Abstract
Steady progress is being made toward the development of a so-called “artificial pancreas,” which may ultimately be a fully automated, closed-loop, glucose control system comprising a continuous glucose monitor, an insulin pump, and a controller. The controller will use individualized algorithms to direct delivery of insulin without user input. A major factor propelling artificial pancreas development is the substantial incidence of—and attendant patient, parental, and physician concerns about—hypoglycemia and extreme hyperglycemia associated with current means of insulin delivery for type 1 diabetes mellitus (T1DM). A successful fully automated artificial pancreas would likely reduce the frequency of and anxiety about hypoglycemia and marked hyperglycemia. Patch-pump systems (“patch pumps”) are likely to be used increasingly in the control of T1DM and may be incorporated into the artificial pancreas systems of tomorrow. Patch pumps are free of tubing, small, lightweight, and unobtrusive. This article describes features of patch pumps that have been approved for U.S. marketing or are under development. Included in the review is an introduction to control algorithms driving insulin delivery, particularly the two major types: proportional integrative derivative and model predictive control. The use of advanced algorithms in the clinical development of closed-loop systems is reviewed along with projected next steps in artificial pancreas development.
Introduction
There has been steady progress over the years toward the development of a so-called “artificial pancreas,” a fully automated, external, closed-loop system for insulin delivery (Fig. 1).1–7 Under experimental conditions, closed-loop glucose control utilizing continuous glucose monitors (CGMs), insulin pumps, and pump-controlling algorithms has shown superiority to open-loop control in being able to achieve greater time in target range, with less hyperglycemia and hypoglycemia.8–10
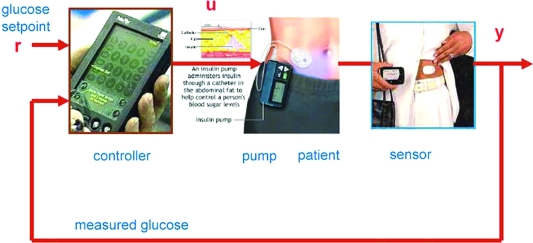
FIG. 1.
The artificial pancreas, a closed-loop system for insulin delivery. The controller compares the desired glucose with the value obtained from a continuous glucose sensor. The controller output signal adjusts the rate of insulin delivered by an insulin infusion pump. The insulin affects the blood glucose, which is sensed and “fed-back” to the controller. Reprinted with permission from Bequette1 and Mary Ann Liebert, Inc., New Rochelle, NY.
Driving these advances are persistent concerns about short-term risks of severe and potentially fatal hypoglycemia as well as severe hyperglycemia and diabetic ketoacidosis. Additionally, there are concerns about long-term risk of complications from hyperglycemia and glycemic variability associated with contemporary methods of insulin administration.11–14 Early glycemic control is particularly important because long-term vascular complications may result from early hyperglycemic stresses, as shown in studies suggesting the importance of “metabolic memory.”15
The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study demonstrated that many patients do not reach target blood glucose levels.16 Intensive therapy should lower glucose values and improve outcomes; however, it will expose patients to an increased risk of hypoglycemia. The Diabetes Control and Complications Trial11 and the Oxford-Steno group12 reported that severe hypoglycemia (requiring emergent or other forms of medical assistance) affected about one-third of patients with type 1 diabetes mellitus (T1DM) (however, all of them were treated with regular insulin at that time), with ∼50% of all episodes occurring in 5% of patients.
Use of insulin pumps has been shown to reduce the incidence of severe hypoglycemic events compared with multiple daily injections (MDI). A meta-analysis of 22 studies found that the rate of severe hypoglycemia per 100 patient-years among patients with T1DM treated with MDI was 100 for adults and 36 for children and that the rate was reduced three- to fourfold among patients receiving continuous subcutaneous insulin infusion.17 Development of a reliable, safe, and effective closed-loop system may further reduce hypoglycemia and hyperglycemia and help overcome these concerns among patients, parents, and physicians. Given the substantial frequency of severe, potentially life-altering (or life-threatening) hypoglycemia, it is not surprising that many children, adolescents, and adults with T1DM experience considerable fears of hypoglycemia. These fears may compromise self-care and treatment adherence, which often lead to hyperglycemia and worsening metabolic control.18–20
Although focused on T1DM management, the present article surveys available or developmental patch pumps designed for type 2 diabetes mellitus as well as those designed for T1DM. This is done because some technologies currently used for type 2 diabetes mellitus patch pumps may be applied to future T1DM pumps. Especially with increasing demand for discreetness, patch pumps are poised to become key components of closed-loop systems. The article also considers pump-controlling algorithms (including in silico [computer-based] simulations) and preclinical and clinical testing of closed-loop systems.
Insulin Patch Pumps
Traditional insulin pumps and software have received broad acceptance because of their ease of use, accuracy, predictability, and ability to calculate bolus insulin doses based on user-input information.21 Most of these traditional pumps deliver insulin through tubing that can kink, catch, and/or detach. These tubing issues, along with the current size of available pumps, often compromise discreet, convenient use. Taken together, these factors helped spawn interest in the development of patch pumps that involve no tubing, readily adhere to the body, are small, lightweight, and completely or partially disposable, and are capable of being worn and manipulated discreetly under clothing. Problems associated with currently available patch pumps will have to be addressed, including temporary unavailability of a controller, pump size (form factor), adhesive intolerance, and poor adherence.
A number of patch pumps are under development. Some patch pumps will require a separate controller device that communicates wirelessly with the pump; others will include all necessary control components. An overview of basic patch-pump information is presented in Table 1. Specifications of pumps under development may change before the pumps reach market. Some pumps listed below as being currently unavailable or under development in the United States may already be available in other countries.
Table 1.
Overview of Insulin Patch Pumps
| Marketing status, pump name (company)a | Insulin delivery mode: basal and/or bolus | E or M control | Days of wear | Reservoir volume | Basal rate, minimal (increments) | Available bolus sizes | Size | Weight | Cannula insertion |
|---|---|---|---|---|---|---|---|---|---|
| Marketed patch pumps | |||||||||
| OmniPod® (Insulet Corp.)b | Basal and bolus | E | 3 | 2.00 mL | 0.05 U/h (0.05, to max 30 U/h) | 0.10-U increments to 10.00 U max bolus | 1.6 × 2.4 × 0.7 in (40.6 × 61.0 × 17.8 mm) | 1.2 oz (34.0 g) (filled) | Internal driver controlled by PDM |
| Patch pump approved for marketing by the U.S. FDA but not yet commercially available in the United States at this writing | |||||||||
| Solo™ (Medingo)c | Basal and bolus | E/M | 3 | 200 U | 0.05 U/h (0.05 U/h; max NA) | 0.05 U | NA | NA | Separate driver |
| Finesse™ (Calibra Medical Inc.)b | Bolus only | M | 3 | 2.00 mL | Bolus only | 0.5, 1, 2, 5 U | 2.5 × 1.2 × 0.3 in (63 × 31 × 7.5 mm) | 0.4 oz (11.3 g) (filled) | Separate driver |
| Patch pumps reported to be under development | |||||||||
| Cellnovo pump (Cellnovo Ltd.) | Basal and bolus | E | 3 | 3 days: 50 units in pump for children; 150 units in pump for adults | NA | NA | NA | NA | NA |
| CeQur™ device (CeQur Ltd.) | Basal and bolus | E/M | 3 | NA | Multiple basal rates | NA | NA | NA | NA |
| Freehand™ (MedSolve Technologies, Inc.) | Basal and bolus | E/M | NA | 3.0 mL | 0.05 U/h (0.05 U/h; max NA) | 0.05 U | 1 in3 (25.4 mm3) | NA | Cannula available in several lengths; insertion method NA |
| Medipacs pump (Medipacs, Inc.) | NA | NA | 3 | NA | 1 mL/24 h | NA | NA | NA | NA |
| Medtronic pump (Medtronic, Inc.) | Basal and bolus | E/M | NA | NA | NA | NA | NA | NA | NA |
| Nanopump™ (Debiotech SA and STMicroelectronics) | Basal and bolus | E | 3 | Several sizes, e.g., 5.00 or 7.50 mL | 0.02 U/h (0.02 U/h; max NA) | 0.02 U (NA) | 2.3 × 1.6 × 0.5 in (60 × 40 × 13 mm) | 1.0 oz (28 g) (5.00-mL reservoir); 1.1 oz (31 g) (7.50-mL reservoir) | Auto-insertion |
| NiliPatch pump (NiliMEDIX Ltd.)d | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| PassPort® (Altea Therapeutics Corp.) | Basal only | M | 12 or 24 h | NA | NA | Basal only | NA | NA | Transdermal delivery; separate applicator |
| SteadyMed patch pump (SteadyMed Ltd.) | Bolus | M | NA | NA | NA | NA | NA | NA | NA |
| V-Go™ (Valeritas, Inc.)e | Basal and bolus | M | 1 | 0.56, 0.66, 0.76 mL | 20 U/24 h (10 U/24 h to max 40 U/24 h) | 2 U, up to 18 boluses | NA | 0.71–1.78 oz (20–50 g) | Transdermal delivery; internal driver |
| Animas Corporation | NA | NA | NA | NA | NA | NA | NA | NA | NA |
E, electronic; FDA, Food and Drug Administration; M, mechanical; max, maximum; NA, not available; PDM, Personal Diabetes Manager.
Insulet Corp., Bedford, MA; Medingo US, Inc., Tampa, FL; Cellnovo Ltd. (formerly Starbridge Systems Ltd.), London, UK; CeQur Ltd., Montreux, Switzerland; Calibra Medical Inc., Redwood City, CA; MedSolve Technologies, Inc., Manhattan Beach, CA; Medipacs, Inc., San Diego, CA; Medtronic, Inc., Minneapolis, MN; Debiotech SA, Lausanne, Switzerland; STMicroelectronics, Geneva, Switzerland; NiliMEDIX Ltd., Tirat-Carmel, Israel; Altea Therapeutics Corp., Atlanta, GA; SteadyMed Ltd., Tel-Aviv, Israel; Valeritas, Inc., Bridgewater, NJ; Animas Corporation, West Chester, PA.
Only OmniPod and Finesse have or will have optional bolus sizes.
Approved for marketing by FDA.
European Union CE Mark certified for marketing.
Revised device is under FDA 510(k) review.
Marketed patch pump
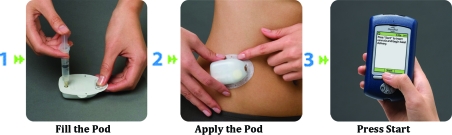
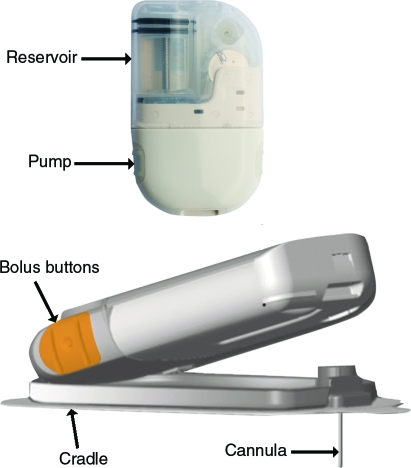
The OmniPod® Insulin Management System (Insulet Corp., Bedford, MA), the first patch pump marketed in the United States, delivers both basal and bolus insulin. It is composed of a pod, which must be replaced every 3 days, and a Personal Diabetes Manager (PDM) (Fig. 2). The pod contains, in addition to the pump, an insulin reservoir with a capacity of 2 mL and a cannula.22 The PDM, which has an onboard glucometer, allows the patient to control the pod wirelessly and also features automated cannula insertion. After receiving a blood glucose value from the fingerstick blood sample tested on the incorporated glucometer and anticipated carbohydrate (CHO) intake information from the patient, the PDM calculates mealtime bolus insulin dosage. The PDM contains a food library and also stores, displays, and downloads data on insulin delivery, blood glucose values, and CHO records. It is equipped with alarms/alerts and a color LCD screen.
FIG. 2.
Operation of the OmniPod insulin management system. Reproduced with permission by Insulet Corporation, Bedford, MA.
Patch pumps approved for marketing by the U.S. Food and Drug Administration but not yet commercially available in the United States at this writing
The Solo™ MicroPump Insulin Delivery System (Medingo US, Inc., Tampa, FL) consists of a basal-bolus micropump, wireless remote controller, and cradle with a built-in cannula. The 2-mL insulin reservoir, which attaches to the pump, must be replaced at least every 2 days when insulin lispro or insulin glulisine is used and at least every 3 days when insulin aspart is used. The pump itself should be replaced every 90 days (Fig. 3).23
FIG. 3.
The Solo MicroPump insulin delivery system. Reproduced with permission from Medingo US, Inc., Tampa, FL.
Finesse™ (Calibra Medical Inc., Redwood City, CA) is a disposable mechanical pump that delivers insulin (bolus only).22 By depressing both its bolus-release buttons simultaneously, the patient will cause a bolus to be delivered. A separate driver will be used for inserting the cannula after the filled pump has been adhered to the body.22
Patch pumps reported to be under development
The following data about patch pumps under development have been derived from information in the public domain available at the time of writing.
The Cellnovo Pump (Cellnovo Ltd., formerly Starbridge Systems, London, UK) is a low-power, basal-bolus pump with an integrated power supply coupled with a reservoir containing a 3-day supply of insulin and a cannula for drug delivery.22 Two pump sizes are to be available: the reservoir in a pump for children is 0.5 mL, and that for adults is 1.5 mL. The empty reservoir and cannula are replaced after 3 days; the entire pump case is retained. This pump will utilize proprietary technology to control mechanical energy. It was projected to be available throughout Europe at the start of 2010.24
The Freehand™ system (MedSolve Technologies, Inc., Manhattan Beach, CA) for basal and bolus insulin delivery will consist of an electronically controlled pump usable for 3 months, a disposable insulin reservoir, a tubeless patch with contained cannula, and a remote control. The system will contain seven basal profiles. Basal delivery will be able to be temporarily suspended, and boluses can be delivered remotely or manually.22
The Nanopump™ (Debiotech SA [Lausanne, Switzerland] and STMicroelectronics [Geneva, Switzerland]) for continuous subcutaneous insulin infusion will be equipped with a reusable aspect, containing the electronics, vibration alarm, buzzer, and capabilities for programming and remote control, and a disposable aspect, containing an insulin reservoir, pump, and batteries. The device's adhesive patch containing an auto-inserted infusion cannula is to be changed every 3 days. Several sizes of insulin reservoir will be available. The pump will be based on micro-electromechanical systems technology.22
The NiliPatch Disposable Insulin Pump system (NiliMEDIX Ltd., Tirat-Carmel, Israel) consists of a disposable insulin pump that delivers basal and bolus insulin.25 The pump uses a patented pressure-triggered release mechanism and is controlled by a system of valves and sensors. The NiliPatch has been certified for marketing in the European Union and Israel.26
The PassPort® system (Altea Therapeutics Corp., Atlanta, GA) for delivery of basal insulin will comprise an applicator and PassPort Patch, which contains a drug reservoir under which there is a small screen (porator) containing metallic filaments. The applicator delivers an electric charge to the porator, galvanizing the filaments and vaporizing the closest skin cells, creating micropores through which insulin passes transdermally.27 Drug delivery will be initiated by folding the patch after attaching it to the skin. The micropores created by controlled bursts of thermal energy permit the flow of not only insulin but also other proteins, peptides, and CHOs into the body without needles or pumps. Phase 1 clinical data indicate that the PassPort system provides sustained, therapeutic insulin levels.28
V-Go™ (Valeritas, Inc., Bridgewater, NJ) is a basal-bolus pump that uses a transdermal h-Patch™ (hydraulic) that needs to be replaced daily. The pump has no electronics, batteries, or programming. The original h-Patch product received Food and Drug Administration (FDA) 510(k) premarket approval in 2005. After product refinements, a new 510(k) for the V-Go and its filling device was submitted and is under FDA review at this writing.22
The CeQur™ (Montreux, Switzerland) insulin infuser, a disposable basal-bolus patch pump intended for type 2 patients, will be available in one of seven basal rates and offer bolus insulin by pressing two buttons simultaneously. It alerts when the device is activated and when it should be replaced.
The following are developmental patch pumps for which there is little available information: the Medipacs patch pump (Medipacs, Inc., San Diego, CA)29; the Medtronic, Inc. Patch Delivery system (Medtronic, Inc., Minneapolis, MN)22; and the SteadyMed patch pump (SteadyMed Ltd., Tel-Aviv, Israel), which is based on an electrochemical battery that expands, propelling a bolus, when a button is pressed.30
Pump-Controlling Algorithms
In general, an algorithm uses a finite sequence of well-defined instructions for completing a task, starting from an initial state and proceeding through computations to a desired end state. With a closed-loop system, an algorithm starts from a state of glucose level as supplied by a CGM and determines, through a series of equations, the desired end state, including the way in which insulin will be infused by a pump to maintain blood glucose within desired limits. Some of these equations may be able to take into account previously collected data reflecting the glucose–insulin responses of large numbers of patients. Most pump-controlling algorithms are computed within pump controllers.
Algorithm design and mathematical modeling
The two major pump-controlling algorithms likely to be used in closed-loop systems are the proportional integrative derivative (PID) algorithm31,32 and the model predictive control (MPC) algorithm.32–34
The PID algorithm calculates the amount of insulin to be delivered based on a model of multiphasic insulin responses to hyperglycemia within pancreatic β-cells.9 The model's three components, which correspond to the phases of β-cell insulin response, are proportional (P), integral (I), and derivative (D). Total insulin delivery by the β-cell, and hence the amount to be delivered by an insulin pump according to the PID algorithm, represents the sum of insulin delivery corresponding to each of the three secretory components.
As indicated in the following equations (in which n denotes the most recent 1-min sensor glucose [SG] value and n – 1 denotes the previous 1-min value), the P component controls insulin delivery, increasing it when glucose is above a prespecified target value and reducing it when it is below target. Because no insulin is delivered when glucose is at target, it does not contribute to the basal insulin requirement.9 The I component mimics the β-cell's slow second-phase insulin excursion, adjusting insulin upward when glucose is above target (and downward when below) but exerting no effect when glucose is at target. The D component corresponds to the β-cell's rapid first-phase insulin rise, increasing insulin delivery when glucose levels are rising and decreasing it when declining. The constants KP, TI, and TD in the equations below balance the amount of insulin delivered by each component:
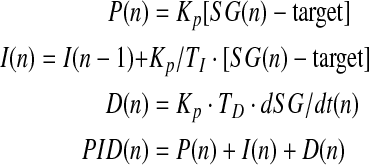 |
The PID equation may also be written in the following form, in which the insulin infusion rate at any time [u(t)] is equal to the basal insulin rate (u0) plus three functions of the error [e, the difference between the measured glucose value and the target value, i.e., SG(n) − target]: P is directly proportional to the error e(t), I is proportional to the integral of the error [ ∫ e(t)dt], and D is proportional to the derivative of the error [de(t)/dt]1:
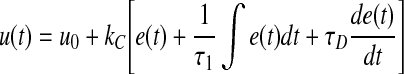 |
The MPC algorithm, which is a composite of multiple algorithms, determines the level and timing of insulin infusion rates based on predictions of the ways in which insulin will affect future glucose concentrations.1 These algorithms have been developed after taking into account many glucose regulatory submodels. One of these, developed by Dalla Man et al.35 and evaluated by Magni et al.33 in an in silico trial, relates to intestinal glucose absorption:
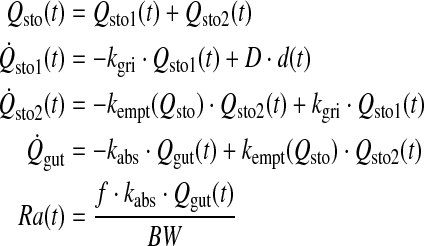 |
where Qsto (in mg) denotes the amount of glucose in the stomach (solid phase, Qsto1; liquid phase, Qsto2), Qgut (in mg) is the glucose mass in the intestine, kgri signifies the rate of grinding, kabs is the rate constant of intestinal absorption, f indicates the proportion of intestinally absorbed glucose that appears in plasma, D (in mg) is the amount of ingested glucose, BW represents body weight (in kg), and Ra (in mg/kg/min) is the rate of glucose appearance in plasma.35
Algorithms have been developed for glucose regulatory submodels in addition to the multiphasic β-cell insulin response to hyperglycemia. These take into account renal excretion, endogenous production, and utilization of glucose, as well as subcutaneous insulin and glucose kinetics. Each model and submodel have their own mathematical expression and corresponding equations.33
Algorithms other than the PID and MPC include a linear quadratic Gaussian algorithm for subcutaneous blood glucose regulation36 and Lehmann and Deutsch model and Elashoff model algorithms for glucose absorption by the intestine.37
Algorithms now include an optimizer function that enables them to find the best set of current and future changes in insulin delivery to maintain desired glucose levels over a prespecified prediction time horizon.1 Algorithms can decrease postprandial insulin infusion to avoid hypoglycemia by projecting a time-limited postprandial glucose excursion.1
In silico algorithm development
The development of artificial β-cell algorithms has been facilitated by extensive testing in silico, that is, testing with computers rather than in laboratory animals or humans.33 Computers performing in silico simulations can draw upon extensive data collected in human studies.5,6,33 Among the in silico subject databases available for simulations are the demographic and metabolic parameters of weight, insulin requirements, fasting plasma glucose, insulin effect on glucose utilization, and the CHO ratio.5 The CHO ratio represents the largest insulin bolus (in U/g) for CHO that does not lower plasma glucose to <95% of fasting plasma glucose after a meal containing 50 g of CHO.5 Simulations can now be performed using data obtained from the same glucose-sensing and insulin-injecting hardware that will be used in a planned clinical trial rather than from other CGMs and insulin pumps.38 Pump-controlling algorithms can marshal information from multiple algorithms and, in some systems, “vote” on the insulin output suggested by several algorithms to determine the precise dosage.6 In silico simulations can provide indispensable information about the safety and limitations of closed-loop control algorithms, guide clinical studies cost-effectively, and rule out ineffective protocols.5,39
In the aggregate, in silico trials have demonstrated that glycemic regulation based on MPC linear output feedback achieves superior glucose regulation (vs. PID-based control).33
Results of in silico trials
In January 2008, the FDA approved an in silico simulation environment as a substitute for animal trials in preclinical closed-loop control experiments. In April 2008, the agency allowed this investigational device to be used in preclinical experiments. The algorithm system used in a follow-up in silico trial included a cohort of 300 simulated subjects based on real data and was reflective of a T1DM patient population. The system included a simulator of errors of marketed CGM sensors and of insulin delivery via marketed insulin pumps. It represented glucose fluctuations observed during prandial challenges in patients with T1DM.5
Buckingham6 demonstrated the ability of algorithms to prevent nocturnal hypoglycemia or to sound an alarm at the approach of hypoglycemia. This system could suspend pump operation to avoid hypoglycemia using a “voting” system for triggering a predictive alarm when two of five algorithms predicted future hypoglycemia. Marchetti et al.31 developed and validated a closed-loop strategy in silico that was based on the physiologic compartment model of Hovorka et al.34 The system enables PID-based postprandial insulin delivery control and filters to reduce sensitivity to CGM sensor noise.
In initial human trials using the FDA-approved paradigm, closed-loop fasting glucose control was excellent, with a fivefold reduction in nocturnal hypoglycemia. Overnight, closed-loop subjects spent significantly more time at glucose targets of 70–140 mg/dL (vs. open-loop systems).39 In silico evaluations of closed-loop control algorithms are likely to be prerequisites to clinical trials of the artificial pancreas.
The Way Forward
Although intravenous or intraperitoneal devices may deliver insulin more physiologically than subcutaneous devices, they entail risks associated with invasive procedures (e.g., infection).10 Use of conventional pump and patch pump subcutaneous systems in combination with CGM cannot effectively “close the loop” because of inherent delays in glucose sensing and insulin delivery, rendering these conventional systems unable to accommodate the effects of large or rapidly absorbed meals or exercise.10 Until improved software and hardware become available, near-term iterations of the artificial pancreas will likely be open- or modified-loop types where the patient will need to direct the pump to deliver insulin at certain times (i.e., meals).40
Anticipated advances in artificial pancreas hardware include the following: more accurate glucose sensors, which are already under development, providing input into controller algorithms; pumps that are more rapidly responsive to algorithm output; single (nonredundant) catheters or cannulae for both glucose sensing and insulin infusion41; patches that incorporate both a CGM and an infusion set; dual-chamber systems capable of administering more than one therapeutic agent42; and a universal hardware interface capable of handling any CGM or insulin pump.43 Additionally, development of an ultra-rapid-acting insulin could provide a pharmacologic “assist” to progress on the artificial pancreas.
A relatively user-friendly patch pump platform—lightweight, comfortable, operable discreetly under clothing, and free of tubing issues—should incorporate high-resolution screens, appealing graphics, and easily manipulated controls.
Finally, refined in silico modeling should expedite the development of improvements in control algorithms and thereby pave the way for the planning and conduct of successful clinical trials needed for U.S. regulatory approval.
Conclusions
Although inherent limitations in current technology make a fully closed-loop system challenging, technologic advances make the “artificial pancreas” an increasingly realistic prospect. Discretion of patch pump platforms is appealing within a closed-loop system, and many are under development. In silico models are being refined and validated with various hardware combinations and will serve to accelerate regulatory review and approval of closed-loop systems on the horizon. Avoiding severe hypoglycemia would be a revolutionary change in diabetes management. Ultimately, an automated artificial pancreas may improve clinical outcomes and quality of life.
Acknowledgments
Assistance in research and manuscript preparation was provided by Hyman W. Fisher, M.D., and Stephen W. Gutkin, Rete Biomedical Communications Corp. (Wyckoff, NJ), with support from the sponsor. This review was supported by Animas Corp., West Chester, PA, which had a role in manuscript preparation and the decision to publish the findings.
Author Disclosure Statement
H.A. is an employee of Animas Corp., a Johnson and Johnson Company, and minor shareholder in Johnson and Johnson Co. N.J.V.B. has conducted studies with both the V-Go patch made by Valeritas and the Finesse patch made by Calibra and prescribes the OmniPod made by Insulet.
References
- 1.Bequette BW. A critical assessment of algorithms and challenges in the development of a closed-loop artificial pancreas. Diabetes Technol Ther. 2005;7:28–47. doi: 10.1089/dia.2005.7.28. [DOI] [PubMed] [Google Scholar]
- 2.Kumareswaran K. Evans ML. Hovorka R. Artificial pancreas: an emerging approach to treat Type 1 diabetes. Expert Rev Med Devices. 2009;6:401–410. doi: 10.1586/erd.09.23. [DOI] [PubMed] [Google Scholar]
- 3.Friedrich MJ. Artificial pancreas may soon be a reality. JAMA. 2009;301:1525–1527. doi: 10.1001/jama.2009.478. [DOI] [PubMed] [Google Scholar]
- 4.Kowalski AJ. Can we really close the loop, how soon? Accelerating the availability of an artificial pancreas: a roadmap to better diabetes outcomes. Diabetes Technol Ther. 2009;11(Suppl 1):S-113–S-119. doi: 10.1089/dia.2009.0031. [DOI] [PubMed] [Google Scholar]
- 5.Kovatchev BP. Breton M. Man CD. Cobelli C. In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Technol. 2009;3:44–55. doi: 10.1177/193229680900300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckingham B. Prediction, automated prevention of hypoglycaemia with a prototype closed-loop system. Presented at EASD/JDRF Symposium Artificial Pancreas Technologies: Progress Towards a Mechanical Closed-Loop System to Restore Euglycemia, at the 45th European Association for the Study of Diabetes (EASD) Annual Meeting; September 29–October 2, 2009; Vienna, Austria. [Google Scholar]
- 7.Weinzimer SA. Tamborlane WV. Sensor-augmented pump therapy in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2008;15:118–122. doi: 10.1097/MED.0b013e3282f7960b. [DOI] [PubMed] [Google Scholar]
- 8.Bruttomesso D. Farret A. Costa S. Marescotti MV. Vettore M. Avogaro A. Tiengo A. Dalla Man C. Place J. Facchinetti A. Guerra S. Magni L. De Nicolao G. Cobelli C. Renard E. Maran A. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: preliminary studies in Padova and Montpellier. J Diabetes Sci Technol. 2009;3:1014–1021. doi: 10.1177/193229680900300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steil GM. Rebrin K. Darwin C. Hariri F. Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55:3344–3350. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 10.Hovorka R. The future of continuous glucose monitoring: closed loop. Curr Diabetes Rev. 2008;4:269–279. doi: 10.2174/157339908785294479. [DOI] [PubMed] [Google Scholar]
- 11.Hypoglycemia in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes. 1997;46:271–286. [PubMed] [Google Scholar]
- 12.Pedersen-Bjergaard U. Pramming S. Heller SR. Wallace TM. Rasmussen AK. Jorgensen HV. Matthews DR. Hougaard P. Thorsteinsson B. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev. 2004;20:479–486. doi: 10.1002/dmrr.482. [DOI] [PubMed] [Google Scholar]
- 13.Leese GP. Wang J. Broomhal J. Kelly P. Marsden A. Morrison W. Frier BM. Morris AD. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26:1176–1180. doi: 10.2337/diacare.26.4.1176. [DOI] [PubMed] [Google Scholar]
- 14.Laing SP. Swerdlow AJ. Slater SD. Botha JL. Burden AC. Waugh NR. Smith AWM. Hill RD. Bingley PJ. Patterson CC. Qiao Z. Keen H. The British Diabetic Association Cohort Study, II: cause-specific mortality in patients with insulin-treated diabetes mellitus. Diabet Med. 1999;16:466–471. doi: 10.1046/j.1464-5491.1999.00076.x. [DOI] [PubMed] [Google Scholar]
- 15.Ceriello A. Ihnat MA. Thorpe JE. Clinical review 2: The “metabolic memory”: is more than just tight glucose control necessary to prevent diabetic complications? J Clin Endocrinol Metab. 2009;94:410–415. doi: 10.1210/jc.2008-1824. [DOI] [PubMed] [Google Scholar]
- 16.Nathan DM. Cleary PA. Backlund JY. Genuth SM. Lachin JM. Orchard TJ. Raskin P. Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickup JC. Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25:765–774. doi: 10.1111/j.1464-5491.2008.02486.x. [DOI] [PubMed] [Google Scholar]
- 18.Beléndez M. Hernández-Mijares A. Beliefs about insulin as a predictor of fear of hypolycemia. Chronic Illn. 2009;5:250–256. doi: 10.1177/1742395309346464. [DOI] [PubMed] [Google Scholar]
- 19.Di Battista AM. Hart TA. Greco L. Gloier J. Type 1 diabetes among adolescents: reduced diabetes self-care caused by social fear of hypoglycemia. Diabetes Educ. 2009;35:465–475. doi: 10.1177/0145721709333492. [DOI] [PubMed] [Google Scholar]
- 20.Patton SR. Dolan LM. Henry R. Powers SW. Parental fear of hypoglycemia: young children treated with subcutaneous insulin infusion. Pediatr Diabetes. 2007;8:362–368. doi: 10.1111/j.1399-5448.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- 21.Zisser H. Robinson L. Bevier W. Dassau E. Ellingsen C. Doyle FJ. Jovanovic L. Bolus calculator: a review of four “smart” insulin pumps. Diabetes Technol Ther. 2008;10:441–444. doi: 10.1089/dia.2007.0284. [DOI] [PubMed] [Google Scholar]
- 22.Bohannon NJ. Patch pumps. Pump Therapy 2009 session; American Diabetes Association 69th Scientific Sessions; Jun 5–9;2009 ; New Orleans, LA. [Google Scholar]
- 23.http://www.solo4you.com. [Dec 1;2009 ]. http://www.solo4you.com
- 24.http://www.adventventures.com/news/story.cfm?ref = 350. [Jan 5;2010 ]. http://www.adventventures.com/news/story.cfm?ref = 350
- 25.http://www.nilimedix.com/?p = products.patch. [Dec 1;2009 ]. http://www.nilimedix.com/?p = products.patch
- 26.http://archive.globes.co.il/searchgl/NiliMedix. [Dec 1;2009 ]. http://archive.globes.co.il/searchgl/NiliMedix
- 27.http://vadim.yuzhakov.com/media/2005%20The%20Gold%20Sheet.pdf. [Dec 1;2009 ]. http://vadim.yuzhakov.com/media/2005%20The%20Gold%20Sheet.pdf
- 28.http://www.aapspharmaceutica.com/meetings/files/32/Yuzhakov.pdf. [Dec 1;2009 ]. http://www.aapspharmaceutica.com/meetings/files/32/Yuzhakov.pdf
- 29.http://www.in-pharmatechnologist.com/Materials-Formulation/Pump-technology-sustains-insulin-patch. [Dec 15;2009 ]. http://www.in-pharmatechnologist.com/Materials-Formulation/Pump-technology-sustains-insulin-patch
- 30.http://www.israel21c.org/health/israel-s-steadymed-pumps-it-up-more-for-less. [Dec 15;2009 ]. http://www.israel21c.org/health/israel-s-steadymed-pumps-it-up-more-for-less
- 31.Marchetti G. Barolo M. Jovanovic L. Zisser H. Seborg DE. An improved PID switching control strategy for type 1 diabetes. IEEE Trans Biomed Eng. 2008;55:857–865. doi: 10.1109/TBME.2008.915665. [DOI] [PubMed] [Google Scholar]
- 32.Cengiz E. Swan KL. Tamborlane WV. Steil GM. Steffen AT. Weinzimer SA. Is an automatic pump suspension feature safe for children with type 1 diabetes? An exploratory analysis with a closed-loop system. Diabetes Technol Ther. 2009;11:207–210. doi: 10.1089/dia.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magni L. Raimondo DM. Bossi L. Man CD. De Nicolao G. Kovatchev B. Cobelli C. Model predictive control of type 1 diabetes: an in silico trial. J Diabetes Sci Technol. 2007;1:804–812. doi: 10.1177/193229680700100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hovorka R. Canonico V. Chassin LJ. Haueter U. Massi-Benedetti M. Orsini Federici M. Pieber TR. Schaller HC. Schaupp L. Vering T. Wilinska ME. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25:905–920. doi: 10.1088/0967-3334/25/4/010. [DOI] [PubMed] [Google Scholar]
- 35.Dalla Man C. Rizza RA. Cobelli C. Meal simulation model of the glucose-insulin system. IEEE Trans Biomed Eng. 2007;54:1740–1749. doi: 10.1109/TBME.2007.893506. [DOI] [PubMed] [Google Scholar]
- 36.Patek SD. Breton MD. Chen Y. Solomon C. Kovatchev B. Linear quadratic Gaussian-based closed-loop control of type 1 diabetes. J Diabetes Sci Technol. 2007;1:834–841. doi: 10.1901/jaba.2007.1-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalla Man C. Camilleri M. Cobelli C. A system model of oral glucose absorption: validation on gold standard data. IEEE Trans Biomed Eng. 2006;53:2472–2478. doi: 10.1109/TBME.2006.883792. [DOI] [PubMed] [Google Scholar]
- 38.Dassau E. Palerm CC. Zisser H. Buckingham BA. Jovanovic L. Doyle FJ. In silico evaluation platform for artificial pancreatic beta-cell development: a dynamic simulator for closed-loop control with hardware-in-the-loop. Diabetes Technol Ther. 2009;11:187–194. doi: 10.1089/dia.2008.0055. [DOI] [PubMed] [Google Scholar]
- 39.Cobelli C. Subcutaneous model predictive closed-loop control of type 1 diabetes: From in-silico to human studies. Presented at EASD/JDRF Symposium Artificial Pancreas Technologies: Progress Towards a Mechanical Closed-Loop System to Restore Euglycemia, at the 45th European Association for the Study of Diabetes (EASD) Annual Meeting; September 29–October 2, 2009; Vienna, Austria. [Google Scholar]
- 40.Weinzimer SA. Steil GM. Swan KL. Dziura J. Kurtz N. Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31:934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 41.Regittnig W. Lindpointner S. Korsatko S. Köhler G. Kaidar R. Yodfat O. Köhler H. Ellmerer M. Pieber TR. Glucose concentrations at the site of subcutaneous insulin delivery in patients with type 1 diabetes. Presented at the 45th European Association for the Study of Diabetes Annual Meeting; Vienna, Austria. 2009. [Google Scholar]
- 42.Damiano ER. Model predictive closed-loop control with insulin, glucagon. Presented at EASD/JDRF Symposium Artificial Pancreas Technologies: Progress Towards a Mechanical Closed-Loop System to Restore Euglycemia at the 45th European Association for the Study of Diabetes (EASD) Annual Meeting; Vienna, Austria. 2009. [Google Scholar]
- 43.Kovatchev B. In silico models: application prior to human testing; Presented at the Artificial Pancreas Workshop; July 21–22; Bethesda, MD. 2008. [Google Scholar]