Abstract
Sphingosine-1-phosphate (S1P), a potent lipid mediator, transduces intracellular signals through the activation of S1P receptors (S1PRs). Although S1PRs have been shown to play an important role in the central nervous system (CNS), accurate localization and the function of S1PR1 in the human CNS are still unclear. In this study, we investigated the localization of S1PR1 in the human CNS of postmortem samples, using a rabbit polyclonal antibody, the specificity of which had been well defined. Immunohistochemical investigation of paraffin-embedded sections revealed diffuse granular staining of the gray matter. The signals of the gray matter were much stronger than those of the white matter. The immunohistochemical expression levels correlated well with the results of quantitative real-time RT-PCR–based analysis and Western blotting. Studies using double immunostaining and immunoelectron microscopy revealed that the antigen was strongly expressed in the membrane of the astrocytic foot processes of glia limitans and astrocytes with radial cytoplasm, but not distributed in neurons. In neurological disorders, hypertrophic astrocytes with strong expression of glial fibrillary acidic protein exhibited significantly decreased expression of S1PR1 in contrast to its strong expression in astrocytes forming fibrillary gliosis. These results indicate that S1PR1 is localized in astrocytes, and its expression level may change during the processes that occur after brain damage. (J Histochem Cytochem 58:847–856, 2010)
Keywords: astrocyte, immunoelectron microscopy, immunohistochemistry, glia, sphingosine-1-phosphate receptor 1
Sphingosine-1-phosphate (S1P), a potent lipid mediator produced from the metabolism of sphingolipids by the action of sphingosine kinase (SPHK), transduces intracellular signals through the activation of five G-protein–coupled receptors, termed S1P receptor (S1PR) 1, S1PR2, S1PR3, S1PR4, and S1PR5 (Toman and Spiegel 2002; Hannun and Obeid 2008). S1P influences different biological processes, such as cell proliferation, survival, migration, and morphogenesis, depending on the relative expression of these S1PRs coupled to different intracellular second messenger systems, including phospholipase C and phosphatidylinositol 3-kinase/protein kinase Akt, as well as Rho- and Ras-dependent pathways (Sanchez and Hla 2004; Kihara et al. 2007).
S1PRs are widely expressed in the central nervous system (CNS), with region-specific distributions (Toman and Spiegel 2002; Jaillard et al. 2005; Ohuchi et al. 2008; Chun and Hartung 2010). The S1P/S1PR signal has been shown to play an important role in neural development, regulation of neural stem cells and glial migration, astrocyte proliferation, protection against apoptosis, and, more recently, modulation of neuronal excitability and glutamatergic neurotransmission (Chun et al. 2000; Toman and Spiegel 2002; Yamagata et al. 2003; Harada et al. 2004; Kajimoto et al. 2007; Kimura et al. 2007; Milstien et al. 2007; Ohuchi et al. 2008). However, in situ localization of S1PRs in the human CNS remains unclear because of the lack of specific antibodies against S1PRs; most findings have been obtained from experiments at the mRNA level (Brinkmann 2009).
FTY720 is a structural analogue of sphingosine and has biological activity in vivo by being phosphorylated by SPHKs (Mandala et al. 2002), which are abundantly expressed in the brain (Blondeau et al. 2007; Bryan et al. 2008). The phosphorylated form of FTY720 (FTY720P) activates S1PRs, mainly S1PR1, in a fashion similar to that of endogenous S1P. It has been shown that it can be used as a drug for the treatment of multiple sclerosis (Kappos et al. 2010). Because FTY720 administration dose dependently induces a peripheral lymphopenia, probably by trapping lymphocytes in lymph nodes, it may prevent autoreactive lymphocytes from moving to the CNS. Otherwise, the drug crosses the blood–brain barrier and may have direct effects on CNS by stimulating the repair process after injury (Miron et al. 2008; Brinkmann 2009; Chun and Hartung 2010). More precise assessment for the expression of S1PRs, especially S1PR1, is required to determine the role of FTY720 in neurological disorders.
We recently investigated S1PR1 expression in formalin-fixed, paraffin-embedded tissue sections of human tissues using an antibody, the specificity of which had been defined by immunostaining of the vasculature in S1PR1−/− and S1PR1+/− mouse embryos (Akiyama et al. 2008). The S1PR1 antigen was stable even in autopsy samples after long-standing fixation and could be used as an immunohistochemical marker (Akiyama et al. 2008,2009; Nishimura et al. 2010). In this study, to clarify the expression of S1PR1 in the human CNS, we investigated postmortem specimens by IHC, immunoelectron microscopy, Western blotting (WB), and quantitative real-time RT-PCR.
Materials and Methods
The study design was approved by the ethics review board of Kawasaki Medical School, Okayama, Japan.
Antibodies
A rabbit polyclonal antibody against aa 322–381 of S1PR1 of human origin [EDG-1 (H60): sc-25489; Santa Cruz Biotechnology, Santa Cruz, CA] was used. This antibody (1:50 dilution) has been thoroughly checked for specificity by comparing the immunostaining results of the vasculature in paraffin sections of S1PR1−/− and S1PR1+/− mouse embryos, an angiosarcoma cell line (ISO-HAS), and mantle cell lymphoma expressing considerable quantities of S1PR1 mRNA (Akiyama et al. 2008; Nishimura et al. 2010). A prediluted rabbit anti-glial fibrillary acidic protein (GFAP) antibody (Nichirei; Tokyo, Japan), polyclonal antibody against aquaporin 4 (AQP4, 1:400 dilution; Millipore, Billerica, MA), and synaptophysin (1:400 dilution; Biogene, Montreal, Canada) were also used.
Tissue Specimens
Tissue samples were retrieved from the files of the Department of Pathology, Kawasaki Medical School. Clinical and pathological information on cases used in this study is summarized in Table 1. The autopsy specimens were fixed in 7.4% buffered formaldehyde for 2 weeks, and samples were taken from the frontal lobe, temporal lobe, parietal lobe, occipital lobe, cingulate gyrus, amygdala, hippocampus with entorhinal and transentorhinal cortex, motor cortex, visual cortex, basal ganglia, thalamus, hypothalamus, mamillary body, subthalamic nucleus, lateral geniculate body, midbrain, pons, medulla oblongata, cerebellum, and spinal cord. These samples were embedded in paraffin and sectioned into 6-μm-thick pieces (hemisphere sections were cut into 8-μm-thick pieces). Routine stainings of hematoxylin-eosin (HE) and Klüver–Barrera were conducted for the diagnosis.
Table 1.
Summary of clinicopathological information on autopsy cases used in this study
| Case | Age/sex | Clinical diagnosis | Site of specimen |
|---|---|---|---|
| 1 | 72/male | Pituitary adenoma | CNS |
| 2 | 57/male | Amyotrophic lateral sclerosis | CNS |
| 3 | 82/male | Cerebral hemorrhage | CNS |
| 4 | 83/female | Sepsis | CNS |
| 5 | 80/female | Lacunar infarction | CNS |
| 6 | 83/female | Cerebellar hemorrhage | CNS |
| 7 | 60/female | Chronic renal failure | CNS |
| 8 | 57/male | Burger disease | Sympathetic ganglion |
| 9 | 49/male | Ischemia of lower leg | Sympathetic ganglion |
| 10 | 51/male | Gastric cancer | Auerbach's plexus |
| 11 | 61/female | Colon cancer | Auerbach's plexus |
| 12 | 57/male | Respiratory failure | Adrenal medulla |
| 13 | 79/male | Metastatic cancer | Cerebrum |
| 14 | 70/male | Subacute infarction | Cerebrum |
| 15 | 98/male | Subacute infarction | Cerebrum |
| 16 | 91/male | Subacute infarction | Cerebellum |
| 17 | 72/female | Old infarction | Cerebrum |
| 18 | 98/male | Old infarction | Cerebrum |
| 19 | 74/male | Old infarction | Cerebellum |
| 20 | 62/male | Multiple system atrophy | Pons |
Central nervous system (CNS) includes cerebrum, cerebellum, brain stem, and spinal cord. In cases 1–12, the examined areas were shown to be within normal limits. There was no lesion of hemorrhage, infarction, tumor, or neurodegeneration. In case 2, abnormal lesions were almost limited to the spinal cord and motor nucleus of the brain stem, and the cerebrum and cerebellum were shown to be within normal limits.
Immunohistochemistry
Seven cases (cases 1–7; Table 1) were used for IHC. The sections were deparaffinized; antigen retrieval was carried out in a pressure cooker containing Tris–EDTA-buffered solution (pH 9.0) for 10 min using a microwave oven (600 W), and the sections were reacted with 3% H2O2 for 5 min to eliminate endogenous peroxidase activity. After incubation in 10% FBS, the sections were first allowed to react with the antibodies for 60 min at room temperature. They were then washed in TBS and allowed to react with the EnVision Detection System (Dako; Glostrup, Denmark) for 30 min.The sections were then incubated in DAB (Dako) for chromogen and counterstained with hematoxylin. Antigen retrieval was not carried out in cases of GFAP and AQP4 staining. For the staining of sections with melanin pigments or Nissl bodies, the sections were incubated in 3,3′,5,5′-tetramethylbenzidine (Trueblue; KPL, Gaithersburg, MD) or were incubated with an alkaline phosphatase-conjugated secondary anti-mouse/rabbit IgG antibody (Histofine simple stain AP; Nichirei) instead of EnVision, and the signals were detected using a New Fuchsin substrate kit (Nichirei). Negative controls were treated in the same manner, but without the primary antibodies.
Double Immunostaining
After antigen retrieval in a pressure cooker containing Tris–EDTA-buffered solution (pH 9.0) for 10 min using a microwave oven (600 W), the tissue sections were first incubated with the antibody mixture for 60 min at room temperature. The sections were then incubated with an alkaline phosphatase-conjugated secondary anti-mouse/rabbit IgG antibody (Histofine simple stain AP), and signals were detected using the New Fuchsin substrate kit. Then, the sections were incubated with an antibody mixture for 60 min at room temperature after the antigen retrieval. The sections were incubated with a peroxidase-conjugated anti-mouse/rabbit IgG antibody (EnVision), and the signals were detected using Trueblue.
Immunocytochemistry
Cerebral and cerebellar cortices from cases 3 and 4 were used for squash preparations (Dawson et al. 2003). These specimens were immediately fixed in 95% ethanol for 1 hr, and endogenous peroxidase activity was eliminated by incubating them in 0.3% H2O2 in methanol for 15 min. Without antigen retrieval, the sections were stained using rabbit anti-S1PR1 antibody (1:20 dilution) for 30 min. They were then washed in TBS and allowed to react with the EnVision Detection System for 30 min. Then, the sections were incubated in DAB for chromogen and counterstained with hematoxylin.
Immunoelectron Microscopy
Preembedded immunoelectron microscopy was performed using the free-floating method (Sadahira et al. 1988). Cerebral cortex autopsy samples from cases 3 and 4 were fixed with 4% paraformaldehyde containing 0.1% glutaraldehyde in a 0.1 mol/liter phosphate buffer solution (pH 7.4) for 2 hr. They were then rinsed in PBS and cut into 50-μm sections using a vibratome. The sections were stained in the free-floating state using the VECTASTAIN ABC kit (Vector Laboratories; Burlingame, CA). After treatment with PBS containing 0.1% saponin for 30 min, the sections were incubated with PBS containing normal serum for 1 hr. Then, they were incubated in a polyclonal rabbit anti-S1PR1 antibody (1:10 dilution) overnight at 4C, washed in PBS, and incubated in a biotinylated secondary antibody for 3 hr at room temperature. The sections were washed in PBS and incubated in an ABC reagent at room temperature for 1 hr. After washing in PBS, the sections were incubated in 0.02% DAB for 10 min and then reacted with DAB containing H2O2. They were refixed with 1% glutaraldehyde, washed in PBS, and stained for peroxidase activity. Negative controls were treated in the same manner, but without the primary antibodies. After the immunohistochemical reaction, the sections were postfixed with 2% osmium tetroxide, dehydrated, and embedded in Epon. Ultrathin sections were examined by electron microscopy after staining with uranyl acetate.
Western Blotting
Four cases (cases 1–4; Table 1) were used for WB. Total proteins were extracted from the brain of autopsy cases for WB. Brain tissues were obtained from the cerebrum (parietal, occipital, or temporal lobe, according to the case); both cerebral cortices and white matter were sampled separately and frozen using liquid nitrogen. Immediately after the addition of a boiled lysis buffer [1% SDS, 1.0 mM sodium ortho-vanadate, and 10 mM Tris (pH 7.4)], the cerebral cortex or the white matter was homogenized, boiled for 5 min, passed through a 26.5-gauge needle five to t10 times, and centrifuged to prepare tissue extracts. The proteins of each tissue extract were quantified using a Qubit Fluorometer (Life Technologies; Carlsbad, CA). An angiosarcoma cell line (ISO-HAS) was cultured and rinsed three times with PBS at room temperature. Immediately after the addition of the boiled lysis buffer, the cells were collected by scraping and were boiled for 5 min, passed through a 26.5-gauge needle five times, and centrifuged to prepare cell extracts. Then, 20 μg of each of the extracts, a molecular marker of the Novex Sharp Pre-Stained Protein Standard, and MagicMark XP Western Protein Standard (Life Technologies) were separated by NuPAGE 4–12% Bis–Tris gel (Life Technologies) electrophoresis and transferred to a polyvinylidene difluoride membrane using iBlot (Life Technologies). The membrane was treated with a blocking reagent (Roche Diagnostics; Basel, Switzerland) at room temperature for 1 hr. A rabbit anti-human S1PR1 antibody (1:500 dilution) was reacted with the membrane at 4C overnight. A mouse anti-β-actin monoclonal antibody (Sigma-Aldrich; St Louis, MO) was reacted with the membrane at room temperature for 1 hr. The membrane was washed with TBS for 30 min and incubated at room temperature for 60 min with a horseradish peroxidase–conjugated anti-mouse/rabbit IgG antibody (Roche Diagnostics). The membrane was washed with TBS for 30 min, treated with ECL Plus Western Blotting Detection System (Roche Diagnostics), and visualized using a cooled charge-coupled device camera, ChemiStage CC-16 (Kurabo; Osaka, Japan).
Quantitative Real-time RT-PCR
Four postmortem cases (cases 1–4; Table 1) were used for real-time RT-PCR (Cummings et al. 2001). Total mRNA was extracted using a RiboPure kit (Life Technologies) and quantified using a Qubit Fluorometer (Life Technologies). cDNA was synthesized from each extract containing 1 μg of mRNA using a QuantiTect Reverse Transcription kit (Qiagen; Hilden, Germany). Real-time PCR primers were purchased from Qiagen (QuantiTect Primer Assay) for human S1PR1 (QT00208733), S1PR2 (QT00230846), S1PR3 (QT00244251), S1PR4 (QT01192744), S1PR5 (QT00234178), SPHK-1 (QT01011927), SPHK-2 (QT00085386), and RPS18 (QT00248682). Gene expression levels were analyzed on an Applied Biosystems 7500 PCR System (Life Technologies) with a QuantiTect SYBR Green PCR kit (Qiagen). S1PR and SPHK expressions were normalized by comparison with the expression of a reference gene, 18S rRNA. The conditions for amplification were 15 min at 95C to activate the HotStarTaq DNA polymerase, 40 cycles of 94C for 15 sec, annealing at 55C for 30 sec, and extension at 72C for 34 sec. As the PCR efficiency of the reaction was comparable between the target and the endogenous reference gene, 18S rRNA, normalized S1PR1, S1PR2, S1PR3, S1PR4, S1PR5, SPHK1, and SPHK2 expressions were calculated using ABI software and 2-ΔΔCt analysis.
Statistical Analysis
The QT-PCR data were analyzed using Student's t-test and Welch's t-test. Statistical significance was defined as a p value <0.01.
Results
Anatomic Localization of S1PR1 in the CNS
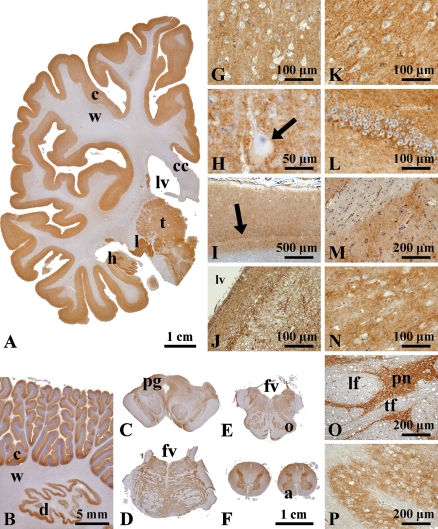
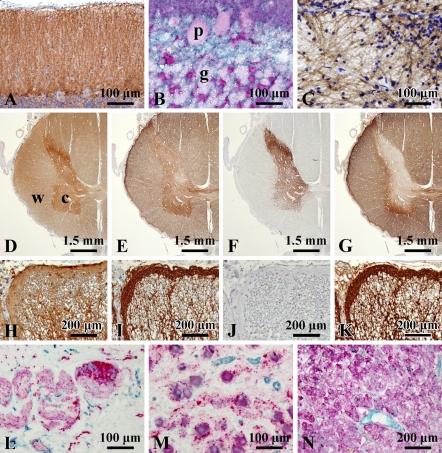
First, we investigated the expression of S1PR1 in the various CNS regions from seven autopsy cases (cases 1–7; Table 1) by IHC. Positive signals were widely distributed in the gray matter throughout the CNS. The S1PR1 expression was much stronger in the gray matter (cerebral cortex, subcortical gray matter, cerebellar cortex, brain stem nuclei, and spinal central gray matter) than in the white matter (Figures 1A–1P).
Figure 1.
Macroscopic and microscopic localization of sphingosine-1-phosphate receptor 1 (S1PR1) in formalin-fixed, paraffin-embedded sections of the central nervous system. Macroscopically, immunoreaction products are localized mainly in gray matter (A, cerebral hemisphere), cerebellar cortex and dentate nucleus (B), and brain stem gray matter (C, midbrain; D, pons; and E, medulla oblongata). Exceptionally, white matter is mildly stained in the spinal cord (F). Microscopically, no difference in S1PR1 expression pattern is seen in the regions of the cerebral cortex, subcortical nuclei, and brainstem nuclei (G–P). Betz cells of precentral gyrus (H, arrow) and the line of Gennari in the striate cortex (I, arrow) are seen. Some areas of the subventricular zone are also stained for S1PR1 (J). In the pons (O), positive signals are seen in the pontine nuclei (pn), but not in the longitudinal fasciculus (lf) and transverse fiber (tf). (G) Parietal lobe, (H) precentral gyrus, (I) striate cortex, (J) subventricular zone, (K) hippocampus, (L) dentate gyrus, (M) putamen, (N) thalamus, (O) pontine nuclei, and (P) inferior olivary nucleus. c, cortex; w, white matter; cc, corpus callosum; lv, lateral ventricle; t, thalamus; l, lateral geniculate body; h, hippocampus; d, dentate nucleus; pg, periaqueductal gray; fv, fourth ventricle; o, inferior olivary nucleus; a, anterior funiculus.
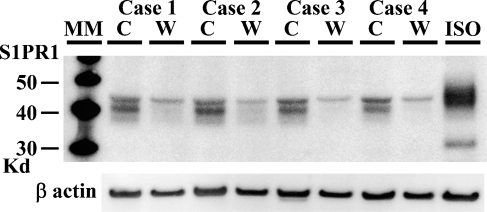
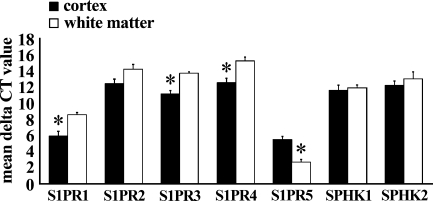
To correlate with the findings of IHC, we next examined the protein of S1PR1 and the mRNA expression of S1PRs in cerebrums from four autopsy cases. On WB, the reaction products were seen with a molecular mass of about 40–45 kDa in the cortex fraction, which was consistent with the findings of S1PR1, whereas the band of 40–45 kDa was very faint in the white matter fraction (Figure 2). We next examined the mRNA expression of S1PRs, SPHK1, and SPHK2 in cerebral cortex and white matter postmortem samples from four autopsy cases. The average ΔCt values for genes expressed in the cortex and white matter are shown in Figure 3. In all cases, the expression of S1PR1 mRNA in the cerebral cortex was significantly greater than that in the white matter. The quantity of S1PR1 mRNA in the cerebral cortex was 6.3-fold greater than that in the white matter. The quantity of S1PR5 mRNA in the white matter was 6.9-fold greater than that in the cortex.
Figure 2.
A comparative study of S1PR1 expression between gray matter and white matter of cerebrum by Western blotting. All gray matter samples from four autopsy cases give strong signals at 40–45 kDa, whereas corresponding signals were faintly observed in white matter. Twenty μg of protein per lane was applied. Total proteins from an angiosarcoma cell line (ISO-HAS) were used as a positive control. Signals of β-actin appear with almost the same intensity between gray matter and white matter. C, cortex; W, white matter; ISO, ISO-HAS.
Figure 3.
A comparative study of expression of S1PR and SPHK between gray matter and white matter of cerebrum of four autopsy cases by quantitative real-time RT-PCR. Data are represented as the mean ΔCt ± SEM (ΔCt = Ct value of target mRNA − Ct value of 18S rRNA); therefore, greater expression is equivalent to smaller ΔCt value of mRNA. S1PR1 and S1PR3 mRNA are more abundantly expressed in gray matter than in white matter (*p<0.01). Inversely, S1PR5 mRNA is more abundantly expressed in white matter than in gray matter (*p<0.01).
S1PR1 Expression in Cerebrum
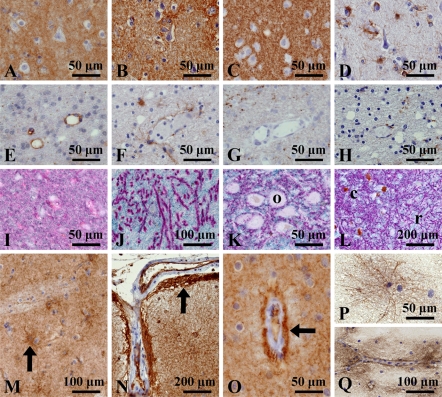
The gray matter exhibited diffuse staining of S1PR1. There was no difference in the staining pattern among the frontal, temporal, parietal, and occipital cortices, primary areas (motor and visual cortices), limbic systems (hippocampus, amygdala, and cingulate cortex), and transentorhinal/entorhinal cortex (Figures 1A, 1G–1I, 1K, and 1L). Subcortical nuclei such as the basal ganglia and thalamus also exhibited diffuse staining (Figures 1M and 1N). The subventricular zone was also positive for S1PR1 (Figure 1J). At high-power magnification, the gray matter of all areas showed fine granular staining, but the nerve cell bodies showed no staining (Figures 1 and 4A).
Figure 4.
A comparative study of expression of S1PR1, synaptophysin, aquaporin 4 (AQP4), and glial fibrillary acidic protein (GFAP) in cerebrum (A–L) and expression of S1PR1 in astrocyte morphology (M–Q). (A–D) Cerebral cortex and (E–H) cerebral white matter. At high-power magnification, the gray matter shows diffusely fine granular staining of S1PR1 (A) similar to those of AQP4 (B) and synaptophysin (C), whereas the white matter shows focal faint staining of S1PR1 (E), AQP4 (F), and synaptophysin (G). GFAP-positive astrocytes are loosely distributed in both cortex (D) and white matter (H). Nerve cell bodies do not stain for S1PR1. Double immunostaining of synaptophysin (red) and S1PR1 (blue) demonstrated a fine intermingled distribution pattern of these antigens in the cerebral cortex (I). In the globus pallidus, a clear differential distribution pattern is seen (J). In the midbrain, differential localization is seen in oculomotor nucleus (K) and substantia nigra (L). Astrocytes with radial cytoplasmic processes in the putamen are stained (M). The glia limitans of the brain surface (N) and blood vessels (O) show strong expression. Radial elongated cytoplasmic staining is clearly seen in the squash preparation, by ICC (P). Immunoreaction products are localized around the blood vessels (Q). o, oculomotor nerve; c, substantia nigra pars compacta; r, substantia nigra pars reticulata.
As this staining pattern superficially suggests synaptic staining, we compared the localization pattern of S1PR1 with that of synaptophysin, a marker of the synaptic vesicle; AQP4, a transmembrane water channel protein that has been shown to be densely distributed in astrocytic foot processes (Preston and Agre 1991; Nielsen et al. 1997); and GFAP, a marker of reactive astrocytes. The S1PR1 staining pattern of the cerebrum was very similar to those of synaptophysin and AQP4 (Figures 4A–4H). However, double immunostaining for S1PR1 and synaptophysin gave distinctive intermingled granular staining, indicating different localizations of the antigens (Figure 4I). In the globus pallidus, anti-synaptophysin antibody stained pipe-shaped structures (Goto et al. 1989), part of the neuron, but these structures were not stained for S1PR1 (Figure 4J).
IHC for S1PR1 also infrequently showed radial cytoplasmic staining in the cerebrum (Figure 4M). Stronger S1PR1 staining was seen in the glia limitans of the brain surface and blood vessels (Figures 4N and 4O). An immunocytochemical squash preparation of the cerebral cortex clearly revealed cells with extended radial cytoplasmic processes (Figure 4P). The glia limitans of the blood vessels was also densely labeled on the squash preparations (Figure 4Q).
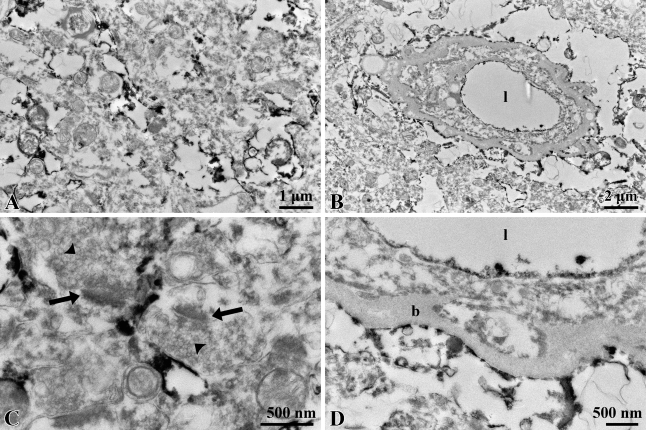
Immunoelectron microscopy demonstrated that reaction products localized on irregular and sheet-like membranous structures are interspersed among the neuronal components and often surround the synapses (Figures 5A and 5C). No signal was seen in the synapses, but strong signals were localized in the glia limitans (Figures 5B and 5D). In the blood vessels, the luminal surfaces of endothelial cells were stained for S1PR1 (Figure 5D).
Figure 5.
Immunoelectron microscopy of S1PR1. Immunoreaction products are localized in thin processes of astrocytes in the (A) neuropil and (B) astrocytic end-feet. (C) No signals are seen in the postsynaptic density (arrows) and synaptic vesicles (arrowheads). (D) The reaction products are also observed on luminal surfaces of the endothelial cells. l, lumen of blood vessel; b, basal membrane.
S1PR1 Expression in Cerebellum
The gray matter was stained for S1PR1 (Figure 1B). Characteristically, the molecular layer exhibited linear S1PR1 staining toward the surface of the cerebellum, which is considered to represent Bergmann fibers (Figure 6A). Purkinje cell bodies were not stained. Double immunostaining for S1PR1 and synaptophysin showed a differential distribution (Figure 6B). An immunocytochemical squash preparation revealed radial elongated cytoplasmic processes (Figure 6C).
Figure 6.
S1PR1 expression in cerebellum (A–C), spinal cord (D–K), and peripheral nervous system (L–N). In the cerebellum, the molecular layer shows linear staining for S1PR1 (A). S1PR1 (blue) and synaptophysin (red) are differentially distributed (B). The area of Bergmann glia shows diffuse staining for S1PR1, but not for synaptophysin. Differential distributions are clearly seen in the granular cell layer. The immunocytochemical squash preparation of the cerebellar cortex shows radial elongated cytoplasmic processes (C). In the spinal cord, comparative immunostaining of S1PR1 (D,H), AQP4 (E,I), synaptophysin (F,J), and GFAP (G,K) is shown. (D–G) Low-power magnification of spinal cord; (H–K) high-power magnification of spinal white matter. The spinal white matter exhibits slightly stronger signals than the cerebral white matter. This localization pattern of S1PR1 is similar to that of AQP4. In the peripheral nervous system, double immunostaining of S1PR1 (blue) and synaptophysin (red) is shown. There is no evidence of S1PR1-positive signals in the neural components. Vascular channels stain for S1PR1. (L) Auerbach's plexus, (M) sympathetic ganglion, and (N) adrenal medulla. p, Purkinje cell; g, granular cell; c, cortex; w, white matter.
S1PR1 Expression in Brain Stem
The gray matter was stained for S1PR1 (Figures 1C–1E, 1O, and 1P). The subpial and subventricular areas were also positive for S1PR1. The substantia nigra pars reticulata exhibited an S1PR1 staining pattern similar to that of the globus pallidus, which was confirmed by double immunostaining for synaptophysin and S1PR1 (Figure 4L). The double immunostaining showed intermingled granular distribution in the brain stem nuclei (Figure 4K).
S1PR1 Expression in Spinal Cord
The gray matter shows diffuse fine granular staining of S1PR1 (Figure 6D) similar to those of AQP4 (Figure 6E) and synaptophysin (Figure 6F). In contrast to cerebral white matter, spinal white matter shows slightly stronger mesh-like signals of S1PR1 (Figures 6D and 6H) similar to those of AQP4 (Figures 6E and 6I) and GFAP (Figures 6G and 6K).
S1PR1 Expression in Peripheral Nervous System
In sympathetic ganglions and the Auerbach's plexus (cases 8–11; Table 1), S1PR1 was not expressed in neuronal components (Figures 6L and 6M). The adrenal medulla (case 12) was negative for S1PR1 (Figure 6N).
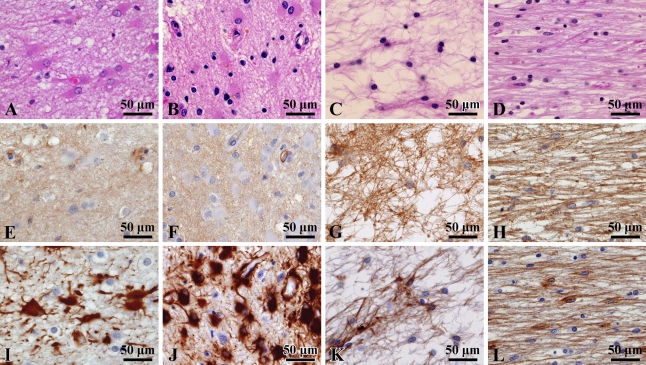
S1PR1 Expression in Astrocytic Pathology
Next, we investigated S1PR1 expression in astrocytosis/fibrillary gliosis by comparison with GFAP expression. Hypertrophic astrocytes proliferating around metastatic lesions in lung cancer (case 13; Table 1) stained strongly for GFAP, but stained faintly for S1PR1 (Figures 7A, 7E, and 7I). In lesions of subacute cerebral infarction (cases 14–16), hypertrophic astrocytes showed strong positivity for GFAP but faint staining for S1PR1 (Figures 7B, 7F, and 7J). In the case of anisomorphic gliosis of old infarctions (cases 17–19), however, S1PR1 was clearly expressed in a dense glial fiber meshwork, which was strongly stained for GFAP (Figures 7C, 7G, and 7K). In a lesion of multiple system atrophy (case 20), isomorphic gliosis was clearly stained for S1PR1 and GFAP (Figures 7D, 7H, and 7L).
Figure 7.
S1PR1 expression in astrocytic pathology. (A–D) Hematoxylin–eosin staining; (E–H) S1PR1 immunostaining; (I–L) GFAP immunostaining. Hypertrophic astrocytes with strong positivity for GFAP show faint staining for S1PR1 in the cases of metastatic carcinoma (A,E,I) and subacute brain infarction (B,F,J). In contrast, strong S1PR1 signals are seen in fibrillary gliosis in the cases of old infarction of cerebral cortex (C,G,K) and multiple system atrophy of middle cerebellar peduncle (D,H,L).
Discussion
S1PRs have been shown to be expressed in the CNS (Toman and Spiegel 2002; Ohuchi et al. 2008; Brinkmann 2009; Chun and Hartung 2010), but in situ localization of S1PR1 in the human CNS remained unclear. In this study, we clearly showed cellular localization of S1PR1 in the human CNS for the first time using IHC, with a well-defined anti-S1PR1 antibody.
To determine the anatomic localization of S1PR1, Waeber and Chiu (1999) studied S1P-stimulated G-protein activity by autoradiography and found that the activity was seen in the gray matter of the forebrain and molecular layer of the cerebellum. Similarly, Sim-Selley et al. (2009) studied the activity stimulated by the S1PR1-selective agonist, SEW2871, by autoradiography. The activity was detected in the cerebral cortex, molecular layer, amygdala, basal ganglia, periaqueductal gray, hippocampus, and hypothalamus, and lower levels were detected in the thalamus and corpus callosum. Indeed, the morphological distinction of astrocytes from neuronal cells is very difficult, especially in gray matter, because cortical astrocytes have abundant lamellate processes surrounding synapses, dendrites, and nerve cell bodies (Hirano and Llena 2006). We investigated S1PR1 expression in the gray matter and white matter separately obtained from autopsy samples and found stronger expression of S1PR1 mRNA in gray matter than in white matter. We also found preferential expression of S1PR3 mRNA in gray matter and S1PR5 mRNA in white matter, which is consistent with the results of previous studies (Jaillard et al. 2005; Brinkmann 2009; Chun and Hartung 2010).
Immunostaining studies allowed us to conclude that S1PR1 is localized in astrocytes, but not in neurons, which could be due to the following findings. First, immunostaining of paraffin sections and squash preparations revealed distinct staining of radial elongated cytoplasmic processes and glia limitans. Second, the expression pattern of S1PR1 in gray matter was similar to that of AQP4, which is abundantly expressed in the astrocyte foot processes (Misu et al. 2007). Third, immunoelectron microscopy revealed an ultrastructural localization of S1PR1 in astrocytic components, but not in synapses. Fourth, double immunostaining revealed differential localization between synaptophysin and S1PR1. Fifth, nerve cells were obviously negative for S1PR1 in the peripheral nervous system.
Astrocytes play important roles in the CNS by regulating ion exchange and neurotransmitter concentrations and by supplying the nutrients and growth factors for neurons (Sofroniew 2005). Matyash and Kettenmann (2009) described the morphological and functional heterogeneity of astrocytes. Recent studies have shown that astrocytes express S1PR1, S1PR2, and S1PR3 at the mRNA level, and S1PRs are considered to be involved in regulating astrocyte function (Brinkmann 2009; Chun and Hartung 2010). Osinde et al. (2007) demonstrated that FTY720P mediates ERK phosphorylation in astrocytes, but not in neurons or oligodendrocytes in primary cultures of the cerebral cortex of rat embryo. Similarly, Mullershausen et al. (2007) showed that FTY720P and the S1PR1-selective agonist SEW2871 stimulate astrocyte migration. However, the role of S1PR1 in astrocyte function in adult humans remains unclear. On the basis of our ultrastructural observation that S1PR1 is preferentially expressed in protoplasmic astrocyte processes surrounding neurons, we conclude that S1P/S1PR1 signaling may be involved in the formation of the processes that maintain astrocyte–neuron contact.
Astrocytes can be activated and proliferated by S1P or FTY720P stimulation (Sorensen et al. 2003; Yamagata et al. 2003; Bassi et al. 2006; Osinde et al. 2007; Wu et al. 2008; Chun and Hartung 2010). In a variety of neurological disorders, astrocytes react by becoming hypertrophic and displaying rapid upregulation of GFAP expression. The reactive astrocytes lead to glial scar with dense GFAP filament formation. In this study, we found that S1PR1 expression is downregulated in hypertrophic astrocytes, with strong GFAP expression. This result suggests a modulation of S1PR1 expression during the activation of astrocytes by inflammatory cytokines, including interleukin-1 and tumor necrosis factor-α (Alvarez et al. 2007). In contrast, S1PR1 was strongly expressed in astrocytes with strong GFAP positivity in fibrillary gliosis of old infarctions and multiple system atrophy. These results are intriguing because FTY720 administration inhibited glial scar formation by directly acting on astrocytes (Miron et al. 2008; Brinkmann 2009). Thus, further immunohistochemical studies with a larger number of cases with CNS disorders are warranted to clarify the involvement of S1P/S1PR1 signaling in neurological disorders.
In conclusion, our study revealed an astrocytic localization of S1PR1 in the human CNS and a change in its expression level in neurological disorders. Further studies using specific antibodies against S1PRs may help to understand the role of S1P/S1PR signaling in human diseases.
Acknowledgments
This study was supported by a grant-in-aid (20590357) from the Ministry of Education, Science, Sports, and Culture of Japan and a project grant (20-209S) from Kawasaki Medical School.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Akiyama T, Hamazaki S, Monobe Y, Nishimura H, Irei I, Sadahira Y (2009) Sphingosine-1-phosphate receptor 1 is a useful adjunct for distinguishing vascular neoplasms from morphological mimics. Virchows Arch 454:217–222 [DOI] [PubMed] [Google Scholar]
- Akiyama T, Sadahira Y, Matsubara K, Mori M, Igarashi Y (2008) Immunohistochemical detection of sphingosine-1-phosphate receptor 1 in vascular and lymphatic endothelial cells. J Mol Histol 39:527–533 [DOI] [PubMed] [Google Scholar]
- Alvarez SE, Milstien S, Spiegel S (2007) Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab 18:300–307 [DOI] [PubMed] [Google Scholar]
- Bassi B, Anelli V, Giussani P, Tettamanti G, Viani P, Riboni L (2006) Sphingosine-1-phosphate is released by cerebellar astrocytes in response to bFGF and induces astrocyte proliferation through Gi-protein-coupled receptors. Glia 53:621–630 [DOI] [PubMed] [Google Scholar]
- Blondeau N, Lai Y, Tyndall S, Popolo M, Topalkara K, Pru JK, Zhang L, et al. (2007) Distribution of sphingosine kinase activity and mRNA in rodent brain. J Neurochem 103:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V (2009) FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol 158:1173–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L, Kordula T, Spiegel S, Milstien S (2008) Regulation and functions of sphingosine kinases in the brain. Biochim Biophys Acta 1781:459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Hartung HP (2010) Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 33:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Weiner JA, Fukushima N, Contos JJ, Zhang G, Kimura Y, Dubin A, et al. (2000) Neurobiology of receptor-mediated lysophospholipid signaling. From the first lysophospholipid receptor to roles in nervous system function and development. Ann N Y Acad Sci 905:110–117 [DOI] [PubMed] [Google Scholar]
- Cummings TJ, Strum JC, Yoon WL, Szymanski MH, Hulette CM (2001) Recovery and expression of messenger RNA from postmortem human brain tissue. Mod Pathol 14:1157–1161 [DOI] [PubMed] [Google Scholar]
- Dawson TP, Neal JW, Llewellyn L, Thomas C (2003) Neuropathology Techniques. London, Arnold, 135–138
- Goto S, Hirano A, Rojas-Corona RR (1989) Immunohistochemical visualization of afferent nerve terminals in human globus pallidus and its alteration in neostriatal neurodegenerative disorders. Acta Neuropathol 78:543–550 [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM (2008) Principles of bioactive lipid signaling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139–150 [DOI] [PubMed] [Google Scholar]
- Harada J, Foley M, Moskowitz MA, Waeber C (2004) Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. J Neurochem 88:1026–1039 [DOI] [PubMed] [Google Scholar]
- Hirano A, Llena J (2006) Fine structure of neuronal and glial processes in neuropathology. Neuropathology 26:1–7 [DOI] [PubMed] [Google Scholar]
- Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, Walsh FS, et al. (2005) Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci 25:1459–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto T, Okada T, Yu H, Goparaju SK, Jahangeer S, Nakamura S (2007) Involvement of sphingosine-1-phosphate in glutamate secretion in hippocampal neurons. Mol Cell Biol 27:3429–3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, et al.; for FREEDOMS Study Group (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362:387–401 [DOI] [PubMed] [Google Scholar]
- Kihara A, Mitsutake S, Mizutani Y, Igarashi Y (2007) Metabolism and biological functions of two phosphorylated sphingolipids, sphingosin 1-phosphate and ceramide 1-phosphate. Prog Lipid Res 46:124–144 [DOI] [PubMed] [Google Scholar]
- Kimura A, Ohmori T, Ohkawa R, Madoiwa S, Mimuro J, Murakami T, Kobayashi E, et al. (2007) Essential roles of sphingosine 1-phoshate/S1P1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem Cells 25:115–124 [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, et al. (2002) Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296:346–349 [DOI] [PubMed] [Google Scholar]
- Matyash V, Kettenmann H (2009) Heterogeneity in astrocyte morphology and physiology. Brain Res Rev 63:2–10 [DOI] [PubMed] [Google Scholar]
- Milstien S, Gude D, Spiegel S (2007) Sphingosine 1-phosphate in neural signalling and function. Acta Paediatr 96:40–43 [DOI] [PubMed] [Google Scholar]
- Miron VE, Schubart A, Antel JP (2008) Central nervous system-directed effects of FTY720 (fingolimod). J Neurol Sci 274:13–17 [DOI] [PubMed] [Google Scholar]
- Misu T, Fujihara K, Kakita A, Konno H, Nakamura M, Watanabe S, Takahashi T, et al. (2007) Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain 130:1224–1234 [DOI] [PubMed] [Google Scholar]
- Mullershausen F, Craveiro LM, Shin Y, Cortes-Cros M, Bassilana F, Osinde M, Wishart WL, et al. (2007) Phosphorylated FTY720 promotes astrocyte migration through sphingosine-1-phosphate receptors. J Neurochem 102:1151–1161 [DOI] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP (1997) Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci 17:171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Akiyama T, Monobe Y, Matsubara K, Igarashi Y, Abe M, Sugihara T, et al. (2010) Expression of sphingosine-1-phosphate receptor 1 in mantle cell lymphoma. Mod Pathol 23:439–449 [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Hamada A, Matsuda H, Takagi A, Tanaka M, Aoki J, Arai H, et al. (2008) Expression patterns of the lysophospholipid receptor genes during mouse early development. Dev Dyn 237:3280–3294 [DOI] [PubMed] [Google Scholar]
- Osinde M, Mullershausen F, Dev KK (2007) Phosphorylated FTY720 stimulates ERK phosphorylation in astrocytes via S1P receptors. Neuropharmacology 52:1210–1218 [DOI] [PubMed] [Google Scholar]
- Preston GM, Agre P (1991) Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA 88:11110–11114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadahira Y, Mori M, Awai M, Watarai S, Yasuda T (1988) Forssman glycosphingolipid as an immunohistochemical marker for mouse stromal macrophages in hematopoietic foci. Blood 72:42–48 [PubMed] [Google Scholar]
- Sanchez T, Hla T (2004) Structural and functional characteristics of S1P receptors. J Cell Biochem 92:913–922 [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Goforth PB, Mba MU, Macdonald TL, Lynch KR, Milstien S, Spiegel S, et al. (2009) Sphingosine-1-phosphate receptors mediate neuromodulatory functions in the CNS. J Neurochem 110:1191–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2005) Reactive astrocytes in neural repair and protection. Neuroscientist 11:400–407 [DOI] [PubMed] [Google Scholar]
- Sorensen SD, Nicole O, Peavy RD, Montoya LM, Lee CJ, Murphy TJ, Traynelis SF, et al. (2003) Common signaling pathways link activation of murine PAR-1, LPA, and S1P receptors to proliferation of astrocytes. Mol Pharmacol 64:1199–1209 [DOI] [PubMed] [Google Scholar]
- Toman RE, Spiegel S (2002) Lysophospholipid receptors in the nervous system. Neurochem Res 27:619–627 [DOI] [PubMed] [Google Scholar]
- Waeber C, Chiu M (1999) In vitro autoradiographic visualization of guanosine-5′-O-(3-[35S]thio) triphosphate binding stimulated by sphingosine 1-phosphate and lysophosphatidic acid. J Neurochem 73:1212–1221 [DOI] [PubMed] [Google Scholar]
- Wu YP, Mizugishi K, Bektas M, Sandhoff R, Proia RL (2008) Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease. Hum Mol Genet 17:2257–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Tagami M, Torii Y, Takenaga F, Tsumagari S, Itoh S, Yamori Y, et al. (2003) Sphingosine 1-phosphate induces the production of glial cell line-derived neurotrophic factor and cellular proliferation in astrocytes. Glia 41:199–206 [DOI] [PubMed] [Google Scholar]