .002). Importantly, analyses of other characteristics suggested no hidden confounds. The percentage of patients reporting alcohol use during the past week did not differ across groups. In addition, the groups did not differ in the number of MDD symptoms endorsed.
Because of the differences detected among the groups in age and other drug dependence, age and the number of other drug dependence symptoms were specified as covariates in all other analyses.
P300 Results
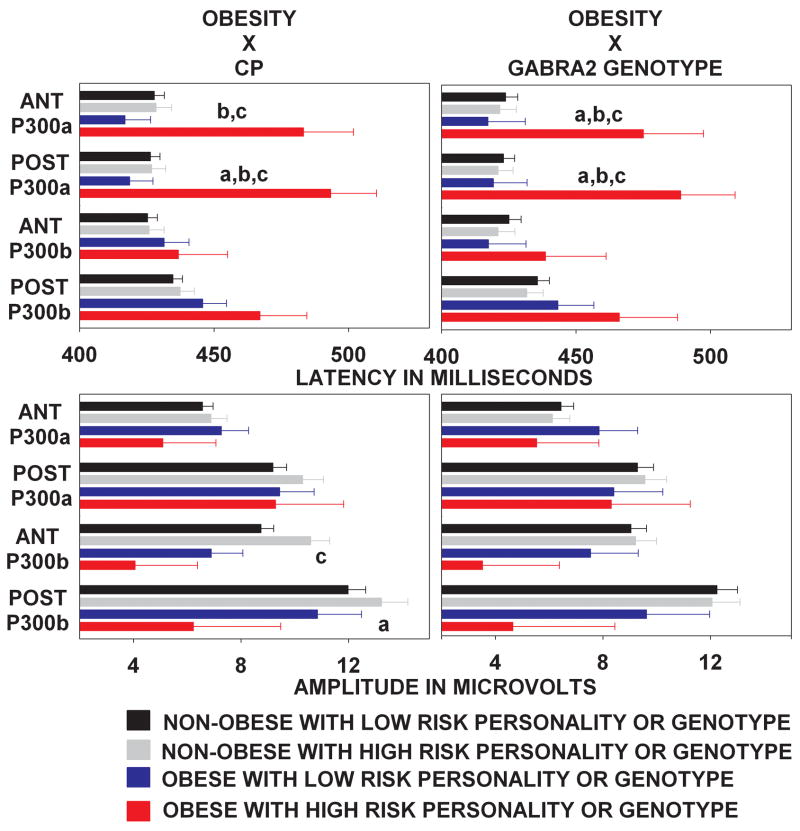
The MANCOVA of P300 data (Figure 1) revealed a significant main effect of Smoking (F8,201 = 2.3, p < .02) and a significant interaction of Conduct Problems with Obesity (F8,201 = 2.2, p < .001). Univariate ANOVAs were used to identify the dependent variables underlying these significant multivariate effects.
Figure 1.
Anterior and posterior region P300a and P300b latency (above) and amplitude (below) as a function of obesity and either personality (left panel) or genetic (right panel) risk. Note the siginificant effect of obesity on P300a latency among subjects with personality or genetic risk, and the absence of this effect among subjects without these risk factors. Subscript definitions: (a) Obesity main effect. (b) Risk status main effect. (c) Obesity × Risk Interaction.
The effect of smoking was limited to the posterior region and only involved the amplitudes of P300a (F1,208 = 4.7, p < .03) and P300b (F1,208 = 5.3, p < .02). As shown in Table 2, a recent history of cigarette smoking, in comparison to its absence, was associated with an amplitude reduction averaging 2.3 μV for P300a and 3.0 μV for P300b. Smoking had no effect on P300a (F1,208 = .1, p = .7) and P300b (F1,208 = 1.1, p =.2) amplitudes over the anterior region and did not change P300 latency over either region (anterior P300a: F1,208 = 1.3, p = .2; anterior P300b: F1,208 = .4, p = .5; posterior P300a: F1,208 = .6, p = .4; posterior P300b: F1,208 = .4, p = .5).
Table 2.
P300 latencies and amplitudes [x̄ (SE)]*
| Anterior P300a Latencyb |
Anterior P300b Latency |
Posterior P300a Latencyb |
Posterior P300b Latency |
Anterior P300a Amplitude |
Anterior P300b Amplitude |
Posterior P300a Amplitudea |
Posterior P300b Amplitudea |
|
|---|---|---|---|---|---|---|---|---|
| CP− OB− Smoking− |
425(5.9) | 424(5.7) | 426(4.9) | 432(5.6) | 7.0(.5) | 9.4(.7) | 10.9(.6) | 14.3(.9) |
| CP+ OB− Smoking− |
411(6.1) | 423(7.7) | 410((5.0) | 440(9.4) | 7.3(1.2) | 12.8(.9) | 11.0(1.4) | 16.8(1.1) |
| CP− OB+ Smoking− |
419(8.9) | 432(11.4) | 430(7.4) | 447(8.3) | 5.9(4.2) | 6.8(1.5) | 10.9(1.2) | 12.1(1.2) |
| CP+ OB+ Smoking− |
472(7.6) | 481(12.8) | 468(10.7) | 477(9.7) | 7.1(3.4) | 4.3(1.6) | 8.2(1.2) | 6.5(1.5) |
| CP− OB− Smoking+ |
434(4.9) | 437(4.9) | 433(4.5) | 445(4.6) | 6.4(.4) | 7.5(.5) | 8.1(1.1) | 10.2(.9) |
| CP+ OB− Smoking+ |
436(7.4) | 426(5.3) | 431(7.3) | 435(5.1) | 7.1((.5) | 9.4(.8) | 9.1(.7) | 11.3(1.1) |
| CP− OB+ Smoking+ |
432(10.9) | 434(10.4) | 428(11.1) | 437(9.9) | 7.4(.9) | 6.6(1.2) | 7.4(1.4) | 8.9(1.6) |
| CP+ OB+ Smoking+ |
456(8.5) | 436(9.2) | 457(9.1) | 452(11.0) | 4.9(.9) | 6.1(1.6) | 8.5(1.6) | 9.3(1.3) |
Subscript definitions:
Smoking, p<.05;
Obesity × Conduct Problems, p<.05
Means are covariate-adjusted by age and the total number of alcohol, opiate, and cocaine dependence symptoms.
The interactive effect of conduct problems and obesity on P300a latency (CP × OB: Anterior region: F1,208 = 7.4, p < .007; Posterior region: F1,208 = 6.7, p < .01) is the most intriguing of all of the results. Table 2 shows, and Tukey post hoc tests confirm, that significant increases in anterior and posterior region P300a latencies were only present in CP+ subjects who were also obese. In other words, neither obesity nor conduct problems alone were sufficient to cause impairments in frontal brain function. The combination was critical.
Analysis Set #2: BMI × frontal region P300a latency correlations
Correlations between BMI and frontal P300a latency were calculated separately for the CP− and CP+ groups. The results of this analysis validated the results shown in the previous ANOVA. BMI was not associated with a change in frontal P300a latency among CP− subjects (r = .09, p =.11). However, among subjects compromised by a childhood history of conduct problems, an increase in BMI occasioned an increase in frontal P300a latency (r = .31, p = .01).
Analysis Set #3: Obesity × Conduct Problems or GABRA2 genotype
When the total number of cases (N = 110) entered in Analysis 2 was distributed across the cells defined by the factorial combination of Obesity with either CP or GABRA2 genotype, the number of cases per cell attained a minimum of n = 8 in one instance. The results of Analysis Set #3 should therefore be interpreted cautiously until replicated in a larger sample.
Background characteristics
Differences across the groups in background characterisics were minor. In the analysis of Obesity × Conduct Problems, the obese group was slightly older (F1,106 = 8.9, p < .04) than their non-obese peers. This group difference in age did not occur in the Obesity × GABRA2 genotype analysis. There were no differences across groups in other characteristics.
P300 Results
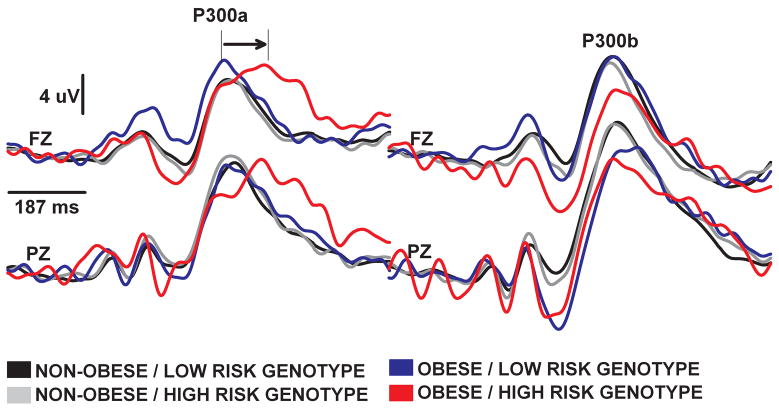
The analyses of P300 data (Figures 1 and 2) revealed remarkable consistency (Figure 2): Conduct Problems (MANCOVA F8,96 = 2.04, p < .05) and GABRA2 genotype at rs279871 (MANCOVA F8,96 = 2.18, p < .05) were consistent in that both amplified the effects of obesity on P300a latency over the anterior and posterior scalp, and produced greater slowing than was associated with the sum of the main effects of the factors. Table 3 summarizes the results of the univariate analyses.
Figure 2.
Group averaged event related potential waveforms at Fz and Pz sites evoked by novel (left panel) and rare target (right panel) stimuli. The 187 ms prestimulus period is indicated with a horizontal line at the beginning of the epoch. Note the synergistic effects of obesity and GABRA2 genotype on P300a latency over the anterior and posterior scalp. This effect was not significant for P300b.
Table 3.
ANOVA Results
| Anterior P300a Latency |
Anterior P300b Latency |
Posterior P300a Latency |
Posterior P300b Latency |
Anterior P300a Amplitude |
Anterior P300b Amplitude |
Posterior P300a Amplitude |
Posterior P300b Amplitude |
|
|---|---|---|---|---|---|---|---|---|
| OB | F=5.7* | F=4.6* | ||||||
| CP | F=4.1* | F=6.1* | ||||||
| OB × CP | F=4.2* | F=7.4* | F=5.4* | |||||
| OB | F=3.8* | F=6.5* | ||||||
| Genotype | F=4.5* | F=5.5* | ||||||
| OB × Genotype | F=3.7* | F=6.7* |
p < .05
Table 3 also shows two circumstances in which obesity influenced P300b amplitude. The significant main effect of obesity on posterior region P300b reflects an average amplitude reduction of 4.1 μV. The significant interactive effect of obesity and conduct problems on anterior region P300b amplitude is uninterpretable because Tukey post hoc analyses revealed no pairwise differences between cell means that were significant.
Analysis Set #4: Path Analysis
Figure 3 illustrates two models by which conduct problems, BMI, and frontal P300a latency may relate. Analyses of each model revealed that only Model #1 was an adequate fit to the data: its 0.1 χ2 (df = 1, p = .91) and 0.00 (p = .94) RMSEA indices were not statistically significant and therefore did not justify a rejection of the model. Furthermore, the 1.0 CFI was greater than 0.95 criterion. In addition, both of the standardized path coefficients in Model #1 were significant: β1 = .14 (p = .02) andβ2 = .12 (p = .02).
Figure 3.
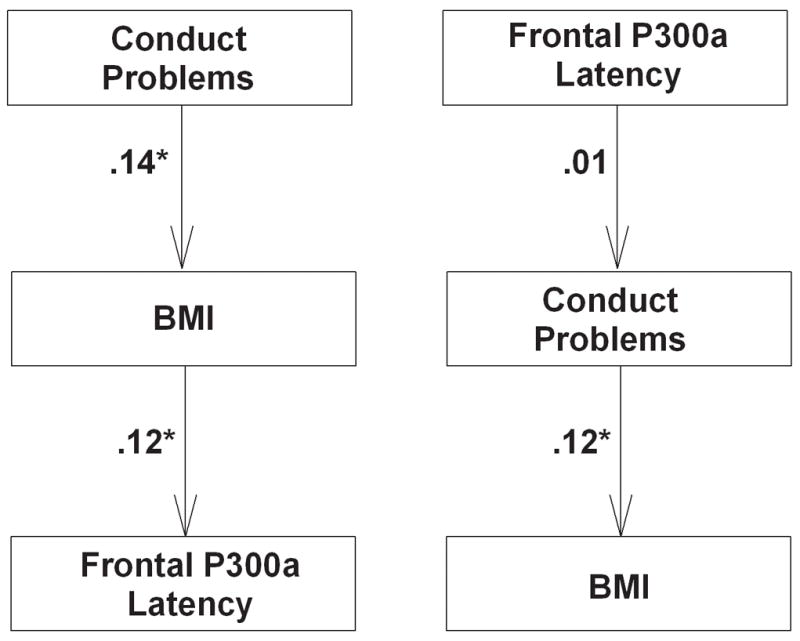
Path Models 1 (left) and 2 (right). Model 1 was a superior fit to the data. See text for explanation. * p < .05
In contrast, Model #2 was a poor fit. Its χ2 (5.6, p = .01) and RMSEA (.11, p = .07) indices were inconsistent. The CFI for Model #2 was .00. The standardized path coefficients were β1 = .01 (p = .78) andβ2 = .12 (p = .02).
Discussion
Because conduct problems, smoking, and obesity overlap, and affect brain function, a relevant question is: are the effects independent or interactive? In the case of smoking, the answer is “independent”. Smoking was not associated with the same changes in P300 latency, or in anterior scalp P300 amplitude, found in the analyses of conduct problems and obesity. In addition, it did not statistically interact with the other factors. Smoking was only associated with a reduction in P300 amplitude over the posterior scalp (Table 2).
An explanation for the greater effect of smoking over the posterior versus anterior scalp may be found in the results of radioligand studies (52) wherein nicotine-binding has been shown to be greater in the thalamus and brainstem than in frontal structures and may, over time, be detrimental to their function. Admittedly, though, the literature is not consistent in demonstrating that posterior brain regions are the primary locus (3, 19, 53). The degree to which anterior brain regions are affected in smokers may be related to the chronicity and severity of the habit, as well as its overlap with other disorders, including depression (54) and personality disorder (55).
In contrast to the simple effects of smoking, the effects of CP and obesity were complex. Their interaction is shown most clearly in Analysis Set #3 which was limited to 110 European-American subjects. The analysis demonstrated a synergism between these two factors in delaying the P300a component. The interaction was duplicated (Figure 2) when a GABRA2 genotype previously associated with conduct problems was substituted for the CP factor. At least two interpretations for the interaction can be offered. The first interpretation hypothesizes that a GABRA2 genotype, associated with conduct problems in previous studies, decreases the age-of-onset or increases the duration of obesity. As a result, the genotype potentiates the adverse effects of obesity on frontal white matter, and delays the emergence of this frontally-generated P300 component. The alternative interpretation views obesity, conduct problems, and increased P300a latency (i.e., frontal brain dysfunction) as behavioral or neurophysiological expressions of a common genetic diathesis involving GABRA2.
Path analysis (Figure 3) was used to evaluate the relative merits of these interpretations. The analyses offered stronger support for the former interpretation than the latter. However, this interpretation cannot be accepted with confidence until we can obtain more information and replicate these results in a separate longitudinal study. For example, although other studies (27) have documented an association between childhood disruptive disorders and the course (i.e., earlier age-of-onset and a stable or increasing level of severity) of weight problems, we could not conduct this longitudinal analysis in the present study. We therefore cannot confirm that GABRA2 genotype and conduct problems do indeed reduce the age-of-onset or increase the chronicity of obesity in our subjects, and thereby effect changes in white matter and P300a latency.
We must also recognize another limitation. The present findings were obtained from a sample of alcohol-dependent females and may be limited in their generality. We implemented this sampling restriction for several reasons. For example, because the subjects possessed a history of alcohol-dependence, their brain function is hypothetically more vulnerable to the effects of additional insults, including those resulting from smoking and obesity. Accordingly, alcohol-dependent subjects constitute a clinically important, high risk group deserving of our attention. In addition, alcohol-dependent subjects possess more conduct problems and nicotine use than non-alcoholic individuals. Limiting the sample to these subjects therefore improves statistical power for demonstrating associations between conduct problems, associated genotypes, smoking, and obesity. Indeed, it is debatable whether the effects of conduct problems and smoking can be studied with adequate power in adults unaffected by alcoholism or other drug use.
In the future, we hope to replicate these findings in subject samples characterized by other forms of substance dependence or personality (e.g., borderline personality disorder) risk. In addition, in place of the DSM-IV clinical diagnostic criteria for Conduct Disorder, we might ask whether other indices of antisocial personality or impulsivity would similarly associate with GABRA2 genotype and with the adverse effects of obesity on the brain. The result may be an expanded theoretical model—not unlike models now discussed in the alcohol research literature--in which impulsivity and conduct problems are viewed as endophenotypes which promote obesity, impair its treatment, and exacerbate its course and complications.
Acknowledgments
Drs. Bierut and Rice are listed as inventors on a patent (US 20070258898) held by Perlegen Sciences, Inc., covering the use of specific SNPs in determining the diagnosis, prognosis, and treatment of addiction. Their affilliation with Perlegen Sciences, Inc. has no relevance to the study described herein. The NIH also had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, or approval of the manuscript. The Collaborative Study on the Genetics of Alcoholism is supported by grant U10AA08401 from the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. The project is directed by 4 principal investigators (Drs. Bierut, Edenberg, Hesselbrock, and Porjesz) and includes 9 different sites where data collection, analysis, and storage take place. These sites and the local project directors are: the University of Connecticut, Farmington (Victor Hesselbrock); Indiana University, Indianapolis (Howard Edenberg, John Nurnberger, Tatiana Foroud); University of Iowa, Iowa City (Samuel Kuperman); State University of New York Downstate Medical Center, Brooklyn (Bernice Porjesz); Washington University in St Louis (Laura Bierut, Alison Goate, John Rice); University of California at San Diego (Marc Schuckit); Howard University, Washington, DC (Robert Taylor); Rutgers University, New Brunswick, New Jersey (Jay Tischfield); and Southwest Foundation for Biomedical Research, San Antonio, Texas (Laura Almasy). Zhaoxia Ren serves as the National Institute on Alcohol Abuse and Alcoholism staff collaborator. This study is in memory of Henri Begleiter and Theodore Reich, original principal and co-principal investigators of COGA. We acknowledge their immeasurable and fundamental scientific contributions to COGA and the field.
References
- 1.Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ. Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol. 2008;63:65–57. doi: 10.1002/ana.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jagust W. What can imaging reveal about obesity and the brain? Curr Alzheimer Res. 2007;4:135–139. doi: 10.2174/156720507780362146. [DOI] [PubMed] [Google Scholar]
- 3.Gallinat J, Meisenzahl E, Jacobsen LK, et al. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24:1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology (Berl) 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- 5.Gallego J, Martinez Vila E, Munoz R. Patients at high risk for ischemic stroke: identification and actions. Cerebrovasc Dis. 2007;24 (Suppl 1):49–63. doi: 10.1159/000107379. [DOI] [PubMed] [Google Scholar]
- 6.Martiniuk AL, Lee CM, Lam TH, et al. The fraction of ischaemic heart disease and stroke attributable to smoking in the WHO Western Pacific and South-East Asian regions. Tob Control. 2006;15:181–188. doi: 10.1136/tc.2005.013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 8.Naya T, Hosomi N, Ohyama H, et al. Smoking, fasting serum insulin, and obesity are the predictors of carotid atherosclerosis in relatively young subjects. Angiology. 2007;58:677–684. doi: 10.1177/0003319707303589. [DOI] [PubMed] [Google Scholar]
- 9.Anokhin AP, Vedeniapin AB, Sirevaag EJ, et al. The P300 brain potential is reduced in smokers. Psychopharmacology (Berl) 2000;149:409–413. doi: 10.1007/s002130000387. [DOI] [PubMed] [Google Scholar]
- 10.Bauer LO. Psychiatric and neurophysiological predictors of obesity in HIV/AIDS. Psychophysiology. 2008;45:1055–1063. doi: 10.1111/j.1469-8986.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kivipelto M, Helkala EL, Hanninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 12.Basseches HI, Karp SA. Field dependence in young anorectic and obese women. Psychother Psychosom. 1984;41:33–37. doi: 10.1159/000287783. [DOI] [PubMed] [Google Scholar]
- 13.Upadhyaya HP, Deas D, Brady KT, Kruesi M. Cigarette smoking and psychiatric comorbidity in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2002;41:1294–1305. doi: 10.1097/00004583-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Abrantes AM, Strong DR, Ramsey SE, Lewinsohn PM, Brown RA. Substance use disorder characteristics and externalizing problems among inpatient adolescent smokers. J Psychoactive Drugs. 2005;37:391–399. doi: 10.1080/02791072.2005.10399812. [DOI] [PubMed] [Google Scholar]
- 15.Sawyer MG, Miller-Lewis L, Guy S, Wake M, Canterford L, Carlin JB. Is there a relationship between overweight and obesity and mental health problems in 4- to 5-year-old Australian children? Ambul Pediatr. 2006;6:306–311. doi: 10.1016/j.ambp.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 17.Ding J, Nieto FJ, Beauchamp NJ, et al. A prospective analysis of risk factors for white matter disease in the brain stem: the Cardiovascular Health Study. Neuroepidemiology. 2003;22:275–282. doi: 10.1159/000071190. [DOI] [PubMed] [Google Scholar]
- 18.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol Aging. 2005;26 (Suppl 1):11–16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Neuhaus A, Bajbouj M, Kienast T, et al. Persistent dysfunctional frontal lobe activation in former smokers. Psychopharmacology (Berl) 2006;186:191–200. doi: 10.1007/s00213-006-0366-7. [DOI] [PubMed] [Google Scholar]
- 20.Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37:335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 21.Lyoo IK, Lee HK, Jung JH, Noam GG, Renshaw PF. White matter hyperintensities on magnetic resonance imaging of the brain in children with psychiatric disorders. Compr Psychiatry. 2002;43:361–368. doi: 10.1053/comp.2002.34636. [DOI] [PubMed] [Google Scholar]
- 22.Bauer LO, Hesselbrock VM. Brain maturation and subtypes of conduct disorder: interactive effects on p300 amplitude and topography in male adolescents. J Am Acad Child Adolesc Psychiatry. 2003;42:106–115. doi: 10.1097/00004583-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Kim MS, Kim JJ, Kwon JS. Frontal P300 decrement and executive dysfunction in adolescents with conduct problems. Child Psychiatry Hum Dev. 2001;32:93–106. doi: 10.1023/a:1012299822274. [DOI] [PubMed] [Google Scholar]
- 24.McAlonan GM, Cheung V, Cheung C, et al. Mapping brain structure in attention deficit-hyperactivity disorder: a voxel-based MRI study of regional grey and white matter volume. Psychiatry Res. 2007;154:171–180. doi: 10.1016/j.pscychresns.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Haltia LT, Viljanen A, Parkkola R, et al. Brain white matter expansion in human obesity and the recovering effect of dieting. J Clin Endocrinol Metab. 2007;92:3278–3284. doi: 10.1210/jc.2006-2495. [DOI] [PubMed] [Google Scholar]
- 26.Clark DB, Cornelius J. Childhood psychopathology and adolescent cigarette smoking: a prospective survival analysis in children at high risk for substance use disorders. Addict Behav. 2004;29:837–841. doi: 10.1016/j.addbeh.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Anderson SE, Cohen P, Naumova EN, Must A. Relationship of childhood behavior disorders to weight gain from childhood into adulthood. Ambul Pediatr. 2006;6:297–301. doi: 10.1016/j.ambp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Mustillo S, Worthman C, Erkanli A, Keeler G, Angold A, Costello EJ. Obesity and psychiatric disorder: developmental trajectories. Pediatrics. 2003;111:851–859. doi: 10.1542/peds.111.4.851. [DOI] [PubMed] [Google Scholar]
- 29.Dick DM, Li TK, Edenberg HJ, et al. A genome-wide screen for genes influencing conduct disorder. Mol Psychiatry. 2004;9:81–86. doi: 10.1038/sj.mp.4001368. [DOI] [PubMed] [Google Scholar]
- 30.Bierut LJ, Rice JP, Goate A, et al. A genomic scan for habitual smoking in families of alcoholics: common and specific genetic factors in substance dependence. Am J Med Genet A. 2004;124:19–27. doi: 10.1002/ajmg.a.20329. [DOI] [PubMed] [Google Scholar]
- 31.Ehlers CL, Wilhelmsen KC. Genomic screen for substance dependence and body mass index in southwest California Indians. Genes Brain Behav. 2007;6:184–191. doi: 10.1111/j.1601-183X.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- 32.Jones KA, Porjesz B, Almasy L, et al. A cholinergic receptor gene (CHRM2) affects event-related oscillations. Behav Genet. 2006;36:627–639. doi: 10.1007/s10519-006-9075-6. [DOI] [PubMed] [Google Scholar]
- 33.Jones KA, Porjesz B, Almasy L, et al. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int J Psychophysiol. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Edenberg HJ, Dick DM, Xuei X, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dick DM, Bierut L, Hinrichs A, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- 36.Cardenas VA, Chao LL, Blumenfeld R, et al. Using automated morphometry to detect associations between ERP latency and structural brain MRI in normal adults. Hum Brain Mapp. 2005;25:317–327. doi: 10.1002/hbm.20103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egan MF, Duncan CC, Suddath RL, Kirch DG, Mirsky AF, Wyatt RJ. Event-related potential abnormalities correlate with structural brain alterations and clinical features in patients with chronic schizophrenia. Schizophr Res. 1994;11:259–271. doi: 10.1016/0920-9964(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 38.McCarley RW, Salisbury DF, Hirayasu Y, et al. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:321–331. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- 39.Dien J, Spencer KM, Donchin E. Localization of the event-related potential novelty response as defined by principal components analysis. Brain Res Cogn Brain Res. 2003;17:637–650. doi: 10.1016/s0926-6410(03)00188-5. [DOI] [PubMed] [Google Scholar]
- 40.Clark VP, Fannon S, Lai S, Benson R, Bauer L. Responses to rare visual target and distractor stimuli using event-related fMRI. J Neurophysiol. 2000;83:3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- 41.Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol. 1998;106:156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- 42.Baskin ML, Ard J, Franklin F, Allison DB. Prevalence of obesity in the United States. Obes Rev. 2005;6:5–7. doi: 10.1111/j.1467-789X.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein RB, Dawson DA, Stinson FS, et al. Antisocial behavioral syndromes and body mass index among adults in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2008;49:225–237. doi: 10.1016/j.comppsych.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reich T, Edenberg HJ, Goate A, et al. Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet. 1998;81:207–215. [PubMed] [Google Scholar]
- 45.Cohen HL, Wang W, Porjesz B, et al. Visual P300: an interlaboratory consistency study. Alcohol. 1994;11:583–587. doi: 10.1016/0741-8329(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 46.Bucholz KK, Cadoret R, Cloninger CR, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 47.Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- 48.Fergusson DM, Horwood LJ. Predictive validity of categorically and dimensionally scored measures of disruptive childhood behaviors. J Am Acad Child Adolesc Psychiatry. 1995;34:477–485. discussion 485–477. [PubMed] [Google Scholar]
- 49.Slutske WS, Heath AC, Dinwiddie SH, et al. Modeling genetic and environmental influences in the etiology of conduct disorder: a study of 2,682 adult twin pairs. J Abnorm Psychol. 1997;106:266–279. doi: 10.1037//0021-843x.106.2.266. [DOI] [PubMed] [Google Scholar]
- 50.Ascioglu M, Dolu N, Golgeli A, Suer C, Ozesmi C. Effects of cigarette smoking on cognitive processing. Int J Neurosci. 2004;114:381–390. doi: 10.1080/00207450490270668. [DOI] [PubMed] [Google Scholar]
- 51.Muthen L, Muthen BO. Mplus User’s Guide (version 5) Muthen & Muthen; Los Angeles: 2007. [Google Scholar]
- 52.Brody AL, Mandelkern MA, London ED, et al. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brody AL, Mandelkern MA, Jarvik ME, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- 54.Monkul ES, Hatch JP, Nicoletti MA, et al. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry. 2007;12:360–366. doi: 10.1038/sj.mp.4001919. [DOI] [PubMed] [Google Scholar]
- 55.Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict Biol. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]