Abstract
During embryonic development, Foxa2 is required for the formation of the node and notochord, and ablation of this gene results in defects in gastrulation, neural tube patterning, and gut morphogenesis. Foxa2 has been shown to be expressed specifically in the glandular epithelium of the murine uterus. To study the uterine function of Foxa2, this gene was conditionally ablated in the mouse uterus by crossing mice with floxed Foxa2 alleles, Foxa2loxP/loxP, with the Pgrcre mouse model. Pgrcre/+ Foxa2loxP/loxP mice showed significantly reduced fertility. Analysis of the uterus on Day 5.5 of pregnancy showed disrupted blastocyst implantation. Pgrcre/+ Foxa2loxP/loxP mice also showed a severe impairment of the uterus to respond to the artificial induction of the decidual response. Morphological examination of the uteri of these mice showed a severe reduction in the number of endometrial glands. The loss of endometrial glands resulted in the reduction of leukemia inhibitory factor (Lif) expression. The lack of a decidual response could be partially rescued by an intrauterine injection of LIF before the initiation of the decidual response. This analysis demonstrates that Foxa2 regulates endometrial gland development and that mice with a loss of endometrial glands cannot support implantation in part due to the loss of LIF, which is a requisite for fertility in the mouse.
Keywords: decidua, female reproductive tract, Foxa2, gland development, implantation, uterus
Conditional ablation of Foxa2 in the mouse uterus results in a reduction in fertility, in the number of endometrial glands, and in leukemia inhibitory factor (Lif) expression; the lack of a decidual response in these mice could be partially rescued by LIF injections.
INTRODUCTION
The uterus consists of heterogeneous cell types that undergo dynamic changes to support embryo development and implantation. The process of implantation consists of attachment and invasion of the uterine luminal epithelium. Successful blastocyst implantation requires the rapid remodeling of the uterine stromal cells in a process termed decidualization [1]. Decidualization is a process characterized by morphological and functional changes in the uterine stromal cells that are characterized by endometrial stromal proliferation and differentiation into large epithelioid decidual cells. This process is critical for the establishment of a fetal-maternal interface during implantation.
The uterine luminal epithelium is the initial site of blastocyst attachment, whereas the glandular epithelium is thought to be the principal source of uterine secretions that are required for the establishment and maintenance of pregnancy [2]. The absence of glandular epithelium and the reduced luminal epithelium in the ovine uterine gland ewe knockout model resulted in a reduction of conceptus survival, supporting a fundamental role for the glandular epithelium and their secretions during early pregnancy [3]. The precise difference between luminal and glandular epithelium is not well characterized; however, it is known that they differ biochemically [4, 5]. In the mouse, the endometrial glands, in response to estrogen, express the cytokine leukemia inhibitory factor, Lif, which is critical for blastocyst implantation. Although the expression of many genes, including steroid hormone receptors, cytokines, growth factors, and several developmental factors, has been implicated in this process, direct in vivo evidence of gene function has been limited. This is largely due to the fact that the ablation of many of the genes implicated in this process results in early lethality or other developmental consequences that preclude further study. Herein, we investigate the role of a developmentally important gene, Foxa2, in the regulation of adult mouse uterine function.
Foxa transcription factors comprise a subfamily of forkhead transcription factors that contain high homology in the winged helix DNA-binding domain [6]. The Foxa family has been found to have important roles in multiple stages of mammalian life, beginning with early development, continuing throughout organogenesis, and during adulthood in metabolism and homeostasis [6]. The Foxa family includes Foxa1, Foxa2, and Foxa3 (previously known as Hnf3α, Hnf3β, and Hnf3γ, respectively) [7]. Foxa1 and Foxa2 cooperate to establish competence in the foregut endoderm and are required for normal development of endoderm-derived organs such as the liver, pancreas, lung, and prostate [7–10]. Foxa1 and Foxa2 are not expressed in the ovary, and Foxa2 is only expressed in the glandular epithelium of the uterus [11]. Foxa2−/− mice die at Embryonic Day (E) 10 or 11 due to severe defects in the node, notochord, neural tube, and gut tube formation, making it difficult to investigate the effect of Foxa2 ablation in the uterus [12]. Herein, we generated a mouse model in which Foxa2 is conditionally ablated in the uterus to investigate the role of Foxa2 in endometrial function. The ablation of Foxa2 resulted in defects in gland formation, decidualization, and fertility. These analyses suggest that Foxa2 has an important role in uterine function and implantation.
MATERIALS AND METHODS
Animals and Tissue Collection
Mice were maintained in the designated animal care facility at Baylor College of Medicine according to the institutional guidelines for the care and use of laboratory animals. Pregnancy samples were obtained by the mating of wild-type C57BL/6 mice, and the day that a vaginal plug was observed was considered Day 0.5 of pregnancy. Uterine tissues were flash frozen at the time of dissection or fixed with 4% paraformaldehyde (vol/vol) and paraffin embedded.
The hormonally induced decidual response has been previously described [13]. Briefly, ovariectomized 129P2/OlaHsd-Foxa2tm1Khk (also known as Foxa2loxP/loxP [control]) and 129P2/OlaHsd-Foxa2tm1Khk × B6;129-Pgrtm2(cre)Lyd (also known as Pgrcre/+ Foxa2loxP/loxP [mutant]) mice were treated with three daily injections of 100 ng of estradiol-17 (E2) per mouse (n = 3 per genotype). After 2 days of rest, mice were then treated with three daily injections of 1 mg of progesterone (P4) and 6.7 ng of E2 per mouse by s.c. injection. The uteri were mechanically stimulated by a scratch of the antimesometrial lumen 6 h after the last hormone injection. Mice were given daily s.c. injections of 1 mg of P4 and 6.7 ng of E2 per mouse for 5 days after stimulation to observe the induction of the uterine decidual response.
Immunohistochemistry
Uteri were fixed overnight in 4% paraformaldehyde (vol/vol), followed by thorough washing in 70% ethanol, and tissues were processed, embedded in paraffin, and sectioned. Uterine sections from paraffin-embedded tissue were cut at 5 μm and mounted on silane-coated slides, deparaffinized, and rehydrated in a graded alcohol series. Sections were preincubated with 10% normal goat serum in PBS (pH 7.5) and then incubated with anti-FOXA2 antibodies in 10% normal serum in PBS (pH 7.5). On the following day, sections were washed in PBS and incubated with biotinylated secondary antibody (5 μl/ml; Vector Laboratories, Burlingame, CA) for 1 h at room temperature. Immunoreactivity was detected using the Vectastain Elite ABC kit (Vector Laboratories); the immunoreactivity was visualized as brown staining.
RNA Isolation and Quantitative Real-Time RT-PCR
Total RNA was extracted from uterine tissues using the Qiagen (Valencia, CA) RNeasy total RNA isolation kit. To investigate the effect of Foxa2 on gene expression changes in the uterus, quantitative real-time RT-PCR analysis was conducted on RNA isolated from mice. Expression levels of Foxa2 and Lif were measured by real-time RT-PCR TaqMan analysis using the ABI Prism 7700 Sequence Detector System according to manufacturer's instructions (Applied Biosystems, Foster City, CA). The RT-PCR was performed using One-Step RT-PCR Universal Master Mix reagent (Applied Biosystems) according to the manufacturer's instructions. Real time probes and primers for Foxa2, Lif, Ihh, Hoxa10, Wnt5a, Wnt7a, Ptch1, Hoxa11, Wnt4, Bmp2, Cebpb, and Hbegf were purchased from Applied Biosystems. Standard curves were generated by serial dilution of a preparation of total RNA isolated from whole mouse uterus. All real-time RT-PCR results were normalized against 18S RNA or Cdhl using ABI rRNA control reagents (Applied Biosystems).
Rescue of the Decidualization Defect by Recombinant LIF
Foxa2 rescue experiments of proliferation and vascularization were accomplished using recombinant LIF. Ovariectomized mice were treated with three daily injections of 100 ng of E2 per mouse. After 2 days of rest, mice were each treated with daily s.c. injections of 1 mg of P4 and 6.7 ng of E2 per mouse. Six hours after the second injection, 10 μl of 10% bovine serum albumin (BSA) or 10 μl of LIF (100 ng/μl in 10% BSA) was injected intraluminally into one horn of the uterus. Both horns were traumatized by a needle scratch on the antimesometrial lumen 6 h after the third injection. Mice continued to receive daily s.c. injections of 1 mg of P4 and 6.7 ng of E2 per mouse each day following the trauma. Three days after the trauma, the mice were killed, and uteri were collected.
Statistical Analysis
All experimental data are presented as the mean ± SEM. The statistical significance of differences was analyzed using one-way ANOVA, followed by Tukey post hoc multiple range test or Student t-test using the Instat software package from GraphPad (San Diego, CA).
RESULTS
Expression of FOXA2 During Early Pregnancy
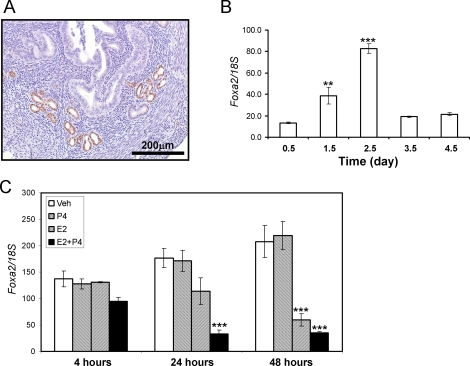
The spatial expression profile of FOXA2 in the uterus was determined by immunohistochemistry (Fig. 1A). FOXA2 is expressed in the glandular epithelium but not the luminal epithelium, stroma, or myometrium of the mouse uterus. Next, the temporal expression of Foxa2 was investigated in the mouse uterus during early pregnancy by performing real-time RT-PCR on RNA isolated from the uteri of pseudopregnant mice. As shown in Figure 1B, Foxa2 expression gradually increases until its peak at Day 2.5 of pseudopregnancy and then sharply decreases throughout the remainder of pseudopregnancy. Because blastocyst implantation in the mouse uterus occurs at Day 4.5, the expression of Foxa2 during the preimplantation period implicates it as a potential regulator of implantation.
FIG. 1.
The expression pattern of Foxa2 in the murine uterus. A) Localization of FOXA2 in the murine uterus. Eight-week-old female mice were killed. Portions of the uterus were fixed in 4% paraformaldehyde, and immunohistochemistry for FOXA2 was performed. Nuclei are lightly counterstained with hematoxylin. B) The expression pattern of Foxa2 by real-time RT-PCR in pseudopregnancy. Total RNA used for the RT-PCR assays was prepared from the pseudopregnant uteri. The expression level of Foxa2 was measured from Day 0.5 to Day 4.5 in the pseudopregnant uterus. C) The expression pattern of Foxa2 by E2 and P4 in the uterus. Total RNA used for the RT-PCR assays was prepared from wild-type mice that were treated with P4, E2, E2 plus P4, or vehicle (sesame oil) for 4, 24, and 48 h. The results represent the mean ± SEM of three independent RNA sets. **P < 0.01; ***P < 0.001.
Steroid Hormone Regulation of Foxa2
Given the temporal regulation of Foxa2 during the preimplantation period, the effect of ovarian steroid hormone regulation on Foxa2 expression was examined. Ovariectomized wild-type female mice were injected daily with one of the following hormones: vehicle (sesame oil), P4 (1 mg per mouse), E2 (0.1 μg per mouse), or E2 plus P4 (1 mg of P4 and 0.1 μg of E2 per mouse). Mice were killed at 4, 24, and 40 h after the initial hormone treatment (n = 3 mice per treatment) and subjected to real-time PCR to characterize the effect of steroid hormone treatment on the relative expression of Foxa2. As shown in Figure 1C, P4 alone had no effect on Foxa2 expression. However, E2 repressed Foxa2 mRNA expression at 48 h but not 4 and 24 h after treatment. The repression of Foxa2 mRNA by E2 was accelerated by cotreatment with P4, as shown by the significant decrease in expression after 24 h of E2 plus P4 treatment. These results suggest that the steroid hormone treatment inhibits the expression of Foxa2 in the uterus.
Conditional Ablation of Foxa2 in the Uterus
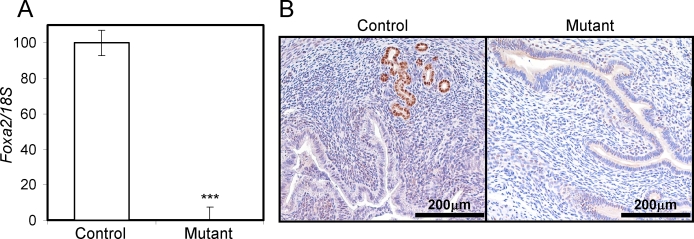
Ablation of Foxa2 leads to embryonic lethality because of severe defects in gastrulation, neural tube patterning, and gut morphogenesis [12]. To further investigate the role of Foxa2 in the adult uterus, conditional ablation of Foxa2 in the uterus was conducted to circumvent the embryonic lethal phenotype. A line of mice in which cre recombinase is under the control of the P4 receptor promoter (Pgrtm2(cre)Lyd) was crossed with a mouse line containing the floxed Foxa2 allele (Foxa2tm1Khk) to provide a tissue-specific knockout of Foxa2 in all Pgr-expressing cells, which includes all compartments of the uterus [14, 15]. Foxa2 gene ablation was validated in the mutant mice by measuring Foxa2 expression in the control Foxa2loxP/loxP and mutant Pgrcre/+ Foxa2loxP/loxP mice at Day 2.5 of pregnancy. Foxa2 expression was not detectable in the mutant mice compared with RNA and protein from the control mice, as determined by real-time PCR and immunohistochemistry (Fig. 2).
FIG. 2.
Analysis of Foxa2 conditionally ablated in the murine uterus. The expression level of Foxa2 was measured in the uteri by real-time RT-PCR (A) and immunohistochemistry (B). Eight-week-old control and mutant mice were killed at Day 2.5. Total RNA used for the RT-PCR assays was prepared from the uteri. The results represent the mean ± SEM of three independent RNA sets. ***P < 0.001.
Fertility and Implantation Defect in Pgrcre/+ Foxa2loxP/loxP Mice
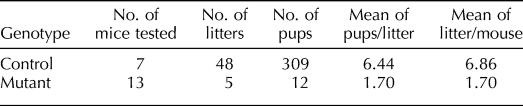
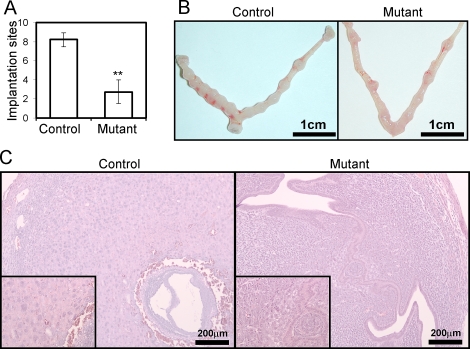
To determine if Foxa2 has a role in implantation, female control and mutant mice were mated to wild-type male mice for 6 mo. Control mice exhibited normal fecundity over this period; however, mutant mice had significantly fewer litters, pups per litter, and total pups born (Table 1). Thus, female mutant mice are severely subfertile, demonstrating the importance of Foxa2 in pregnancy. To determine the cause of the subfertility, we examined whether blastocysts were able to undergo successful implantation. We killed mice on the morning of Day 5.5 of pregnancy and counted the number of implantation sites. Implantation sites were significantly decreased in the mutant mice (2.75 ± 1.25) compared with the control mice (8.20 ± 0.73) (Fig. 3, A and B). Histological examination showed that blastocysts are able to attach to the uterine lumen and initiate a decidual response in mutant mice (Fig. 3C). However, the decidual region and size of the embryos were decreased in the mutant mice compared with control mice. The morphology of the stroma cells showed an abnormal decidual phenotype. Also, the embryos did not exhibit invasion into the uterine stroma through the epithelium. This observation suggests that the mutant uterus is incapable of supporting embryo invasion and normal decidualization.
TABLE 1.
Fertility defect of mutant mice.
FIG. 3.
Implantation defect in the mutant mice. A) Implantation sites in the control and mutant uterus at Day 5.5. Control and mutant mice were killed at Day 5.5. The number of implantation sites was counted in the uteri. The results represent the mean ± SEM of five independent mice. **P < 0.01. B) Gross anatomy of the mutant uteri shows a decrease in the number of implantation sites compared with controls. C) Hematoxylin-eosin staining of control and mutant uteri with blastocysts at Day 5.5. Insets are high-power views of attachment sites.
Defect of Decidualization in Pgrcre/+ Foxa2loxP/loxP Mice
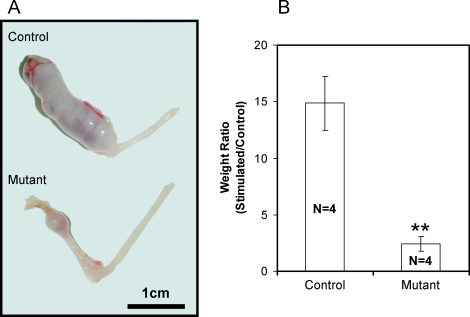
To confirm that mutant mice exhibit a defect in the ability of uterine stroma cells to undergo a decidual reaction, the ability of their uteri to undergo decidualization after artificial hormonal induction was determined. Ovariectomized mutant (n = 4) and littermate control (n = 4) mice were treated with E2 and P4 to mimic pregnancy, and the uterus was traumatized to mimic the stimulation of the blastocysts at implantation and to induce decidualization (See Materials and Methods). As expected, the uterine horn of control mice exhibited a robust decidual response 5 days after receiving the artificial stimulation. However, mutant mice displayed a reduction in the decidual response (Fig. 4A). Quantification of the decidual response by the measurement of uterine wet weight indicated that the stimulated uterine horn of mutant mice was significantly smaller compared with littermate controls (Fig. 4B). These results demonstrate that the mutant mice exhibit a decidualization defect both during natural pregnancy and in the hormonally induced decidualization reaction.
FIG. 4.
Decidualization defect in the mutant mice. Six-week-old mice were ovariectomized and 2 wk later were subjected to a hormone regimen and a decidual stimulus. A) Gross anatomy of the mutant uteri shows a decrease in size of the decidual horn compared with controls. B) Stimulated horn weight:unstimulated horn weight ratio was significantly decreased in the mutant uteri. The results represent the mean ± SEM. **P < 0.01.
Defect of Glandular Development in Pgrcre/+ Foxa2loxP/loxP Mice
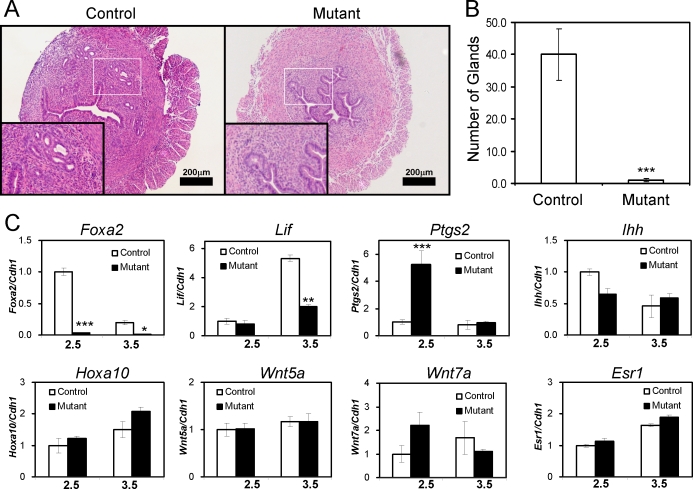
To determine if the cause of the subfertility in Foxa2 mutant mice had a morphological origin, histological analysis of nonpregnant uteri was conducted. Examination of the uteri of control and mutant mice showed a reduction in the number of uterine glands upon ablation of Foxa2 (Fig. 5A). The number of uterine glands was quantified by counting the number of glands per uterine section, which demonstrated that the reduction in uterine glands was statistically significant (Fig. 5B). One endometrial gland protein, LIF, which is a member of the interleukin 6 family of cytokines and is a secreted glycoprotein, is known to be critical for implantation, as female Lif−/− mice are sterile due to an implantation defect. Lif is expressed in the uterine glands in a bimodal fashion with peaks at Day 0.5 and Day 3.5 of pregnancy, and LIF can substitute for the nidatory surge in E2 in regulation of blastocyst implantation in mice [16]. The expression of Lif was significantly decreased at Day 3.5 in the mutant mice compared with control mice (Fig. 5C). Examination of other regulators of implantation, Ihh, Hoxa10, Wnt5a, Wnt7a (Fig. 5C), Ptch1, Hoxa11, Wnt4, Bmp2, Cebpb, and Hbegf (data not shown), were not significantly altered during the preimplantation period; only Ptgs2 expression was altered during this period. The expression value of mRNAs was normalized against cadherin 1 (Cdh1, also known as E-cadherin) mRNA to eliminate the effect of reduced epithelium due to glandular loss in Pgrcre/+ Foxa2loxP/loxP. Therefore, the most likely cause of the impaired decidual response in the Pgrcre/+ Foxa2loxP/loxP mice is the lack or ablation of Lif secretion due to the loss of uterine glands.
FIG. 5.
Defect of gland formation in the mutant mice. A) Hematoxylin-eosin staining of uteri from 8-wk-old control and mutant mice. Insets represent high-power views of the boxed region. B) The number of glands was counted from the same area of histological slides. The results represent the mean ± SEM of three independent mice. ***P < 0.001. C) The expression of Lif at Day 2.5 and Day 3.5 in the mutant mice. The expression levels of Foxa2, Lif, Ptgs2, Ihh, Hoxa10, Wnt5a, Wnt7a, and Esr1 were measured at Day 2.5 and Day 3.5 of the pseudopregnant uterus. Total RNA used for the RT-PCR assays was prepared from the pseudopregnant uteri. The results represent the mean ± SEM of three independent RNA sets. *P < 0.05; **P < 0.01; ***P < 0.001.
Rescue of the Decidualization Defect by Recombinant LIF
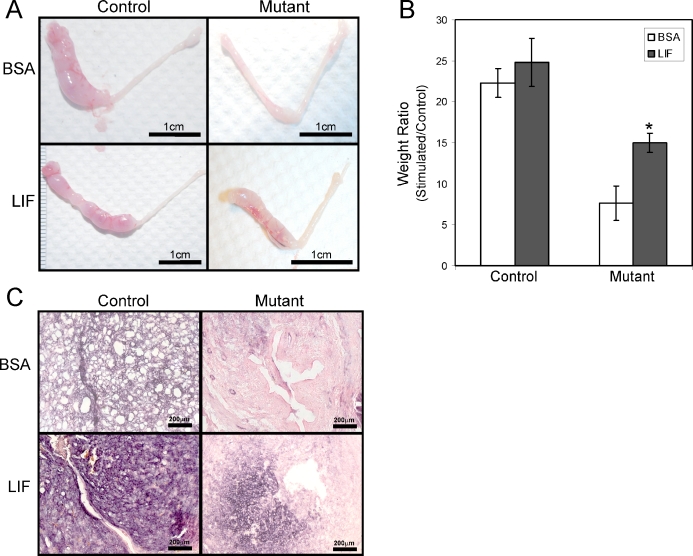
To determine if the uterine decidualization defect was due to the lack of LIF secreted by the uterine glands, we investigated whether the administration of recombinant LIF could rescue the decidual defect in the mutant mice. Control and mutant mice were ovariectomized and induced to undergo the decidual reaction as already described. At 24 h before endometrial trauma, 10 μl of vehicle (10% BSA) or recombinant LIF (100 ng/μl in 10% BSA) was injected intraluminally into one uterine horn. The mice were killed 5 days after receiving the decidual trauma. Decidualization was unaffected by administration of exogenous LIF, as seen by the robust decidual response in the control mice. As expected, the mutant stroma showed a decreased decidual response when injected with vehicle, whereas LIF partially restored the decidualization response in mutant mice (Fig. 6, A and B). This result was confirmed by staining for alkaline phosphatase activity, a well-known marker for decidual cells. Robust alkaline phosphatase activity, as seen by dark purple staining, could be seen in both the vehicle- and LIF-treated control mice. Alkaline phosphatase staining was significantly decreased in uteri of mutant mice treated with BSA; however, the staining was intensified in the mutant mice that were administered recombinant LIF (Fig. 6C). Thus, LIF can partially rescue the impaired decidual response in the mutant mice. Taken together, these results indicate that the impaired decidual response observed in Foxa2 mutant mice is at least in part attributable to a lack of Lif induction during implantation.
FIG. 6.
Partial rescue of the decidualization defect by recombinant LIF administration in the mutant mice. A) Gross morphology of the decidual response in the mutant uteri 5 days after the decidual stimulus and treatment of 10% BSA or recombinant LIF in 10% BSA. B) Stimulated horn weight:unstimulated horn weight ratio was significantly increased in the mutant uteri treated with recombinant LIF compared with BSA. The results represent the mean ± SEM. *P < 0.05. C) Differentiation by alkaline phosphatase staining in the mutant uterus treated with 10% BSA or recombinant LIF in 10% BSA after the decidual trauma. Bars = 1 cm (A) and 200 μm (C).
DISCUSSION
FOXA2 has important roles in early embryonic development, organogenesis, and glucose homeostasis [6, 9, 12, 17–19]. Among the FOXA proteins, FOXA2 is the only FOXA protein that has been detected in the murine uterus, and this expression is restricted to the glandular epithelium [11]. Foxa2 knockout mice die at E10 or E11 because of severe defects in node, notochord, neural tube, and gut tube development [12]. However, the function of Foxa2 in the uterus has remained elusive. To study the role of Foxa2 in the uterus, we generated mice in which Foxa2 was ablated (Pgrcre/+ Foxa2loxP/loxP) in the reproductive tract using previously generated mice with floxed Foxa2 alleles and the Pgrcre mouse model [14, 15]. The female Pgrcre/+ Foxa2loxP/loxP mice were severely subfertile, with an inability of the uterus to support embryo invasion and decidualization. The major morphological phenotype of these mice was the absence of uterine glands. Because the Pgrcre mouse model recombines alleles in all compartments of the uterus, as well as the pituitary, mammary gland, and ovary, we had to determine if the subfertility was due to the uterine defect or was in part due to pituitary or ovarian defects. No ovarian phenotype was detected in these mice. Although a pituitary contribution to this phenotype has not been ruled out, the severe inability of the uterus to support implantation and undergo an experimentally induced decidual response with exogenous steroid hormones indicates that the fertility defect is due in part to a uterine defect. Also in support of a uterine defect as the cause of the subfertility is the lack of uterine glands in this model. As shown in sheep, P4 administration to neonatal sheep resulted in the ablation of uterine glands, which rendered the adult ewes sterile [20].
Postnatal uterine morphogenesis involves the differentiation and development of the endometrial glandular epithelium from the luminal epithelium, as well as the development of the endometrial stroma and inner circular and outer longitudinal layers of the myometrium from the uterine mesenchyme [2, 20]. Little is known about the mechanisms regulating postnatal uterine morphogenesis and, in particular, endometrial gland development. Within the uterus, the Pgrcre mouse model recombines alleles in both the endometrial epithelial and stromal compartments, as well as in the myometrium. However, given that the expression of Foxa2 is limited to the glandular epithelium, it is likely that Foxa2 directly regulates adenogenesis in the mouse uterus. Foxa2 has an important role in epithelial budding and morphogenesis in many organs, including the pancreas, liver, lung, and prostate [11, 21–25]. Thus, Foxa2 is likely involved in bud formation and epithelial specification during uterine gland formation. Foxa2 may have a role in gland specification by forming a gene regulatory network capable of inducing a competence in the luminal epithelium to become glandular epithelium via its ability to alter chromatin. Foxa transcription factors are able to open highly compacted chromatin, facilitating the binding of other transcription factors, and this process has been shown to occur for glucocorticoid receptor, androgen receptor, and estrogen receptor 1 [8, 19, 26–29]. One possible signaling pathway that Foxa2 may regulate that is integral to gland formation is the Wnt/β-catenin pathway. Mouse models in which Wnt5a, Wnt7a, and catenin beta 1 (Ctnnb1) were ablated all displayed a lack of uterine glands [30–33]. Foxa2 has been shown to regulate the expression of multiple Wnt signaling members, including Wnt3a, Wnt8a, and Wnt7b, the expression of which was decreased in mouse models lacking Foxa2 [10, 34]. Foxa2 also transactivates the Wnt7b promoter in vitro [35]. The reciprocal interaction between Foxa2 and Wnt signaling has been noted also in that Ctnnb1, a downstream effector of the Wnt pathway, can promote Foxa2 [36–38]. There is also evidence that Foxa2 is able to engage in autoregulation by binding to its own promoter to enhance transcription [39]. The lack of altered expression of Wnt7a and Wnt5a in the preimplantation Pgrcre/+ Foxa2loxP/loxP mouse uterus (Fig. 5C) does not rule out the possibility that Foxa2 regulates adenogenesis through Wnt signaling during the neonatal period, when glands are initially formed. Future studies are needed to determine the potential role of complex interactions between Wnt and Foxa2 signaling to either initiate or maintain glandular identity.
Because uterine gland development is repressed in the ovine model by treatment with P4, one might speculate that Foxa2 expression is repressed by P4 in the mouse uterus [20]. However, we determined that P4 alone has no effect on Foxa2 expression but that E2 and cotreatment of E2 plus P4 repress Foxa2 expression (Fig. 1C). Therefore, treatment of the neonatal mouse with E2 plus P4 may repress Foxa2 and attenuate endometrial glandular development.
Endometrial glands and their secretions are critical regulators of peri-implantation survival of blastocysts and implantation, as well as the establishment of uterine receptivity in numerous species [40–43]. These glands produce histotrophic factors that nurture the blastocysts and support attachment and, depending on the species, invasion of blastocysts [2, 44, 45]. During normal pregnancy, the presence of an active blastocyst in the uterus is the stimulus for implantation; however, the uterus itself is only receptive to the blastocyst for a limited period of time, known as the “window of receptivity.” Deficiencies in uterine receptivity, embryo development, and blastocyst-uterine communication compromise fertility [46, 47]. Several signaling pathways necessary for implantation have been identified [48, 49]. However, the mechanism by which the attachment of a blastocyst to the uterine luminal epithelium is triggered remains unclear. After the attachment of the blastocyst to the luminal epithelium, the surrounding stromal cells undergo decidualization, eventually embedding the embryo into the stroma. While insight into the mechanisms by which this decidualization occurs has been elucidated, much still remains to be known about this vital process [1, 48, 49]. The Foxa2 mutant mice exhibit a defect in implantation and decidualization. Notably, some of the implanting blastocysts can induce decidualization; however, further development and implantation of blastocysts are defective (Fig. 4C). These results suggest that uterine glands are necessary to produce growth factors and potential histotrophic factors that are critical to prime the endometrial stroma for implantation, as well as to stimulate the embryo trophoblasts to invade the uterus.
The Foxa2 mutant mouse serves as a model to determine which products of the endometrial glands are responsible for allowing trophoblast invasion of the uterus and the induction of decidualization. One product of these glands that may regulate this function is LIF. LIF is secreted by the uterine glands in response to nidatory estrogen at Day 3.5 [16, 50, 51] and is expressed in the subluminal stroma at the implantation site [52]. Lif−/− mice are unable to undergo blastocyst implantation and decidualization of the stroma [44]. LIF can substitute for E2 action in the termination of artificially delayed implantation and in the reinitiation of blastocyst implantation in mice [16]. Our results show that the expression of Lif is significantly decreased at Day 3.5 in the mutant mice compared with control mice (Fig. 3) and that the decidualization defect of the mutant mice was partially rescued by administration of recombinant LIF (Fig. 6). These results confirm that Lif is a critical product of the endometrial glands that is important for regulating changes in the endometrium during implantation [16]. However, determining if Foxa2 directly regulates Lif expression or if the lack of Lif expression is simply the result of loss of endometrial glands is difficult in this model because uterine glands are completely ablated. Transfections and chromatin immunoprecipitation analysis would be required to determine if Foxa2 is a direct regulator of Lif transcription. However, in silico analysis of 2 kilobases of the Lif promoter region using the Transcription Element Search System (http://www.cbil.upenn.edu/cgi-bin/tess/tess?RQ=WELCOME) did not detect any Foxa2-binding sites (data not shown).
In conclusion, we have demonstrated a role for Foxa2 in endometrial function using mice with conditional ablation of Foxa2 in the uterus. Uterine-specific ablation of Foxa2 results in a defect in uterine gland formation. Furthermore, these mice exhibit both implantation and decidualization defects, demonstrating that Foxa2 regulation is critical for adult uterine function. The results of this investigation provide significant insights into our understanding of the importance of Foxa2 in female reproduction.
Acknowledgments
We thank Jinghua Li and Jie Yang for technical assistance and Heather L. Franco and Janet DeMayo, MS, for manuscript preparation.
Footnotes
Supported by NIH grant R01HD057873 (to J.W.J.), NIH grant R01CA77530 (to J.P.L.), and NIH grants R01HD042311 and U54HD0077495 (to F.J.D.).
These authors contributed equally to this work and should be considered co-first authors.
REFERENCES
- Lee KY, DeMayo FJ.Animal models of implantation. Reproduction 2004; 128: 679–695. [DOI] [PubMed] [Google Scholar]
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE.Developmental biology of uterine glands. Biol Reprod 2001; 65: 1311–1323. [DOI] [PubMed] [Google Scholar]
- Gray CA, Taylor KM, Ramsey WS, Hill JR, Bazer FW, Bartol FF, Spencer TE.Endometrial glands are required for preimplantation conceptus elongation and survival. Biol Reprod 2001; 64: 1608–1613. [DOI] [PubMed] [Google Scholar]
- Bell SC, Drife JO.Secretory proteins of the endometrium: potential markers for endometrial dysfunction. Baillieres Clin Obstet Gynaecol 1989; 3: 271–291. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Bazer FW, Roberts RM.Purification and properties of a progesterone-induced plasmin/trypsin inhibitor from uterine secretions of pigs and its immunocytochemical localization in the pregnant uterus. J Biol Chem 1982; 257: 6886–6897. [PubMed] [Google Scholar]
- Friedman JR, Kaestner KH.The Foxa family of transcription factors in development and metabolism. Cell Mol Life Sci 2006; 63: 2317–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner KH.The hepatocyte nuclear factor 3 (HNF3 or FOXA) family in metabolism. Trends Endocrinol Metab 2000; 11: 281–285. [DOI] [PubMed] [Google Scholar]
- Gao N, Zhang J, Rao MA, Case TC, Mirosevich J, Wang Y, Jin R, Gupta A, Rennie PS, Matusik RJ.The role of hepatocyte nuclear factor-3 alpha (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol Endocrinol 2003; 17: 1484–1507. [DOI] [PubMed] [Google Scholar]
- Lee CS, Sund NJ, Behr R, Herrera PL, Kaestner KH.Foxa2 is required for the differentiation of pancreatic alpha-cells. Dev Biol 2005; 278: 484–495. [DOI] [PubMed] [Google Scholar]
- Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA.Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J Biol Chem 2005; 280: 13809–13816. [DOI] [PubMed] [Google Scholar]
- Besnard V, Wert SE, Hull WM, Whitsett JA.Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns 2004; 5: 193–208. [DOI] [PubMed] [Google Scholar]
- Weinstein DC, Ruiz i Altaba A, Chen WS, Hoodless P, Prezioso VR, Jessell TM, Darnell JE., JrThe winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell 1994; 78: 575–588. [DOI] [PubMed] [Google Scholar]
- Finn CA, Martin L.Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod 1972; 7: 82–86. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP.Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005; 41: 58–66. [DOI] [PubMed] [Google Scholar]
- Sund NJ, Ang SL, Sackett SD, Shen W, Daigle N, Magnuson MA, Kaestner KH.Hepatocyte nuclear factor 3beta (Foxa2) is dispensable for maintaining the differentiated state of the adult hepatocyte. Mol Cell Biol 2000; 20: 5175–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, Stewart CL.Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology 2000; 141: 4365–4372. [DOI] [PubMed] [Google Scholar]
- Lantz KA, Vatamaniuk MZ, Brestelli JE, Friedman JR, Matschinsky FM, Kaestner KH.Foxa2 regulates multiple pathways of insulin secretion. J Clin Invest 2004; 114: 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sund NJ, Vatamaniuk MZ, Casey M, Ang SL, Magnuson MA, Stoffers DA, Matschinsky FM, Kaestner KH.Tissue-specific deletion of Foxa2 in pancreatic beta cells results in hyperinsulinemic hypoglycemia. Genes Dev 2001; 15: 1706–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Rubins NE, Ahima RS, Greenbaum LE, Kaestner KH.Foxa2 integrates the transcriptional response of the hepatocyte to fasting. Cell Metab 2005; 2: 141–148. [DOI] [PubMed] [Google Scholar]
- Bartol FF, Wiley AA, Floyd JG, Ott TL, Bazer FW, Gray CA, Spencer TE.Uterine differentiation as a foundation for subsequent fertility. J Reprod Fertil Suppl 1999; 54: 287–302. [PubMed] [Google Scholar]
- Ashizawa S, Brunicardi FC, Wang XP.PDX-1 and the pancreas. Pancreas 2004; 28: 109–120. [DOI] [PubMed] [Google Scholar]
- Lee CS, Friedman JR, Fulmer JT, Kaestner KH.The initiation of liver development is dependent on Foxa transcription factors. Nature 2005; 435: 944–947. [DOI] [PubMed] [Google Scholar]
- Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT, Fulkerson PC, Rothenberg ME, Whitsett JA.Foxa2 regulates alveolarization and goblet cell hyperplasia. Development 2004; 131: 953–964. [DOI] [PubMed] [Google Scholar]
- Mirosevich J, Gao N, Matusik RJ.Expression of Foxa transcription factors in the developing and adult murine prostate. Prostate 2005; 62: 339–352. [DOI] [PubMed] [Google Scholar]
- Gao N, Ishii K, Mirosevich J, Kuwajima S, Oppenheimer SR, Roberts RL, Jiang M, Yu X, Shappell SB, Caprioli RM, Stoffel M, Hayward SW, et al. Forkhead box A1 regulates prostate ductal morphogenesis and promotes epithelial cell maturation. Development 2005; 132: 3431–3443. [DOI] [PubMed] [Google Scholar]
- Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS.Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell 2002; 9: 279–289. [DOI] [PubMed] [Google Scholar]
- Holmqvist PH, Belikov S, Zaret KS, Wrange O.FoxA1 binding to the MMTV LTR modulates chromatin structure and transcription. Exp Cell Res 2005; 304: 593–603. [DOI] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 2005; 122: 33–43. [DOI] [PubMed] [Google Scholar]
- Laganiere J, Deblois G, Lefebvre C, Bataille AR, Robert F, Giguere V.From the cover: location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc Natl Acad Sci U S A 2005; 102: 11651–11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta L, Sassoon D.Wnt7a is a suppressor of cell death in the female reproductive tract and is required for postnatal and estrogen-mediated growth. Biol Reprod 2004; 71: 444–454. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, Lydon JP, DeMayo FJ.beta-Catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 2009; 28: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mericskay M, Kitajewski J, Sassoon D.Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development 2004; 131: 2061–2072. [DOI] [PubMed] [Google Scholar]
- Miller C, Sassoon DA.Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 1998; 125: 3201–3211. [DOI] [PubMed] [Google Scholar]
- Tamplin OJ, Kinzel D, Cox BJ, Bell CE, Rossant J, Lickert H.Microarray analysis of Foxa2 mutant mouse embryos reveals novel gene expression and inductive roles for the gastrula organizer and its derivatives. BMC Genomics 2008; 9: e511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA.beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 2003; 278: 40231–40238. [DOI] [PubMed] [Google Scholar]
- Sinner D, Rankin S, Lee M, Zorn AM.Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development 2004; 131: 3069–3080. [DOI] [PubMed] [Google Scholar]
- Sawada A, Nishizaki Y, Sato H, Yada Y, Nakayama R, Yamamoto S, Nishioka N, Kondoh H, Sasaki H.Tead proteins activate the Foxa2 enhancer in the node in cooperation with a second factor. Development 2005; 132: 4719–4729. [DOI] [PubMed] [Google Scholar]
- Yu X, Wang Y, Jiang M, Bierie B, Roy-Burman P, Shen MM, Taketo MM, Wills M, Matusik RJ.Activation of beta-catenin in mouse prostate causes HGPIN and continuous prostate growth after castration. Prostate 2009; 69: 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom DT, Dowell RD, Jacobsen ES, Nekludova L, Rolfe PA, Danford TW, Gifford DK, Fraenkel E, Bell GI, Young RA.Core transcriptional regulatory circuitry in human hepatocytes. Mol Syst Biol 2006; 2: 2006.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi IC, Cheon YP, Li Q, Bagchi MK.Progesterone receptor-regulated gene networks in implantation. Front Biosci 2003; 8: s852–s861. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E.Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab 2002; 87: 2954–2959. [DOI] [PubMed] [Google Scholar]
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K.Embryo implantation. Dev Biol 2000; 223: 217–237. [DOI] [PubMed] [Google Scholar]
- Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE.Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 2002; 124: 289–300. [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ.Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 1992; 359: 76–79. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP.Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 1997; 277: 1109–1113. [DOI] [PubMed] [Google Scholar]
- Castro-Rendon WA, Castro-Alvarez JF, Guzman-Martinez C, Bueno-Sanchez JC.Blastocyst-endometrium interaction: intertwining a cytokine network. Braz J Med Biol Res 2006; 39: 1373–1385. [DOI] [PubMed] [Google Scholar]
- Tranguch S, Daikoku T, Guo Y, Wang H, Dey SK.Molecular complexity in establishing uterine receptivity and implantation. Cell Mol Life Sci 2005; 62: 1964–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ.In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol 2008; 19: 178–186. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ.Mouse models of implantation. Trends Endocrinol Metab 2007; 18: 234–239. [DOI] [PubMed] [Google Scholar]
- Bhatt H, Brunet LJ, Stewart CL.Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci U S A 1991; 88: 11408–11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MM, Leder P.Leukemia inhibitory factor is expressed by the preimplantation uterus and selectively blocks primitive ectoderm formation in vitro. Proc Natl Acad Sci U S A 1992; 89: 8240–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Lim H, Das SK, Paria BC, Dey SK.Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol 2000; 14: 1147–1161. [DOI] [PubMed] [Google Scholar]