Abstract
Murine models suggest that natural killer (NK) cells are important for normal implantation site development, in part, through the production of interferon gamma (IFNG). As KLRK1 (NKG2D) is expressed on human and murine uterine NK (uNK) cells, we examined the role of KLRK1 in the interaction between murine trophoblasts and NK cells. Flow cytometric analysis revealed that both murine trophoblast stem (TS) cells and differentiated trophoblast giant cells expressed the KLRK1 ligand retinoic acid early transcript 1, or RAET1. Coculture of activated NK cells with either TS cells or giant cells led to the production of IFNG, as measured by ELISA. In addition, coculture with TS cells led to the downregulation of KLRK1. Both responses were inhibited by soluble KLRK1 ligand, but not by irrelevant protein. Further studies demonstrated the presence of KLRK1 ligand on uterine cells derived from either virgin or pregnant mice, although uterine RAET1 protein expression was upregulated in vitro by progesterone, but not estradiol. We suggest that the interaction of KLRK1 and RAET1 may be involved in IFNG production by uNK cells, and thus, this receptor-ligand pair may contribute to successful murine implantation site development.
Keywords: immunology, KLRK1, natural killer cells, pregnancy, RAET1, reproduction, trophoblast, uterus
RAET1 expressed in murine trophoblast cells elicits an interferon-gamma response from NK cells in a KLRK1-dependent manner.
INTRODUCTION
Natural killer (NK) cells are bone marrow–derived lymphocytes that are classically characterized by their antiviral and antitumor abilities [1]. In addition to their fundamental role in host defense, NK cells also support pregnancy-associated uterine vascularization [2]. Decidual natural killer (dNK) cells are present at the maternal-fetal boundary during gestation, and they are thought to be important to normal pregnancy in both mice and humans [2, 3]. Uterine natural killer (uNK) cell-deficient mice, although fertile, demonstrate abnormal implantation sites, having incomplete widening of uterine vessels and significant decidual pathology [2]. Interferon-gamma (IFNG) secreted by dNK cells facilitates physiologic uterine artery alterations during pregnancy and supports normal endometrial decidualization [2].
NK cell activity is regulated by the integration of signals derived from activating and inhibitory receptors. It is suggested that in order to establish a successful pregnancy, the activation status of dNK cells must be above a certain threshold. Indeed, the presence of genes encoding receptors that could provide a strong maternal killer cell immunoglobulin-like receptor/fetal human leukocyte antigen-C inhibitory signal is associated with the development of preeclampsia [4]. Human dNK cells express NCR1 (NKp46), NCR2 (NKp44), NCR3 (NKp30), and KLRK1, as well as other activating receptors [5, 6]. The expression of activating receptors on murine dNK cells was also documented [7, 8]. Moreover, KLRK1 transcripts were detected in murine implantation sites [9].
KLRK1 is a member of the C type lectin-like receptor family that is expressed on NK cells, CD8+ T cells, γδ+ T cells, and NKT cells [10]. KLRK1 is a homodimeric protein that couples to the adapter molecule hematopoietic cell signal transducer (HCST, also known as DAP10) in humans and both HCST and TYRO protein tyrosine kinase binding protein (TYROBP, also known as DAP12) in mice [10]. The adapter proteins are critical for KLRK1-mediated signal transduction and cell surface expression of the receptor [11]. Ligation of this receptor on NK cells leads to target cell killing and cytokine secretion [10].
KLRK1 recognizes a number of inducible ligands that are major histocompatibility complex (MHC) class I–like proteins. Murine KLRK1 ligands include retinoic acid early transcript 1 (RAET1), H60A (H60), and UL16-binding protein 1 (ULBP1) (MULT1) [12]. There are five known Raet1 isoforms: a, b, c, d, and e (RAE-1 α, β, γ, δ, and ε) [12]. RAET1 is expressed early during embryo development but it is usually not present or it is expressed at low levels on normal adult tissues [12]. Cellular stress, such as transformation and viral or bacterial infection, can lead to the induction of KLRK1 ligands on cells, thus facilitating their recognition and elimination by the immune system [13]. Interestingly, Raet1a, d, and e transcripts were detected in murine implantation sites from E6.5 through E10.5. The transcripts localized to vascular endothelium, trophoblasts, and stromal cells [9].
At the blastocyst stage of preimplantation embryonic development, there are two cell lineages. The inner cell mass gives rise to the embryo proper, while the trophectoderm gives rise to the trophoblast cells of the placenta [14]. Trophoblast stem (TS) cells were derived from the trophectoderm of mouse blastocysts [15]. They can also be derived from the extra-embryonic ectoderm from E6.5 conceptuses and the chorionic ectoderm from E7.5 to E10 embryos [16]. TS cells are pluripotent trophoblast progenitor cells that possess the ability to differentiate into all trophoblast lineages present in the mouse placenta [15]. During pregnancy, fetal trophoblast cells invade the uterus and transform the uterine spiral arteries into high-capacity vessels, thus increasing blood flow to the developing fetus. In humans, dNK cells regulate uterine trophoblast invasion [5]. Thus, TS cells provide a valuable system wherein the interaction between trophoblasts and NK cells, important for trophoblast invasion and placentation, may be investigated.
As KLRK1 protein and Raet1 transcripts are present in murine implantation sites, we examined the role of KLRK1 and RAET1 in the interaction between trophoblasts and NK cells. Herein we demonstrate the presence of RAET1 protein on TS cells, trophoblast giant cells, and on uterine cells derived from both virgin and pregnant mice. Progesterone, but not estradiol, upregulated RAET1 protein expression on uterine stromal cells in vitro. Importantly, TS cells and trophoblast giant cells elicited an IFNG response from splenic NK cells by HCST- and TYROBP-dependent and independent mechanisms. Moreover, TS cells induced the downregulation of KLRK1 on splenic NK cells. Although splenic NK cells and not dNK cells were used in these studies, our model system provides proof of principle for KLRK1/RAET1 signaling between NK cells and trophoblast cells. We suggest that the interaction of KLRK1 and RAET1 may be involved in IFNG production by dNK cells, which was previously shown to be important for normal murine implantation site development.
MATERIALS AND METHODS
Mice/Embryo Recovery
Embryos were recovered as previously described [17]. In short, 3-wk-old female C57BL/6 mice (National Cancer Institute) were superovulated and mated with males. All procedures described here were reviewed and approved by the animal studies committee at Washington University and were performed in accordance with Institutional Animal Care and Use Committee approval. Blastocysts were recovered as described elsewhere [18]. TS cells were derived from murine blastocysts as previously described [16]. In brief, blastocysts were harvested and cultured, one per well, on an irradiated mouse embryonic fibroblast (MEF) feeder layer. The MEFs were derived from C57BL/6 embryos at E15.5 according to a previously published protocol [16]. The embryos were cultured in TS cell media [18] supplemented with 25 ng/ml fibroblast growth factor 4 (FGF4) and 1 μg/ml heparin. During the 3- to 4-wk course of TS cell derivation, the embryo outgrowths were disaggregated in the wells and the media was replaced intermittently.
Cells
To confirm the trophoblast lineage of the TS cell line, several genetic markers were analyzed (Fig. 1). TS cells were maintained in the absence of an MEF feeder layer as previously described [18]. TS cells were differentiated into trophoblast giant cells by culturing them in TS cell media that lacked MEF-conditioned medium, FGF4, and heparin for 6 days, as described elsewhere [16].
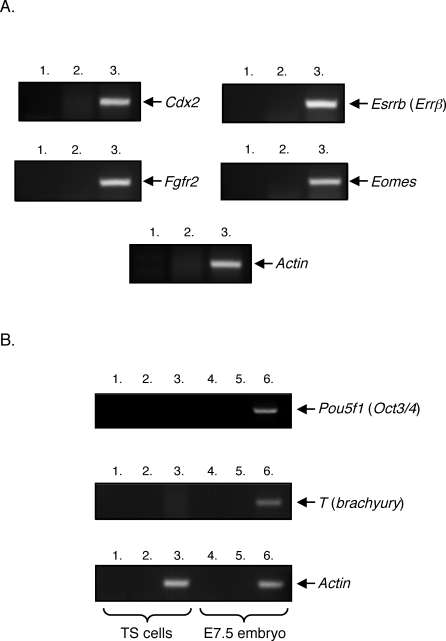
FIG. 1.
Characterization of C57BL/6 TS cell line using genetic markers. RT-PCR was performed on mRNA isolated from C57BL/6 TS cells. A) This cell line expressed the TS cell markers Cdx2, Esrrb, Fgfr2, and the mouse homologue of eomesodermin (Eomes). Lanes 1: no template control; lanes 2: no RT control; and lanes 3: RT included. All RT-PCR reactions contained the indicated gene-specific primers. B) C57BL/6 TS cells did not express the inner cell mass marker Pou5f1 or the mesoderm marker T as determined by RT-PCR. Lanes 1: no template control; lanes 2: no RT control; and lanes 3: RT included. As a positive control, RT-PCR was performed on mRNA isolated from E7.5 mouse embryos. Lanes 4: no template control; lanes 5: no RT control; and lanes 6: RT included.
Lymphokine-activated killer (LAK) cells were generated as described elsewhere [19, 20]. In brief, adult splenocytes were isolated from C57BL/6 mice. Cells that did not adhere to nylon wool were cultured in vitro in the presence of 800 IU/ml interleukin 2 (IL2) for 4 days. Cells that did not adhere to the plastic were discarded, and the remaining population was cultured in vitro for an additional 3–4 days. LAK cell preparations contained <20% non-NK cells.
Antibodies and Flow Cytometry Reagents
Mouse KLRK1 and irrelevant West Nile virus glycoprotein DIII tetramers were prepared as previously described [21, 22]. A pan anti-RAET1 phycoerythrin (PE) antibody, a pan anti-RAET1 fluorescein isothiocyanate (FITC) antibody, a rat immunoglobulin G2a (IgG2a) PE isotype control, and a rat IgG2a FITC isotype control were purchased from R&D Systems. A rat IgG2b PE isotype control antibody, a mouse IgG2a PE isotype control antibody, an anti-protein tyrosine phosphatase receptor type C (PTPRC; also known as leukocyte common antigen [CD45] PE antibody), an anti-PTPRC FITC antibody, an anti-PTPRC allophycocyanin (ASPC) antibody, an anti-killer cell lectin-like receptor subfamily B member 1C (KLRB1C; also known as NK1.1 PE antibody), an anti-KLRK1 APC antibody, an anti-intercellular adhesion molecule 1 (ICAM1) PE antibody, an anti-intercellular adhesion module 2 (ICAM2), Alexa Fluor 488 antibody, PE-conjugated streptavidin, and APC-conjugated streptavidin were purchased from eBioscience. An anti-histocompatibility 2-D region (H2-D) PE antibody (clone 8F12), an anti-histocompatibility 2, K1, K region (H2-K1B, H-2KB) PE antibody, and 7-aminoactinomycin D (7-AAD) were purchased from BD Biosciences.
Flow Cytometry
TS cells or trophoblast giant cells were harvested and the cells were blocked in 5% normal mouse sera and 5% normal rat sera in PBS/10% fetal bovine serum (FBS) for 15 min at 4°C. Cells were then stained with an irrelevant DIII tetramer-streptavidin PE, a KLRK1 tetramer-streptavidin PE, a rat IgG2a PE isotype control antibody, or a pan anti-RAET1 PE antibody. Cells were incubated for 30 min on ice, with gentle agitation after 15 min. Data were collected using a BD FACSCalibur flow cytometer (Becton Dickinson) and analyzed with FlowJo software (TreeStar, Inc.). Live cell gates were based on forward and side scatter plots. All flow cytometry experiments were conducted three times.
Reverse Transcription-Polymerase Chain Reaction
RT-PCRs were carried out on 1 μg mRNA using the Titan One Tube RT-PCR System (Roche) according to the manufacturer's protocol. The RT-PCR program was as follows: 48°C for 30 min, 94°C for 2 min, 94°C for 30 sec, 51°C for 30 sec, and 68°C for 45 sec. The PCR program contained 45 cycles followed by a 30-min extension at 68°C. The program was performed on a DNA Engine Peltier Thermal Cycler (BioRad). The primer sequences were as follows: Cdx2 forward primer: 5′ TTAAACTCCACTGTCACCCAG, Cdx2 reverse primer: 5′ GTAGATGCTGTTCGTGGGTAG (product size 143 bp), Esrrb (Errβ forward primer: 5′ CATGAAATGCCTCAAAGTGGG, Esrrb reverse primer: 5′ AAATCGGCAGGTTCAGGTAG (product size 124 bp), Fgfr2 forward primer: 5′ ATTAACCGTGTCCCCGA, Fgfr2 reverse primer: 5′ TTACCAACACGTTTCTGGC (product size 136 bp), Eomes forward primer: 5′ TTTCGTGGAAGTGGTTCTGG, Eomes reverse primer: 5′ CAGTGTTAGGAGATTCTGGGTG (product size 130 bp), Pou5f1 (Oct3/4) forward primer: 5′ GCAGAAGGAGCTAGAACAGT, Pou5f1 reverse primer: 5′ TCGAAGCGACAGATGGT (product size 138 bp), T (brachyury) forward primer: 5′ TACCCAGCTCTAAGGAACC, T reverse primer: 5′ CGAGGCTAGACCAGTTATCA (product size 136 bp), Prl3d1 (placental lactogen I) forward primer: ATCTTCTCAGAAATGCAGCTG, Prl3d1 reverse primer: GATCATTGCTTTCAGAAGGTC (product size 333 bp), Raet1 forward primer: 5′ CCAAGGCAGCAGTGACCAAG, Raet1 reverse primer: 5′ CATTCCCCCACTTCAGTGGC, Hprt (hypoxanthine guanine phosphoribosyl transferase) forward primer: 5′ GTAATGATCAGTCAACGGGGGAC, Hprt reverse primer: 5′ CCAGCAAGCTTGCAACCTTAACCA. RT-PCR products were cloned and their identity was confirmed by sequencing.
Interferon Gamma ELISA
A total of 3 × 104 TS cells/well or 1.5 × 104 trophoblast giant cells/well were plated in a 96-well plate and allowed to adhere overnight at 37°C and 5% CO2. Increasing numbers of LAK cells (500–25 000) derived from either wild-type or Hcst × Tyrobp double-deficient mice were added to the adherent TS cells or trophoblast giant cells. The cells were cocultured for 16 h in vitro in TS cell media supplemented with 800 IU/ml IL2. Increasing concentrations of soluble ULBP1 or an irrelevant DIII protein were added to parallel wells that contained the maximum number (25 000) of LAK cells. ULBP1 was used as a specific competitor due to its high affinity [23]. Competition with ULBP1 is representative of that with all other homologous ligands, since each of the latter recognizes the same “epitope” on KLRK1 [24]. Indeed, identical results were obtained in a subset of experiments performed simultaneously with soluble RAET1 (data not shown). The amount of IFNG in the supernatants was quantified via IFNG ELISA (eBioscience) according to the manufacturer's protocol. Each condition was run in triplicate wells. This experiment was performed three times.
KLRK1 Downregulation Assay
A total of 8 × 105 TS cells or 4 × 105 trophoblast giant cells were plated per well in 6-well plates and allowed to adhere overnight at 37°C and 5% CO2. There were 5 × 105 LAK cells alone or LAK cells plus 1 μM-soluble ULBP1 or irrelevant DIII protein added to the adherent TS cells or trophoblast giant cells. The cells were cocultured for 8 h in vitro and subsequently harvested using cell dissociation solution (Sigma). Cells were blocked in 5% normal mouse sera in PBS/10% FBS for 15 min and then triple stained with an anti-PTPRC FITC antibody, an anti-KLRB1C PE antibody, and an anti-KLRK1 APC antibody. Cells were incubated with monoclonal antibodies for 30 min on ice with gentle agitation after 15 min. KLRK1 levels on KLRB1C+ cells within a PTPRC+ gate were analyzed. Live cell gates were based on forward and side scatter plots and 7-AAD fluorescence. This experiment was performed three times.
Uterine Preparations-KLRK1 Ligand Expression
Six- to 7-wk-old female C57BL/6 mice in estrus were mated with males of proven fertility. Virgin or pregnant mice at E8.5 and E10.5 were killed, the uteri were isolated, and a single cell suspension was generated as previously described [8]. Briefly, virgin or pregnant mice were euthanized, perfused with PBS (Sigma-Aldrich, St. Louis, MO) containing 1 μg/ml heparin (Sigma-Aldrich), and the uteri were isolated. Virgin uteri were cut longitudinally, minced into small pieces of approximately 1 mm3, and transferred to tubes containing 4°C Hanks Balanced Salt Solution (HBSS). Interimplantation sites were removed from uteri derived from pregnant mice. The fetus and placenta were removed from each implantation site, and the placenta was placed in a separate dish. The uteri were then minced into small pieces. The decidua basalis was removed from the placenta, minced, and added back to the dissected uterine tissue. All uteri were washed twice in 4°C HBSS to remove blood contamination. The tissue was digested in 10 ml of HBSS containing 2 mg/ml of collagenase type I (Invitrogen). Uteri were digested at 37°C for 60 min with intermittent vortexing. After digestion, cells were pelleted and resuspended in 5 ml of 4°C FBS (Sigma-Aldrich). The cells were pelleted and resuspended in PBS/10% FBS. Finally, the cells were passed through a 70-μm cell strainer (BD Falcon; Fisher Scientific, Pittsburgh, PA), counted, and stained for analysis by flow cytometry, as described above. The cells were stained with an irrelevant DIII tetramer-streptavidin APC, a KLRK1 tetramer-streptavidin APC, a rat IgG2a FITC isotype control antibody, or a pan anti-RAET1 FITC antibody. For blocking experiments, cells were incubated with 10 μM ULBP1 or 10 μM DIII irrelevant protein for 30 min at 4°C prior to the addition of the tetramers or antibodies. This experiment was performed three times.
In Vitro Culture of Uterine Stromal Cells
The stage of estrous cycle was determined by histological analysis of vaginal smears [25]. Virgin C57BL/6 mice in diestrus were used for these experiments. Uterine horns were harvested and cells were isolated as described above. The cells were passed through a 40-μm cell strainer (BD Falcon, Fisher Scientific), counted, and plated at 8 × 105 cells/well in 6 well plates. The uterine stromal cells were cultured for 48 hours in phenol red-free DMEM/Hams F-12 media (Fisher Scientific) containing 2.5% charcoal-stripped FBS and 50 μg/ml penicillin/streptomycin. Uterine stromal cells were cultured in media alone or media containing the following: ethanol (vehicle control), 1 μM estradiol (Sigma), 1 μM progesterone (Sigma), 1 μM estradiol and 1 μM progesterone, 1 μM mifepristone (RU486, Sigma), or 1 μM progesterone and 1 μM mifepristone. The cells were harvested and blocked in 5% normal mouse sera/5% normal rat sera in PBS/10% FBS for 15 min at 4°C and then stained with either a rat IgG2a PE isotype control antibody or a pan anti-RAET1 PE antibody for 30 min at 4°C. In addition, uterine stromal cells cultured in media alone were dual stained with an anti-PTPRC APC antibody and either the DIII tetramer streptavidin-PE or the KLRK1 tetramer streptavidin-PE. This experiment was performed at least four times.
Statistical Analyses
Differences between control values and experimental values among more than one experimental group were determined by a one-way ANOVA with a Tukey post hoc test (PASW, IBM). A P value <0.001 was considered statistically significant.
RESULTS
TS Cells and Trophoblast Giant Cells Express RAET1 Protein
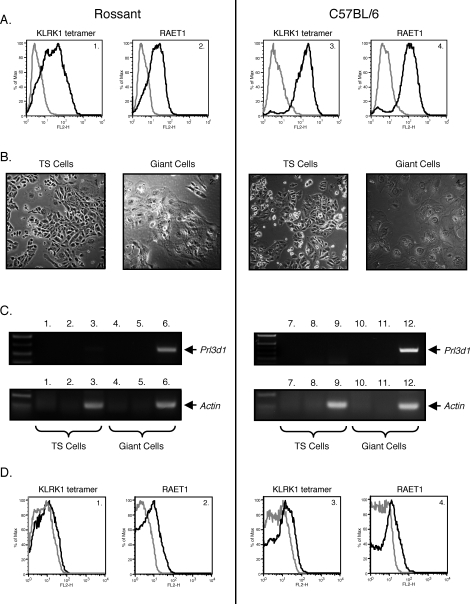
We wished to address whether trophoblasts and NK cells communicate in a cell contact–dependent manner. Specifically, we investigated whether trophoblast cells send a stimulatory signal to NK cells through KLRK1, leading to the production of IFNG that is important for normal murine implantation site development. To establish an in vitro cell culture system in which trophoblast-NK cell interactions could be modeled, we derived a TS cell line from murine C57BL/6 blastocysts. This TS cell line expressed the TS cell markers Cdx2, Esrrb (Errβ), and Fgfr2, and the mouse homologue of eomesodermin (Eomes) (Fig. 1) [15]. These cells did not express the inner cell mass or epiblast-specific marker Pou5f1 (Oct3/4) or the mesoderm-specific marker T (brachyury) [15]. Moreover, the TS cells could be differentiated into placental lactogen-expressing trophoblast giant cells (Fig. 2, B and C). A previously established TS cell line kindly provided by Dr. Janet Rossant, Hospital for Sick Children Research Institute, Toronto, Ontario, Canada [15], and the TS cell line derived herein were stained with an irrelevant tetramer (West Nile virus glycoprotein DIII tetramer), a KLRK1 tetramer, an isotype control antibody, or a pan anti-RAET1 antibody (Fig. 2, A1, A2, A3, and A4). Both TS cell lines expressed RAET1 protein. The TS cells were differentiated into trophoblast giant cells, as depicted in Fig. 2B. Murine trophoblast giant cells invade the uterus and may be considered similar to human extravillous trophoblast cells [26, 27]. To verify that the differentiated TS cells were indeed trophoblast giant cells, RT-PCR was performed using Prl3d1 (placental lactogen I)-specific primers. Prl3d1 is a giant cell–specific marker. Prl3d1 mRNA was largely detected in differentiated but not undifferentiated TS cells (Fig. 2C, top panel, 1–12). Low amounts of Prl3d1 mRNA could be detected in both TS cell lines, as some giant cells are always present during normal TS cell culture. The trophoblast giant cells also expressed RAET1 protein, although to a lesser extent (Fig. 2, D1, D2, D3, and D4). Interestingly, blastocysts from which the TS cells were derived expressed Raet1 transcripts (Supplemental Fig. S1; all Supplemental Data are available online at www.biolreprod.org). Finally, TS cells expressed other proteins known to be important for NK cell recognition including ICAM1, ICAM2, and the MHC class I molecules H2-D and H2-K1B (Supplemental Fig. S2).
FIG. 2.
RAET1 protein is expressed on the cell surface of TS cells and trophoblast giant cells. A) Rossant TS cells or C57BL/6 TS cells were stained with irrelevant DIII tetramer-streptavidin PE (A1 and A3 gray line), KLRK1 tetramer-streptavidin PE (A1 and A3 black line), rat IgG2a PE isotype control antibody (A2 and A4 gray line), or a pan anti-RAET1 PE antibody (A2 and A4 black line). B) Morphology of TS cells differentiated into trophoblast giant cells. Rossant TS cells (B1) and C57BL/6 TS cells (B3) were differentiated into trophoblast giant cells (B2 and B4, respectively). C) RT-PCR for Prl3d1. Top left panel depicts RT-PCR reactions using Rossant TS cell mRNA. C1: no template control, C2: no RT control, and C3: RT included. All three reactions contained Prl3d1-specific primers. The top left panel also depicts RT-PCR reactions containing Rossant trophoblast giant cell mRNA. C4: no template control, C5: no RT control, and C6: RT included. All three reactions contained Prl3d1-specific primers. Depicted in the lower left panel are control RT-PCR reactions using actin-specific primers. The lane descriptions for the actin RT-PCR are the same as C1–C6. C7–C12 recapitulate C1–C6 except that TS cell and trophoblast giant cell mRNA was derived from the C57BL/6 cell line. D) Trophoblast giant cells were stained as described in A. One representative experiment is depicted. Each of these experiments was performed three times.
TS Cells and Trophoblast Giant Cells Induce IFNG Production by Syngenic LAK Cells, in Part, Through KLRK1
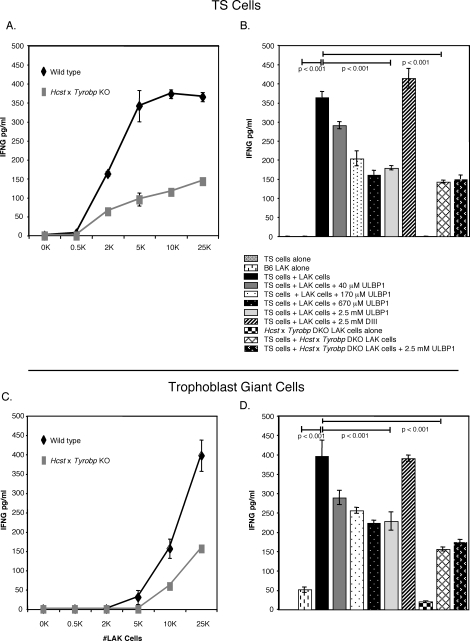
We chose to use IL2-activated splenic NK cells (LAK cells) instead of dNK cells in our coculture system because dNK cells present in implantation sites are activated and are already secreting IFNG [2]. In addition, while collagenase treatment does not remove many NK cell receptors from the cell surface [8], KLRK1 expression on dNK cells was somewhat affected by our isolation procedure (data not shown). Thus, TS cells derived from C57BL/6 mice were cocultured with LAK cells derived from both C57BL/6 wild-type mice and Hcst × Tyrobp double knockout mice [28]. TS cells induced IFNG production by wild-type LAK cells in a manner directly dependent on the number of NK cells present in the culture (Fig. 3A). Cytotoxicity caused by the splenic NK cells paralleled IFNG production (data not shown). Interestingly, Hcst × Tyrobp double-knockout LAK cells maximally made less than half the amount of IFNG (148 ± 6 pg/ml) as the wild-type control (366 ± 23 pg/ml). To determine whether the IFNG produced by the activated LAK cells was mediated via KLRK1, a ligand-blocking experiment was performed (Fig. 3B). The addition of soluble KLRK1 ligand (ULBP1) to the coculture system partially blocked IFNG production, whereas an irrelevant protein did not. Maximal inhibition by soluble ULBP1 reduced the amount of IFNG produced by wild-type C57BL/6 LAK cells (161 ± 12 pg/ml) to approximately the same degree as inactivation of the Hcst and Tyrobp gene loci (143 ± 4 pg/ml). These groups were not significantly different at the 0.05 level as determined by a one-way ANOVA with a Tukey post hoc test. Thus, TS cells induced IFNG production by LAK cells through HCST- and TYROBP-dependent and independent mechanisms. Similar results were obtained when trophoblast giant cells were used in place of the TS cells in the coculture assay (Fig. 3, C and D).
FIG. 3.
C57BL/6 TS cells and trophoblast giant cells induce IFNG production in syngeneic LAK cells by Hcst- and Tyrobp-dependent and independent mechanisms. A) IFNG secreted by C57BL/6 wild-type LAK cells cocultured for 16 h in vitro with TS cells (black line) or Hcst × Tyrobp double-knockout LAK cells cocultured with TS cells (gray line). B) IFNG levels in supernatants derived from TS cells alone, B6 LAK cells alone, TS cells + LAK cells, TS cells/LAKs + 40 μM ULBP1, TS cells/LAKs + 170 μM ULBP1, TS cells/LAKs + 670 μM ULBP1, TS cells/LAKs + 2.5 mM ULBP1, TS cells/LAKs + 2.5 mM DIII, Hcst × Tyrobp double-knockout (DKO) LAKs alone, TS cells + Hcst × Tyrobp DKO LAKs, and TS cells/Hcst × Tyrobp LAKs + 2.5 mM MULT-1. C) IFNG secreted by C57BL/6 wild type LAK cells cocultured with trophoblast giant cells (black line) or Hcst × Tyrobp double-knockout LAK cells cocultured with trophoblast giant cells (gray line) as described in A. D) Trophoblast giant cells were substituted for TS cells in the ligand-blocking experiment described in B. One representative experiment is depicted. Values are the mean ± SEM of triplicate wells. Significance was determined by one-way ANOVA with a Tukey test. This experiment was performed three times.
KLRK1 Is Downregulated on LAK Cells Cultured with TS Cells
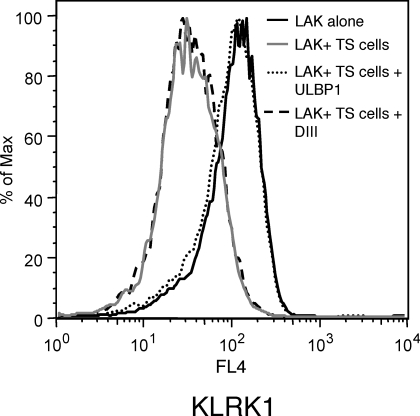
It is known that constant stimulation of NK cells with cells expressing a KLRK1 ligand can lead to the downregulation of KLRK1 protein and impaired NK cell function [29]. We investigated whether this phenomenon occurred in our coculture system. KLRK1 levels were assessed on LAK cells cocultured with TS cells by flow cytometry. TS cells induced the downregulation of KLRK1 protein expressed on LAK cells (Fig. 4). This response was inhibited by soluble ULBP1, but not by an irrelevant protein. Thus, stimulation of NK cells by RAET1 expressed on TS cells led to decreased KLRK1 protein expression.
FIG. 4.
KLRK1 is downregulated on LAK cells that were cocultured with TS cells. KLRK1 protein levels were assessed on 5 × 105 LAK cells cultured alone, cocultured with TS cells, cocultured with TS cells in the presence of 1 μM soluble ULBP1, or cocultured with TS cells in the presence of 1 μM DIII protein for 8 hours in vitro. The cells were then stained with an anti-PTPRC FITC antibody, an anti-KLRB1C PE antibody, and an anti-KLRK1 APC antibody. KLRK1 levels on KLRB1C+ cells within a PTPRC+ gate were analyzed. One representative experiment is depicted. This experiment was performed three times.
RAET1 Protein Is Expressed in the Virgin and Gravid Mouse Uterus
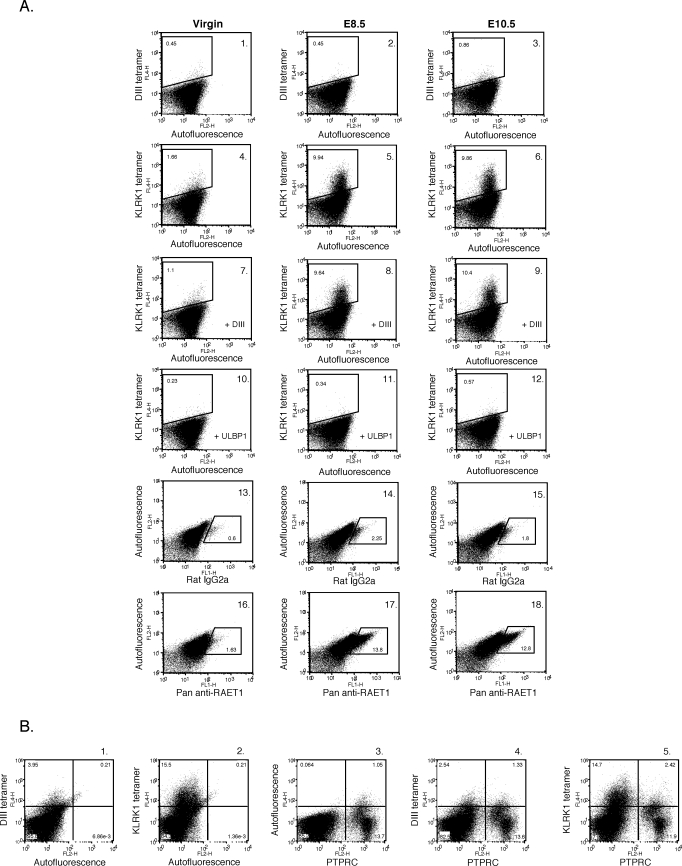
We next examined RAET1 protein expression on uteri isolated from virgin mice and uteri isolated from implantation sites at E8.5 and E10.5. Uteri were isolated and a single-cell suspension was generated. The cells were stained with an irrelevant tetramer (DIII tetramer) or a KLRK1 tetramer and analyzed by flow cytometry. A low level of KLRK1 ligand expression was detected on cells derived from virgin uteri (Fig. 5, A4) as compared to the control (Fig. 5, A1). KLRK1 ligand was clearly expressed on cells derived from E8.5 and E10.5 implantation sites (Fig. 5, A5 and A6, respectively) as compared to controls (Fig. 5, A2 and A3). KLRK1 tetramer staining of uterine cells was blocked by the presence of soluble ULBP1 (Fig. 5, A10, A11, and A12), but not by an irrelevant protein (Fig. 5, A7, A8, and A9). The vast majority of KLRK1 ligand expressed in the uterus at E8.5 was present on PTPRC (CD45)-negative cells (Fig. 5, B5).
FIG. 5.
RAET1 ligand is expressed in the uterus of both virgin and gravid mice. A) Uteri were isolated from virgin mice and from implantation sites at E8.5 and 10.5. A single-cell suspension was generated and stained with irrelevant DIII tetramer–streptavidin APC (A1, A2, and A3), KLRK1 tetramer-streptavidin APC (A4, A5, and A6), rat IgG2a FITC isotype control antibody (A13, A14, and A15), or pan anti-RAET1 FITC antibody (A16, A17, and A18). Cells stained with KLRK1 tetramer were also pre-incubated with either 10 μM of irrelevant DIII protein (A7, A8, and A9) or soluble ULBP1 (A10, A11, and A12). B) Uteri from E8.5 implantation sites were isolated and the cells were stained with irrelevant DIII tetramer-streptavidin APC (B1), KLRK1 tetramer-streptavidin APC (B2), an anti-PTPRC PE antibody (B3), irrelevant DIII tetramer- streptavidin APC and an anti-PTPRC PE antibody (B4), or KLRK1 tetramer-streptavidin APC and an anti-PTPRC PE antibody (B5). One representative experiment is depicted. This experiment was performed three times.
Cells isolated from virgin uteri and from implantation sites at E8.5 and E10.5 were also stained with either an isotype control antibody or a pan anti-RAET1 antibody. RAET1 protein was not easily detected in the virgin uterus when this staining reagent was used; however, some cells did express this protein (Fig. 5, A16). We performed RT-PCR followed by a nested PCR on mRNA isolated from virgin uteri using Raet1-specific primers. Raet1 transcripts were detected in the virgin uterus (data not shown). RAET1 protein was clearly detected with the pan anti-RAET1 antibody on uterine cells isolated from implantation sites at E8.5 and E10.5 (Fig. 5, A17 and A18, respectively) as compared to cells stained with an isotype control antibody (Fig. 5, A14 and A15). Thus, RAET1 protein is expressed in the virgin and gravid uterus.
Progesterone Upregulates RAET1 Protein Expression on Uterine Stromal Cell Cultures
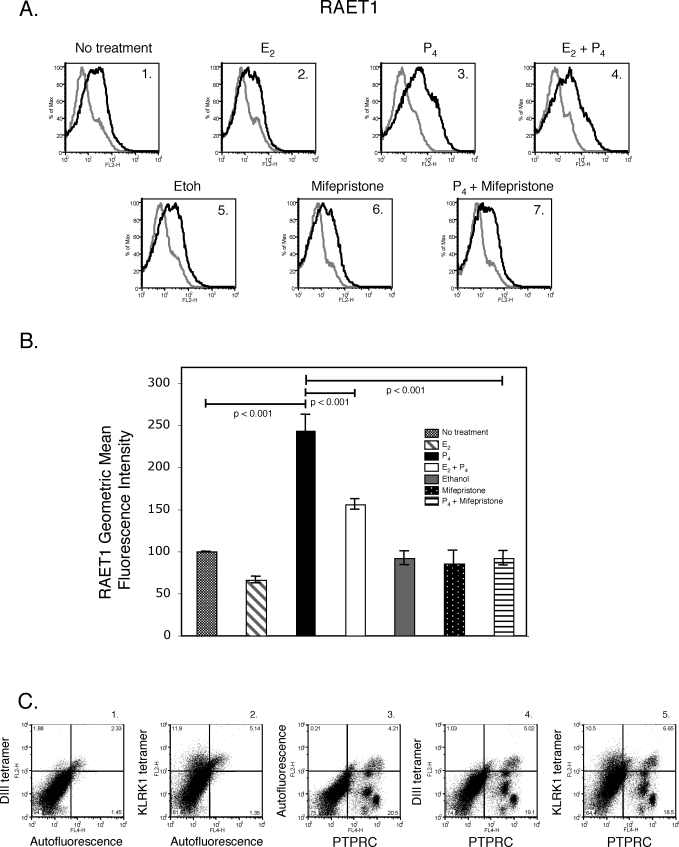
Progesterone, a steroid hormone, is required to establish and maintain pregnancy in mammals [30]. In mice, if fertilization occurs, progesterone levels remain elevated until just prior to parturition, at which point serum progesterone concentrations decline [31]. Given that uterine RAET1 protein expression increased in parallel with progesterone concentrations, we next determined whether progesterone regulates RAET1 protein expression. Uteri were isolated from virgin mice during diestrus in an attempt to avoid confounding effects of endogenous estrogen and progesterone surges on KLRK1 ligand expression. The isolated uteri were pooled and a single-cell suspension was generated. The uterine stromal cells were cultured in vitro for 48 h in media alone, ethanol (vehicle control for mifepristone), estradiol (E2), progesterone (P4), estradiol and progesterone, mifepristone (a progesterone receptor antagonist), or progesterone and mifepristone. Uterine stromal cells cultured in vitro were stained with either an isotype control antibody or a pan anti-RAET1 antibody and analyzed by flow cytometry. Uterine stromal cells cultured in media alone expressed RAET1 protein (Fig. 6, A1). Interestingly, progesterone, but not estradiol, increased RAET1 protein expression on these cells (Fig. 6, A3 and A2, respectively). In fact, estradiol inhibited the degree to which progesterone increased RAET1 protein levels (Fig. 6, A3 and A4). Mifepristone inhibited progesterone-induced upregulation of RAET1 protein levels (Fig. 6, A7). The geometric mean fluorescence intensity of RAET1 staining on treated uterine stromal cells was as follows: estradiol 66 ± 4, progesterone 243 ± 19, estradiol and progesterone 156 ± 6, ethanol 92 ± 8, mifepristone 83 ± 16, or progesterone and mifepristone 92 ± 9 (Fig. 6B). The geometric mean fluorescence intensity is relative to the untreated control cells. Thus, progesterone upregulated RAET1 protein on uterine stromal cells by a progesterone receptor–dependent mechanism. Furthermore, the majority of RAET1 protein expressed on uterine stromal cells was present on PTPRC (CD45)-negative cells (Fig. 6, C5).
FIG. 6.
Progesterone upregulates RAET1 protein expression on uterine stromal cells. A) Uteri were isolated from virgin C57BL/6 mice in diestrus and cultured for 48 h in vitro in media alone (A1), 1 μM estradiol (A2), 1 μM progesterone (A3), 1 μM estradiol and 1 μM progesterone (A4), ethanol-vehicle control for mifepristone (A5), 1 μM mifepristone (A6), or 1 μM progesterone and 1 μM mifepristone (A7). Cells were stained with either a rat IgG2a PE isotype control (gray histogram) or a pan anti-RAET1 PE antibody (black histogram). B) RAET1 geometric mean fluorescence intensity for treatments described in A. Values are the mean ± SEM of four to six independent experiments. Samples were compared by one-way ANOVA with a Tukey test. C) Uterine stromal cells cultured in media alone were stained with DIII tetramer-streptavidin PE (C1), KLRK1 tetramer-streptavidin PE (C2), anti-PTPRC APC antibody (C3), DIII tetramer-streptavidin PE and anti-PTPRC APC antibody (C4), or KLRK1tetramer-streptavidin PE and anti-PTPRC APC (C5). One representative experiment of three is depicted.
DISCUSSION
Murine KLRK1 ligands include RAET1, H60A, and ULBP1 [12]. H60A is not expressed in C57BL/6 mice [32] and, under normal conditions, ULBP1 is degraded by the lysosome [33]. Although RAET1 is usually not present or it is expressed at low levels on normal adult tissues [12], we chose to focus on RAET1 expression in TS cells and uterine stromal cells, as retinoic acid, which induces RAET1 expression, is secreted by endometrial stromal cells during the process of decidualization in a progesterone-dependent manner [34]. Herein we demonstrated that TS cells and trophoblast giant cells express RAET1 protein. Furthermore, we demonstrated the presence of RAET1 protein in murine virgin and gravid uteri. The expression of RAET1 on fetal trophoblast cells would appear undesirable, as the presence of this protein should facilitate the elimination of these cells by the maternal immune system. However, it is becoming clear that uNK cell activation during gestation is beneficial to the developing fetus [2, 35].
In the pregnant mouse uterus, one would expect that dNK cells interact with trophoblast giant cells. While the trophoblast giant cells expressed lower levels of RAET1 protein than TS cells did, we demonstrated that they did elicit an IFNG response from NK cells. Although the trophoblast giant cells were less efficient than TS cells at inducing NK cell IFNG production, they did induce roughly the same amount of IFNG as undifferentiated TS cells did at the highest dose of NK cells used. Thus, in our model system, trophoblast giant cells activated NK cells. Based on these data, we speculate that trophoblast giant cells could also activate NK cells at the maternal-fetal interface. The physiological function of RAET1 expressed on TS cells is unclear. Potentially, TS cells could interact with endometrial NK cells, which are present in the endometrium of both murine and human uteri prior to pregnancy. Endometrial NK cells are incompletely characterized and their function is currently ill-defined. We speculate that the physiologic relevance of RAET1 expressed on TS cells may relate to the activation of endometrial NK cells very early during the implantation process.
Our data highlight the potential utility of undifferentiated or differentiated TS cells as model systems to help elucidate the mechanisms by which dNK cells may contribute to placental development and thus the establishment of a successful pregnancy. We demonstrated that in addition to RAET1, TS cells express other proteins known to be important for NK cell recognition including the classical MHC I proteins H2-D and H2-K1B (Supplemental Fig. S2). Classical MHC I protein expression patterns on trophoblast cells are much less well characterized in mice than in humans. It is thought that the labyrinthine trophoblast cells of the mouse placenta do not express classical MHC I. Conversely, spongiotrophoblast cells were shown to express polymorphic MHC I proteins that were paternally derived [36, 37]. Interstitial trophoblast cells were also reported to express classical MHC I antigens [36]. A study conducted by Erlebacher et al. also demonstrated the presence of classical MHC I on multiple independently derived TS cell lines [38]. We speculate that as TS cells differentiate into distinct trophoblast subtypes, they either maintain or lose their ability to express classical MHC I proteins according to the expression pattern described above for trophoblast lineages of the mouse placenta. Physiologically differential MHC I expression on trophoblast subtypes may, in part, reflect the likelihood that each lineage will interact with specific components of the maternal immune system.
We chose to utilize IL2-activated splenic NK cells in our coculture experiments, as the dNK cells isolated by our methods were nonresponsive in vitro. IL2 is not present in the gravid uterus. Moreover, IL2 treatment of dNK cells was shown to alter the levels and types of cytokines secreted by these cells [39, 40]. Thus, IL2-activated splenic NK cells may be phenotypically and functionally distinct from dNK cells. Although IL2-activated NK cell responses may be different from dNK cell responses, we used our model system to establish that trophoblast cells have the ability to activate NK cells via KLRK1 and speculate that this interaction may contribute to normal murine implantation site development.
We demonstrated that TS cells elicit an IFNG response by LAK cells, in part through a KLRK1-dependent mechanism. Moreover, coculture of RAET1-expressing TS cells with LAK cells led to the downregulation of KLRK1 protein. Previously, Coudert et al. demonstrated that chronic stimulation of NK cells with tumor cells expressing KLRK1 ligand led to sustained IFNG production and the suppression of NK cell cytotoxic activity [41]. In this system, KLRK1 was downregulated on NK cells. A subsequent report demonstrated that chronic stimulation of KLRK1 impaired not only KLKR1 function, but also the function of other NK cell activation pathways including antibody-dependent cell-mediated cytotoxicity and missing self-recognition [42]. In light of these studies, chronic KLRK1 activation by RAET1 present in the uterus during gestation may represent one mechanism by which the maternal immune system is rendered tolerant of fetal trophoblast cells. In addition, IFNG production by murine dNK cells is critical for restructuring the maternal/fetal blood supply and for endometrial decidualization [2]. Thus, prolonged IFNG production by dNK cells in the absence of cytotoxicity may facilitate normal murine implantation site development.
A number of hypotheses have been put forth to explain why the semi-allogeneic fetus is not rejected by the maternal immune system. One theory postulates that the placenta acts as an immunoregulatory tissue. Previously, syncytiotrophoblast cells of human placenta were shown to express the KLRK1 ligands MICA and MICB [43]. Elevated concentrations of MIC proteins were detected in the blood of pregnant women. In addition, KLRK1 levels were decreased on peripheral blood mononuclear cells (PBMC) derived from pregnant women as compared to nonpregnant controls. Interestingly, serum derived from pregnant women induced the downregulation of KLRK1 on PBMC derived from nonpregnant women. An anti-MICA/B antibody blocked this effect. A recent report demonstrated that ULBPs, a family of human KLRK1 ligands, are also expressed by syncytiotrophoblasts [44]. Exosomes expressing KLRK1 ligands were secreted by human placenta and induced the downregulation of KLRK1 on peripheral blood NK cells, CD8+ T cells, and γδ T cells, leading to impaired cytotoxicity [44]. Similarly, chronic stimulation of dNK cells through RAET1 expressed on trophoblast cells or uterine stromal cells may lead to KLRK1 downregulation on dNK cells. In mice, KLRB1C (NK1.1)−ITGA2 (DX5)− dNK cells expressed lower amounts of KLRK1 protein than splenic NK cells [7]. The low level of KLRK1 protein detected on the KLRB1C−ITGA2− dNK cells is consistent with the hypothesis that RAET1 expressed in the uterus during gestation may lead to the downregulation of this receptor. Again, the benefits of KLRK1 downregulation on NK cells are at least twofold. First, chronic stimulation may lead to prolonged IFNG production, and second, it may impair multiple NK cell activation pathways, allowing fetally derived tissue to escape attack by the maternal immune system [41–44].
The ovarian steroid hormones estradiol and progesterone regulate numerous aspects of the innate immune system [45]. Previously, estradiol was shown to increase MICA protein expression on a uterine adenocarcinoma cell line [46]. In addition, MICA expressed on epithelial cells in human endometrial tissue was highest in the secretory phase of the menstrual cycle [46]. The authors stated that initial studies did not demonstrate progesterone regulation of human KLRK1 ligand expression in primary human endometrial cells and a human uterine epithelial cell line. In contrast, our data demonstrated that progesterone, but not estradiol, upregulated RAET1 protein expression on uterine stromal cells. The differential effects of estradiol and progesterone on uterine KLRK1 ligand expression may be due to several factors. First, different uterine cell types were examined, i.e., uterine epithelial versus stromal cells. Second, working in mice afforded the opportunity to harvest uteri at a specific point during the estrous cycle. This likely conferred a level of homogeneity among tissue samples and allowed us to collect tissue during a time when endogenous estradiol and progesterone levels were relatively low. Finally, it is possible that the effects of ovarian steroid hormones on KLRK1 ligand expression may be species specific. Interestingly, putative progesterone responses elements are present within the promoters of Raet1 genes.
The uterine stromal cells cultured in vitro demonstrated relatively high levels of RAET1 protein as compared to freshly isolated uterine cells. This may be due to differences in RAET1 expression in the whole uterus as compared to a relatively pure uterine stromal cell population. It is also possible that maintaining the cells in vitro led to increased basal levels of RAET1 protein. Although the levels of RAET1 expression may differ between cultured and freshly isolated cells, progesterone upregulated RAET1 expression on uterine stromal cells and uterine expression of RAET1 increased during gestation when progesterone concentrations are elevated. Furthermore, we demonstrated a clear difference in the amount of RAET1 expressed on uterine cells derived from virgin mice as compared to pregnant mice. These uteri were processed identically and simultaneously, negating the possibility that differences in RAET1 expression were due to processing of the tissue. Whether progesterone-induced upregulation of RAET1 protein expression is the result of progesterone effects on uterine retinoic acid production remains to be determined.
In contrast to most adult cells, TS cells and uterine stromal cells expressed relatively high levels of RAET1. Moreover, pregnancy induced increased uterine expression of this ligand. We speculate that the interaction of KLRK1 and RAET1 is involved in IFNG production by dNK cells, which is important for normal murine implantation site development. It is also possible that TS cells or trophoblast giant cells secrete exosomes expressing KLRK1 ligands, which could influence dNK cell function. RAET1 expressed on multiple cell types in the uterus during gestation may maximize dNK cell activation. In addition, a recent report demonstrated that epidermal upregulation of RAET1 alone induced the reorganization of immune cells present in this tissue [47]. Similarly, RAET1 upregulation within the uterus may restructure the immune compartment and regulate the local immune response. The data described herein suggest that an interaction between RAET1 and KLRK1 may be physiologically important for establishing a successful pregnancy, in part through the production of IFNG. In addition, ovarian steroid hormones regulated KLRK1 ligand expression and thus may affect the immune response during gestation. Although murine pregnancy is in many ways distinct from human pregnancy, the findings presented herein hopefully provide insight into mechanisms important for human embryo implantation. Further studies are warranted to elucidate the precise role of KLRK1 ligands in the uterus of mice and humans during pregnancy.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Janet Rossant (Hospital for Sick Children Research Institute, Toronto, ON, Canada) for providing TS cells; Dr. Toshiyuki Takai (Tohoku University, Sendai, Japan) for providing Hcst × Tyrobp deficient mice; Liping Yang (Washington University, St. Louis, MO) for help in generating the TS cell lines; Dr. Jessica Campbell (Washington University, St. Louis, MO) for providing the KLRK1 tetramers; Dr. David Fremont (Washington University, St. Louis, MO) for providing the West Nile virus DIII tetramers; and Antonina Frolova (Washington University, St. Louis, MO) for statistical review.
Footnotes
Supported by the National Institutes of Health Grant 5K12HD001459 to J.K.R. and K08AI57361 to L.N.C. W.M.Y. is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- Cooper MA, Colonna M, Yokoyama WM.Hidden talents of natural killers: NK cells in innate and adaptive immunity. EMBO Rep 2009; 10: 1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy BA, van den Heuvel MJ, Borzychowski AM, Tayade C.Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev 2006; 214: 161–185. [DOI] [PubMed] [Google Scholar]
- Manaster I, Mandelboim O.The unique properties of human NK cells in the uterine mucosa. Placenta 2008; 29(suppl A):S60–S66. [DOI] [PubMed] [Google Scholar]
- Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A.Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med 2004; 200: 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006; 12: 1065–1074. [DOI] [PubMed] [Google Scholar]
- Vacca P, Pietra G, Falco M, Romeo E, Bottino C, Bellora F, Prefumo F, Fulcheri E, Venturini PL, Costa M, Moretta A, Moretta L, et al. Analysis of natural killer cells isolated from human decidua: evidence that 2B4 (CD244) functions as an inhibitory receptor and blocks NK-cell function. Blood 2006; 108: 4078–4085. [DOI] [PubMed] [Google Scholar]
- Yadi H, Burke S, Madeja Z, Hemberger M, Moffett A, Colucci F.Unique receptor repertoire in mouse uterine NK cells. J Immunol 2008; 181: 6140–6147. [DOI] [PubMed] [Google Scholar]
- Mallidi TV, Craig LE, Schloemann SR, Riley JK.Murine endometrial and decidual NK1.1+ natural killer cells display a B220+CD11c+ cell surface phenotype. Biol Reprod 2009; 81: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, He H, Colonna M, Seya T, Takai T, Croy BA.Pathways participating in activation of mouse uterine natural killer cells during pregnancy. Biol Reprod 2005; 73: 510–518. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Groh V, Spies T.Immunobiology of human NKG2D and its ligands. Vivier E, Colonna M.Immunobiology of Natural Killer Cell Receptors. Heidelberg:Springer;2006: 121–138. [DOI] [PubMed] [Google Scholar]
- Tassi I, Klesney-Tait J, Colonna M.Dissecting natural killer cell activation pathways through analysis of genetic mutations in human and mouse. Immunol Rev 2006; 214: 92–105. [DOI] [PubMed] [Google Scholar]
- Eagle RA, Trowsdale J.Promiscuity and the single receptor: NKG2D. Nat Rev Immunol 2007; 7: 737–744. [DOI] [PubMed] [Google Scholar]
- Stern-Ginossar N, Mandelboim O.An integrated view of the regulation of NKG2D ligands. Immunology 2009; 128: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas GC, VandeVoort CA, Kumar P, Chang TC, Golos TG.Trophoblast stem cells: models for investigating trophectoderm differentiation and placental development. Endocr Rev 2009; 30: 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J.Promotion of trophoblast stem cell proliferation by FGF4. Science 1998; 282: 2072–2075. [DOI] [PubMed] [Google Scholar]
- Quinn J, Kunath T, Rossant J.Mouse trophoblast stem cells. Soares MJ, Hunt JS.Placenta and Trophoblast, Vol. 1. Totowa, New Jersey:Humana Press Inc.;2006: 125–158. [Google Scholar]
- Moley KH, Chi MM, Mueckler MM.Maternal hyperglycemia alters glucose transport and utilization in mouse preimplantation embryos. Am J Physiol 1998; 275: E38–E47. [DOI] [PubMed] [Google Scholar]
- Riley JK, Carayannopoulos MO, Wyman AH, Chi M, Ratajczak CK, Moley KH.The PI3K/Akt pathway is present and functional in the preimplantation mouse embryo. Dev Biol 2005; 284: 377–386. [DOI] [PubMed] [Google Scholar]
- Idris AH, Iizuka K, Smith HR, Scalzo AA, Yokoyama WM.Genetic control of natural killing and in vivo tumor elimination by the Chok locus. J Exp Med 1998; 188: 2243–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho EL, Carayannopoulos LN, Poursine-Laurent J, Kinder J, Plougastel B, Smith HR, Yokoyama WM.Costimulation of multiple NK cell activation receptors by NKG2D. J Immunol 2002; 169: 3667–3675. [DOI] [PubMed] [Google Scholar]
- Campbell JA, Trossman DS, Yokoyama WM, Carayannopoulos LN.Zoonotic orthopoxviruses encode a high-affinity antagonist of NKG2D. J Exp Med 2007; 204: 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybakken GE, Oliphant T, Johnson S, Burke S, Diamond MS, Fremont DH.Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature 2005; 437: 764–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM.Cutting edge: murine UL-16-binding protein-like transcript 1: a newly described transcript encoding a high-affinity ligand for murine NKG2D. J Immunol 2002; 169: 4079–4083. [DOI] [PubMed] [Google Scholar]
- Deng L, Mariuzza RA.Structural basis for recognition of MHC and MHC-like ligands by natural killer cell receptors. Semin Immunol 2006; 18: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E.The oestrus cycle in the mouse. Am J Anat 1922; 30: 297–348. [Google Scholar]
- Rossant J, Cross JC.Placental development: lessons from mouse mutants. Nat Rev Genet 2001; 2: 538–548. [DOI] [PubMed] [Google Scholar]
- Watson ED, Cross JC.Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 2005; 20: 180–193. [DOI] [PubMed] [Google Scholar]
- Inui M, Kikuchi Y, Aoki N, Endo S, Maeda T, Sugahara-Tobinai A, Fujimura S, Nakamura A, Kumanogoh A, Colonna M, Takai T.Signal adaptor DAP10 associates with MDL-1 and triggers osteoclastogenesis in cooperation with DAP12. Proc Natl Acad Sci U S A 2009; 106: 4816–4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, Ljunggren HG.Tumor cell recognition by the NK cell activating receptor NKG2D. Eur J Immunol 2008; 38: 2957–2961. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Spencer TE, Johnson GA, Burghardt RC, Wu G.Comparative aspects of implantation. Reproduction 2009; 138: 195–209. [DOI] [PubMed] [Google Scholar]
- Virgo BB, Bellward GD.Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology 1974; 95: 1486–1490. [DOI] [PubMed] [Google Scholar]
- Malarkannan S, Shih PP, Eden PA, Horng T, Zuberi AR, Christianson G, Roopenian D, Shastri N.The molecular and functional characterization of a dominant minor H antigen, H60. J Immunol 1998; 161: 3501–3509. [PubMed] [Google Scholar]
- Nice TJ, Coscoy L, Raulet DH.Posttranslational regulation of the NKG2D ligand Mult1 in response to cell stress. J Exp Med 2009; 206: 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteen KG, Bruner-Tran KL, Ong D, Eisenberg E.Paracrine mediators of endometrial matrix metalloproteinase expression. Ann N Y Acad Sci 2002; 955: 139–146. [DOI] [PubMed] [Google Scholar]
- Hanna J, Mandelboim O.When killers become helpers. Trends Immunol 2007; 28: 201–206. [DOI] [PubMed] [Google Scholar]
- Redline R, Lu CY.Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta. Implications for maternal-fetal immunological relationship. Lab Invest 1989; 61: 27–36. [PubMed] [Google Scholar]
- Zuckermann FA, Head JR.Expression of MHC antigens on murine trophoblast and their modulation by interferon. J Immunol 1986; 137: 846–853. [PubMed] [Google Scholar]
- Erlebacher A, Lukens AK, Glimcher LH.Intrinsic susceptibility of mouse trophoblasts to natural killer cell–mediated attack in vivo. PNAS 2002; 26: 16940–16945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006; 12: 1065–1074. [DOI] [PubMed] [Google Scholar]
- Vacca P, Cantoni C, Prato C, Fulcheri E, Moretta A, Moretta L, Mingari MC.Regulatory role of NKp44, NKp46, DNAM-1 and NKG2D receptors in the interaction between NK cells and trophoblast cells. Evidence for divergent functional profiles of decidual versus peripheral NK cells. Int Immunol 2008; 20: 1395–1405. [DOI] [PubMed] [Google Scholar]
- Coudert JD, Zimmer J, Tomasello E, Cebecauer M, Colonna M, Vivier E, Held W.Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood 2005; 106: 1711–1717. [DOI] [PubMed] [Google Scholar]
- Coudert JD, Scarpellino L, Gros F, Vivier E, Held W.Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood 2008; 111: 3571–3578. [DOI] [PubMed] [Google Scholar]
- Mincheva-Nilsson L, Nagaeva O, Chen T, Stendahl U, Antsiferova J, Mogren I, Hernestal J, Baranov V.Placenta-derived soluble MHC class I chain-related molecules down-regulate NKG2D receptor on peripheral blood mononuclear cells during human pregnancy: a possible novel immune escape mechanism for fetal survival. J Immunol 2006; 176: 3585–3592. [DOI] [PubMed] [Google Scholar]
- Hedlund M, Stenqvist AC, Nagaeva O, Kjellberg L, Wulff M, Baranov V, Mincheva-Nilsson L.Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol 2009; 183: 340–351. [DOI] [PubMed] [Google Scholar]
- Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L.Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev 2005; 206: 306–335. [DOI] [PubMed] [Google Scholar]
- Basu S, Pioli PA, Conejo-Garcia J, Wira CR, Sentman CL.Estradiol regulates MICA expression in human endometrial cells. Clin Immunol 2008; 129: 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, Kaplan DH, Hayday AC, Girardi M.Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol 2008; 9: 146–154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.