Abstract
Homeostasis of many tissues is maintained by self-renewal and differentiation of stem cells. Spermatogenesis is one such system relying on the activity of spermatogonial stem cells (SSCs). Several key regulators of SSC self-renewal have been identified, yet knowledge of molecules that control SSC differentiation is undefined. In this study, we found that transient impairment of STAT3 signaling enhances SSC self-renewal in vitro without affecting general spermatogonial proliferation, indicating an alteration in the balance of SSC fate decisions that inhibited differentiation. Confirming this observation, short hairpin RNA-mediated stable reduction of STAT3 expression in cultured SSCs abolished their ability to differentiate beyond the undifferentiated spermatogonial stage following transplantation into recipient testes. Collectively, these results demonstrate that STAT3 promotes the differentiation of SSCs. In contrast, STAT3 plays a central role in maintaining self-renewal of mouse embryonic stem cells, and STAT signaling is essential for self-renewal of male germline stem cells in Drosophila.
Keywords: differentiation, spermatogonial stem cell, STAT3
Impairment of STAT3 signaling altered spermatogonial stem cell fate decisions, leading to an increased rate of self-renewal and blockade of spermatogonial differentiation in mouse seminiferous tubules.
INTRODUCTION
Homeostasis of most, if not all, tissues is maintained by the self-renewal and differentiation of stem cells. Spermatogenesis is a model tissue-specific stem cell system in which self-renewal and differentiation of spermatogonial stem cells (SSCs) forms the foundation for continual male fertility [1]. Currently, SSCs are the only tissue-specific stem cell population in mammals with the availability of a long-term culture system that supports their self-renewal and differentiation [2], and a robust transplantation method to unequivocally measure stem cell number and activity in an experimental cell population [3–5]. Specific markers of SSCs have not been identified making the study of these cells in vivo is challenging. However, functional transplantation in which SSCs colonize recipient testes and reestablish spermatogenesis is an efficient assay to study stem cell content and functionality in an experimental cell population. In addition, THY1 or CD90 has been identified as a surface marker of SSCs in rodents [6], nonhuman primates [7], and cattle [8]. Isolation of the THY1+ testis cell fraction results in enrichment of SSCs and culture of mouse THY1+ germ cells in serum-free conditions with supplementation of glial cell line-derived neurotrophic factor (GDNF) supports expansion of SSC numbers for extended periods of time [2]. Within these THY1+ germ cell cultures both SSC self-renewal and differentiation is supported which provides a model to identify and study mechanisms regulating SSC fate decisions. Because of the heterogeneity of SSC content in cultures of THY1+ germ cells experimental manipulations must be coupled with transplantation analyses to examine specifically effects on SSCs versus the other non-stem cell germ cells and effects on self-renewal versus differentiation or survival. Over the last decade several molecules that regulate SSC self-renewal have been identified and studied using transplantation analyses and germ cell cultures [9–15]; however, mechanisms that regulate SSC differentiation remain unknown.
The undifferentiated spermatogonial population in mouse testes consists of type Asingle (As), Apaired (Apr), and Aaligned (Aal) germ cells [16–18]. Traditionally, As spermatogonia have been considered the SSC population in vivo. During steady-state spermatogenesis, two major steps of differentiation occur within the undifferentiated spermatogonial population. First, SSC differentiation produces Apr, followed by Aal4, Aal8, and Aal16 spermatogonia [16, 18]. Second, the Aal16 spermatogonia differentiate into type A1 spermatogonia, which subsequently give rise to type A2, A3, A4, intermediate, and B spermatogonia. Collectively, type A1-A4, intermediate and B germ cells are referred to as differentiating spermatogonia. The transcriptional repressor PLZF is expressed by the undifferentiated spermatogonial population, but not differentiating spermatogonia in the male mouse germline [9, 10]. In addition, a distinguishing feature of the transition from undifferentiated to differentiating spermatogonia is the attainment of KIT expression by Aal16, and possibly Aal8 spermatogonia, which persists in A1 through B spermatogonia [19–21]. Currently, specific molecular makers that distinguish Apr and Aal4–16 spermatogonia from SSCs (As spermatogonia) have not been described; however, transplantation analyses provide a means for unequivocal distinction of SSCs from non-stem cell spermatogonia in experimental cell populations.
In the Drosophila male germline, stem cell self-renewal is critically dependent on JAK/STAT signaling [22, 23]. Unlike invertebrates, mammals express five different STAT isoforms of which STAT3 has been identified as a central regulator of mouse embryonic stem (ES) cell pluripotency and self-renewal [24, 25]. Stimulation by the key cytokines leukemia inhibitory factor (LIF) and basic fibroblast growth factor (bFGF [official symbol, FGF2]) leads to down-stream activation of STAT3 via phosphorylation of the tyrosine 705 (p-Tyr705) residue. In the germline of neonatal mouse testes expression of STAT3 is localized to gonocytes (precursor germ cells of undifferentiated spermatogonia) and the undifferentiated spermatogonial population which contains SSCs [26]. However, the role of STAT3 in spermatogenesis or male germ cell biology has not been discovered. The objective of this study was to determine the role of STAT3 signaling in regulating function of mammalian SSCs.
MATERIALS AND METHODS
Animals and Reagents
Donor mice for establishing SSC cultures were B6;129S-Gt(ROSA)26Sor/J (designated ROSA; The Jackson Laboratory) at 6 days of age. All germ cell stages in these mice express a LacZ transgene and are easily identifiable in recipient seminiferous tubules following transplantation. Stat3 and nontargeting control short interfering RNA (siRNA) oligonucleotides were purchased from Sigma Inc. (St. Louis, MO). Sequences of the Stat3 siRNA oligonucleotides are sense: 5′-GGACGACUUUGAUUUCAAC-3′ and antisense: 5′-GUUGAAAUCAAAGUCGUCC-3′. Stat3 and control short hairpin RNAs (shRNAs) were also purchased from Sigma Inc. Cell-permeable STAT3 Inhibitor Peptide (cat. no. 573095), Inactive Control Peptide (cat. no. 573105), and AG490 were purchased from Calbiochem Inc. (Gibbstown, NJ). To inhibit STAT3 signaling, cultured THY1+ germ cells were treated with 1 mM of STAT3 inhibiting peptide or 5 μM of AG490, and controls were treated with 1 mM of control peptide or equal volume of DMSO (solvent for AG490). Antibodies for STAT3 and pTyr705 STAT3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All animal procedures were approved by the University of Pennsylvania or Pennsylvania State University Institutional Animal Care and Use Committees.
Flow Cytometric Analyses of KIT Expression
Cultured THY1+ germ cells were removed from SIM mouse 6-thioguanine and ouabian resistant embryonic fibroblast feeder cells (STO) by gentle pipetting and digested with trypsin-ethylenediamine-tetraacidic acid (EDTA) to generate single cell suspensions. Cells were resuspended in DPBS-S (PBS with 1% fetal bovine serum, 10 mM Hepes, 1 × 104 U/ml penicillin, 1 × 104 μg/ml streptomycin, 1 mM sodium pyruvate, and 1 mg/ml glucose) and incubated with monoclonal rat anti-mouse KIT (c-KIT) antibody conjugated to phycoerythrin/Cy5 (1:100; AbCam, Cambridge, MA) on ice for 20 min. Cells were then washed three times with DPBS-S and analyzed with a Guava Easy Cyte Plus flow cytometer (Millipore Corp., Billerica, MA). Controls were cells not incubated with primary antibody. Single cell suspensions of seminiferous tubules from adult mice were used as a positive control for KIT staining. Testes were digested with collagenase (1 mg/ml in Hanks Balanced Salt Solution [HBSS]) to separate seminiferous tubules, allowed to settle on ice, and the supernatant removed. Tubules were then washed five times in HBSS with settling on ice and removal of supernatant each time to eliminate interstitial cells. Single cell suspensions of tubules were then created by digestion with trypsin-EDTA.
THY1+ Germ Cell Cultures
Isolation of THY1+ germ cells from 6-day-old ROSA mice was accomplished by magnetic activated cell sorting, as described previously [2, 5]. Cells were then washed in mouse SSC serum-free medium (mSFM) [2] and plated onto STO feeders in mSFM supplemented with 20 ng/ml recombinant human GDNF (R&D Systems Inc., Minneapolis, MN) and 1 ng/ml recombinant human FGF2 (bFGF) (BD Biosciences, San Jose, CA). Cultures were maintained in these conditions at 37°C in an atmosphere of 5% CO2 in air and subcultured at 1:2 to 1:3 ratios onto fresh STO feeders every 7 days. Primary cultures were used for experiments between 1 and 3 mo after establishment.
siRNA Transfections and Lentiviral shRNA Transductions
Cultured THY1+ germ cell clumps were separated from STO feeders by gentle pipetting which yields a cell population that is greater than 90% pure THY1+ germ cells [8]. Single cell suspensions were then created by trypsin-EDTA digestion and 1–2 × 105 cells were seeded onto new STO feeders in mSFM with GDNF and FGF2 supplementation. For transient siRNA treatments, cells were transfected with either nontargeting control or predesigned Stat3-specific siRNA oligonucleotides overnight by lipofection, as described previously [11, 12]. All predesigned siRNAs were purchased from Ambion Inc. (a division of Applied Biosystems, Carlsbad, CA). Cells were then washed with HBSS and fresh mSFM with GDNF, and FGF2 was added. After 7 days of culture, single cell suspensions were collected by digestion with trypsin-EDTA and suspended in mSFM at a concentration of 1 × 106 cells/ml for transplantation analyses. In addition, the total number of germ cells within each sample was counted with a hemacytometer. Cultures consist of STO feeders and clump-forming THY1+ germ cells only, and differences in cellular morphology between the two cell populations allow for distinguishing them microscopically. In addition, all clump-forming cells express markers of undifferentiated spermatogonia. Thus, counting the number of round germ cells allows for quantification of spermatogonial numbers in each sample. For stable shRNA transductions, cells were exposed overnight to either nontargeting control or Stat3-specific lentiviral particles in mSFM supplemented with GDNF, FGF2, and polybrene (8 μg/ml). All predesigned shRNAs (MISSION shRNAs) were purchased from Sigma Inc. The vector for both the Stat3 and nontargeting control shRNA was pLKO.1-puro, which incorporates the human U6 promoter to drive transcription of shRNA sequences and the puromycin resistance gene for selection of stably transduced cells. On the next day after exposure to lentiviral particle, cells were washed three times with HBSS and fresh mSFM containing GDNF; FGF2 and puromycin (1 μg/ml) was then added to the cells. Cells with stable incorporation of the lentiviral shRNA expression construct were selected by incubation with puromycin for 6 days. For transplantation analyses, single cell suspensions were collected by trypsin-EDTA digestion, washed, and suspended in mSFM at a concentration of 2 × 106 cells/ml. The experiment was repeated two times with different primary cultures of THY1+ germ cells.
Quantitative RT-PCR Analyses
Following overnight siRNA transfection or 6-day selection after lentiviral shRNA transduction, RNA was isolated from cultured THY1+ germ cells with Trizol reagent (Invitrogen). All samples were then treated with DNase to remove possible contaminating genomic DNA. The purity of RNA was determined based on spectrophotometric analyses of 260:280 ratios, and only samples with a value of 1.8 or higher were used for subsequent PCR analyses. For each sample, 500 ng of RNA was reverse transcribed by oligo(d)T priming and M-MLV reverse transcriptase. Quality of the resulting cDNAs were determined with conventional PCR analyses for glyceraldehyde 3-phosphate dehydrogenase (Gapdh) expression and agarose gel electrophoresis. SYBR green assays were conducted with an ABI 7500 sequence detection system to determine relative Stat3 gene expression. Quantitative comparisons between treatments were made by normalizing relative expression of Stat3 to the expression of ribosomal protein S2 (Rps2) in each sample, as described previously [8, 9]. Primer pairs used were: Gapdh, 5′ AACTTTGGCATTGTGGAAGGGCTC 3′, 5′ TGGAAGAGTGGGAGTTGCTGTTGA 3′; Rps2, 5′ CCATGCCTCATCACTTACCCTAT 3′, 5′ GTCCGGAAGAGCTTGCAGAA 3′; and Stat3, 5′ GACCTGCAGCAATACCATTGAC 3′, 5′ CCGTTATTTCCAAACTGCATCA 3′.
Western Blot Analyses
For protein analyses, THY1+ germ cells were separated from STO feeders by gentle pipetting and cells were suspended in lysis buffer containing protease and phosphatase inhibitors. Approximately 30 μg of total protein was separated by SDS-PAGE and transferred to nitrocellulose membranes. Blots were then blocked in PBS containing 5% bovine serum albumin (BSA) and incubated overnight with goat anti-human pTyr705-STAT3 primary antibody at 4°C. On the next day, blots were washed in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) and incubated with horseradish peroxidase-conjugated donkey anti-goat IgG secondary antibody for 2 h at room temperature. Blots were again washed in TBS-T, developed with a chemiluminescent substrate, and viewed with a ChemiDoc imager (Bio-Rad, Hercules, CA). Digital images were captured for further analyses, and blots were striped for incubation with rabbit anti-human STAT3 or rabbit anti-human tubulin-beta antibody to detect total STAT3 or tubulin-beta within each sample.
Immunocytochemistry Analyses
For examination of PLZF expression by cultured THY1+ germ cells, clumps were removed from STO feeders by gentle pipetting and single cell suspensions created by trypsin-EDTA digestion. Cells were then adhered to poly-lysine-coated glass coverslips and fixed in 4% paraformaldehyde (PFA) for 10 min at room temperature, followed by incubating in PBS containing 0.1% Triton X-100 for permeabilization. Cells were then incubated for 30 min in PBS with 10% normal goat serum to block for nonspecific antibody binding. To detect PLZF, cells were then incubated with rabbit anti-human PLZF primary antibody (1:50; Santa Cruz Biotechnology). Secondary detection included incubation with Alexa 488-conjugated goat anti-rabbit IgG antibody (1:1000; Santa Cruz Biotechnology). Coverslips were then mounted on glass slides with aqueous mounting medium containing 4′,6′-diamidino-2-phenylindole (DAPI) to stain cell nuclei. Controls consisted of cells incubated with normal rabbit IgG in place of primary antibody. For determination of PLZF-expressing cells, coverslips were viewed by fluorescent microscopy at 20× magnification, and the number of fluorescent green-stained cells was counted in five random fields of view, followed by counting the number of DAPI-stained nuclei within each field. Only round germ cells with nuclear staining of PLZF expression were counted. The percentage of PLZF-expressing cells for each sample was determined by dividing the number of PLZF+ cells by the number of corresponding DAPI-stained cells. For examination of STAT3 expression, THY1+ germ cell clumps were fixed in 4% PFA for 10 min at room temperature, followed by incubation in ice-cold acetone for permeabilization. Cells were then incubated with 10% normal goat serum to block nonspecific binding, followed by incubation with rabbit anti-mouse STAT3 primary antibody (1:200; Santa Cruz Biotechnology). Cells were then washed and incubated with Alexa 488-conjugated goat anti-rabbit IgG secondary antibody (1:1000; Invitrogen), followed by viewing with a fluorescent microscope, and digital images were captured. Cells incubated with normal rabbit IgG in place of primary antibody served as a negative control. All fluorescently stained samples were viewed at room temperature by fluorescent microscopy (Leica AxioScope 2 Plus; Leica, Bannockburn, IL) with 20×, 0.5 NA objective lenses (Zeiss Neo Fluar; Carl Zeiss Inc., Thornwood, NY), and digital images were captured with a DP2 camera and software (Olympus Inc., Center Valley, PA). Adobe Photoshop software (Adobe Inc., San Jose, CA) was used to assemble images into figures. No postacquisition modifications were made to the original images.
SSC Transplantations
SSC content and activity of experimental cell populations was examined by functional transplantation into the seminiferous tubules of recipient mice, as described previously [3–5]. Briefly, single cell suspensions were suspended in mSFM at a concentration of 1 × 106 cells/ml and 8–10 μl of cell suspension was microinjected into each recipient testis via the rete. LacZ-expressing ROSA donors were used for all experiments to allow for visualization of re-established spermatogenesis based on blue staining following incubation with X-Gal. All recipient testes were evaluated for re-establishment of colonies of spermatogenesis 2 mo after transplantation. Each colony of donor-derived spermatogenesis is clonally derived from a single stem cell [27]; thus, counting colonies provides an accurate measure of cells with SSC potential within an experimental cell population. To make quantitative comparisons between treatments, the number of colonies was determined manually using a dissecting microscope, and digital images were captured.
Statistical Analyses
Differences between means were determined by the mixed-model analysis of variance function of SPSS statistical software (SPSS Inc., Chicago, IL). A P value of ≤0.05 was considered significant.
RESULTS
The Cultured Mouse THY1+ Germ Cell Population Consists of SSCs and Other Undifferentiated Spermatogonia
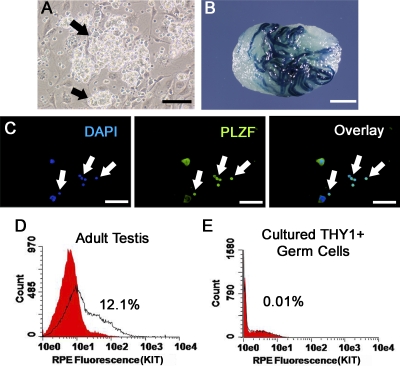
To examine whether STAT3 plays a role in SSC functions, we utilized cultures of THY1+ spermatogonia established from prepubertal ROSA mice that express a marker LacZ transgene in germ cells. When maintained in serum-free conditions and supplemented with GDNF and FGF2, THY1+ spermatogonia form clumps of germ cells consisting of SSCs and non-stem cells (Fig. 1A). Colonies of spermatogenesis are produced upon transplantation of these cells, demonstrating the presence of SSCs (Fig. 1B). However, the cultured cell populations are not pure SSCs, and likely also contain other undifferentiated spermatogonia [2, 28]. To define further the non-SSC component, we examined the cultured clump-forming germ cells for expression of markers for undifferentiated and differentiating spermatogonia. First, expression of the undifferentiated spermatogonial marker PLZF was observed in greater than 95% of the clump-forming germ cells upon analysis of single cell suspensions by immunocytochemistry (Fig. 1C). Second, expression of the differentiating spermatogonial marker KIT by clump-forming germ cells was examined by flow cytometric analyses. While KIT (c-KIT)-positive cells were readily detected in total testis cell populations from adult mice (Fig. 1D), essentially no KIT+ cells could be detected in THY1+ clump-forming germ cell populations (Fig. 1E). Collectively, these observations demonstrate that the THY1+ clump-forming germ cell population is composed of undifferentiated spermatogonia. Thus, both self-renewal and differentiation of SSCs is supported in serum-free medium conditions, with GDNF and FGF2 supplementation providing an excellent model system to study mechanisms regulating SSC functions, including determining the role of STAT3. However, the only means to distinguish between these different cell populations is by transplantation in which only the SSCs will colonize and re-establish spermatogenesis in recipient testes.
FIG. 1.
Characterization of spermatogonial subtypes in cultured THY1+ germ cells. A) Germ cell clumps (indicated by arrows) formed by THY1+ germ cells from 6-day-old mice maintained in serum-free medium with GDNF and FGF2 supplementation. Bar = 100 μm. B) Colonization of a recipient testis microinjected with cultured THY1+ germ cells. Colonies of spermatogenesis derived from the cultured cells provide unequivocal evidence of SSC presence in the cultured clump-forming THY1+ germ cell population. Bar = 2 mm. C) Representative images from immunocytochemical analysis of single-cell suspensions of cultured THY1+ germ cells for expression of the marker PLZF, which is expressed by As (SSC), Apr, and Aal spermatogonia. Arrows indicate PLZF+ cells. On average, 95.7 ± 5% (n = 3 different primary cultures examined) of cells were determined to be PLZF+. Bars = 100 μm. D and E) Representative histogram plots for examination of KIT expression by cultured THY1+ germ cells. Single cell suspensions from seminiferous tubules of adult mice were used as a positive control for staining of the differentiating spermatogonial marker KIT. Cells expressing KIT were readily identified in positive controls, but essentially no KIT+ cells were identified in cultured THY1+ germ cells (n = 2 different primary cultures examined), indicating lack of differentiating spermatogonia in cultured clump-forming THY1+ germ cells. Red fill represents unstained control, and open area under black line represents KIT staining. Collectively, the finding that nearly all cells express PLZF, some cells colonize recipient testes, and that there is a lack of detectable KIT staining demonstrates that the cultured THY1+ germ cell population consists of a heterogeneous mixture of undifferentiated spermatogonia composed of SSCs and non-SSCs.
Disruption of STAT3 Signaling Enhances SSC Self-Renewal In Vitro
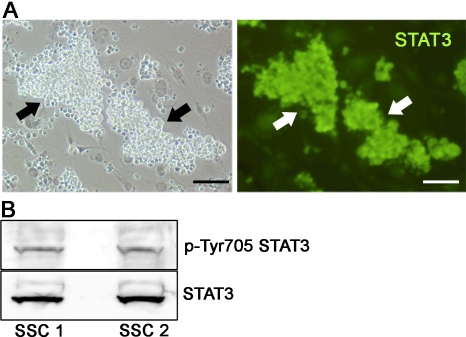
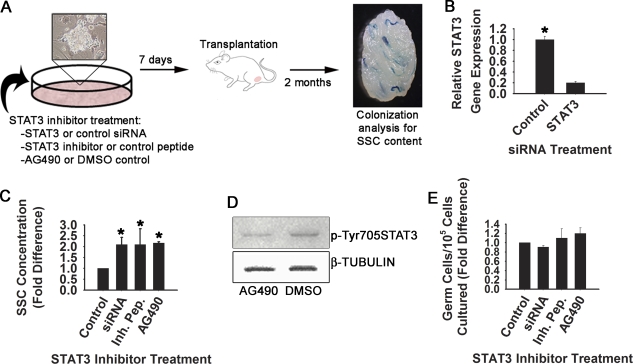
Through immunofluorescent staining, the expression of STAT3 was observed by clumps of THY1+ germ cells (Fig. 2A), and Western blot analysis revealed the presence of p-Tyr705 STAT3, suggesting an activated STAT3 signaling mechanism within this heterogeneous undifferentiated spermatogonial population that contains SSCs (Fig. 2B). Because Stat signaling is essential for self-renewal of germline stem cells in Drosophila, and STAT3 is a major regulator of ES cell pluripotency, this observation suggests that the function of mouse SSCs may also be regulated by a similar signaling mechanism. To examine this possibility, STAT3 function was transiently impaired in cultured THY1+ spermatogonia by three different methods. The effects on SSC content specifically were then examined after 7 days using functional transplantation as an endpoint of analysis (Fig. 3A). This timeline covers greater than one self-renewal cycle of 5.6 days for SSCs in cultured THY1+ germ cell populations [2]. With this assay, decrease of SSC numbers following experimental manipulation indicates impaired self-renewal and survival, whereas increased SSC content suggests enhanced self-renewal. First, siRNA treatment was used to reduce expression of Stat3 in cultured THY1+ germ cells, resulting in greater than 80% reduction of Stat3 gene expression compared with cells treated with control siRNA (Fig. 3B). Transplantation analyses revealed an increase of SSC content by greater than 2-fold in Stat3 siRNA-treated cells compared with those treated with control siRNA (Fig. 3C). Second, cultured THY1+ germ cells were treated with a cell-permeable STAT3 inhibitor peptide, and transplantation analyses also revealed a greater than 2-fold increase of SSC content after one self-renewal cycle compared with cells treated with an inactive control peptide (Fig. 3C). Third, cultured THY1+ germ cells were exposed to the pharmacological inhibitor AG490, which binds the up-stream effecter JAK2 to prevent down-stream activation of STAT3. Treatment with a low dose of 5 μM AG490 effectively impaired STAT3 phosphorylation in cultured THY1+ germ cells (Fig. 3D). Normalization to the expression level of tubulin-beta revealed that treatment with AG490 reduced the level of phosphorylated STAT3 to 32% of that in control cells treated with DMSO only. Similar to both siRNA and inhibitor peptide treatments, after one self-renewal cycle, transplantation analyses revealed an increase of over 2-fold in SSC content of THY1+ germ cells treated with AG490 compared with vehicle-treated controls (Fig. 3C). Importantly, impairment of STAT3 did not significantly alter total germ cell numbers for any of the treatments compared to controls, suggesting lack of a general effect on germ cell proliferation or survival (Fig. 3E).
FIG. 2.
Identification of STAT3 expression in cultured THY1+ germ cells. A) Immunocytochemical examination of STAT3 protein expression by cultured clump-forming THY1+ germ cells. Arrows indicate STAT3-expressing germ cell clumps. Bar = 100 μm. B) Western blot examination of activated STAT3 protein in cultured THY1+ germ cells. The activated form of STAT3 with posphorylation at tyrosine residue 705 was detected in cell lysates from cultured germ cell clumps. The top panel is the result of analysis for activated STAT3, and the bottom panel represents reprobing of the same blot for total STAT3. Two different primary cultures of THY1+ germ cells, SSC1 and SSC2, were examined for expression of activated STAT3.
FIG. 3.
Effects of impaired STAT3 function on SSC activity in cultured THY1+ germ cell populations. A) Experimental strategy for examining the role of STAT3 in SSC functions in vitro. Cultured THY1+ germ cells were treated with Stat3 siRNA, a cell-permeable STAT3 inhibitor peptide, or the pharmacological inhibitor of STAT3, AG490. Controls were cells treated with nontargeting siRNA, an inactive peptide, or DMSO. After 7 days, which is slightly greater than one SSC self-renewal cycle of 5.6 days in cultured THY1+ germ cell cultures, SSC content of experimental cell populations was determined by transplantation into recipient mouse testes that were then examined for colonization 2 mo later. Quantification of colony number is a direct measure of SSC content in a microinjected cell suspension. B) Quantitative RT-PCR analyses of relative Stat3 gene expression 24 hours after treatment with Stat3 or control siRNA. Stat3 treatment resulted in 80.2 ± 2.6% (n = 3 different primary cultures) reduction of Stat3 expression compared with control siRNA treatment. C) Quantification of SSC concentration in cultures of THY1+ germ cells treated with different STAT3 inhibitors or control treatments after one self-renewal cycle. SSC numbers are derived from transplantation analyses, and are expressed as fold difference compared to control. SSC content was 2.1± 0.3-, 2.1 ± 0.7-, and 2.2 ± 0.1-fold greater for siRNA-, inhibitor peptide-, or AG490-treated cells compared with controls, respectively. Data are mean ± SEM for two independent experiments of different primary cultures of THY1+ germ cells and 16–20 transplanted recipient mouse testes. *Significantly different from control at P ≤ 0.05. SSC numbers for all controls are set at 1, and SSC numbers for treatments are presented as fold difference compared to the corresponding control. D) Representative images of Western blot analysis for phosphorylated STAT3 expression in cells treated with the pharmacological inhibitor AG490, or DMSO as the vehicle control. The top panel represents expression of STAT3 phosphorylated at tyrosine residue 705, and the bottom panel represents reprobing of the same blot for expression of the housekeeping gene Tubulin-beta. E) Quantification of total germ cell numbers in cultures of THY1+ germ cells treated with different STAT3 inhibitors or control treatments after one self-renewal cycle of 7 days. Data are mean ± SEM for two independent experiments of different primary cultures of THY1+ germ cells, and are derived from the same cell populations used for transplantation analyses. Germ cell numbers for all controls are set at 1, and germ cell numbers for treatments are presented as fold difference compared to the corresponding control.
Impairment of STAT3 Signaling in SSCs Disrupts the Capacity for In Vivo Differentiation and Regeneration of Spermatogenesis
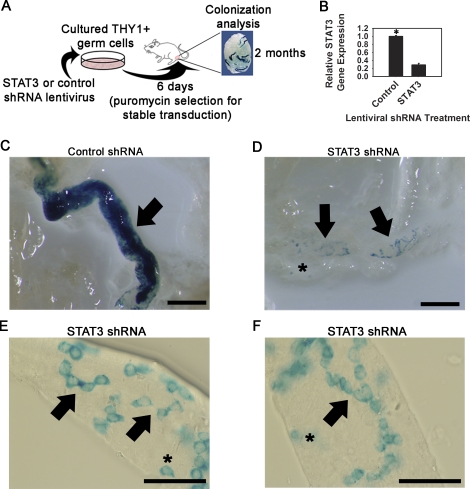
Next, we aimed to determine whether STAT3 expression also plays a role in SSC differentiation in vivo. Global inactivation of STAT3 in mice results in embryonic lethality, and is not a feasible model for examining postnatal germ cell function [29]. To overcome this limitation, cultured ROSA THY1+ germ cells were stably transduced with shRNA expression constructs via lentiviral infection to impair expression of Stat3, followed by transplantation into recipient mouse testes to examine SSC colonization and re-establishment of spermatogenesis (Fig. 4A). Stable transduction with a Stat3 shRNA lentivirus resulted in 72.1 ± 4.1% (n = 2 experiments performed with different cultures) reduction of Stat3 gene expression compared with cells transduced with nontargeting control shRNA lentivirus (Fig. 4B). The number of germ cells recovered from control (4.8 ± 0.5 × 104 cells) and Stat3 shRNA (4.3 ± 0.6 × 104 cells) treatments were not different (P = 0.31). Following transplantation, control shRNA-transduced cells generated colonies of complete spermatogenesis, evidenced by dense blue staining within recipient seminiferous tubules (Fig. 4C). In contrast, Stat3 shRNA-transduced cells generated colonies consisting only of cohorts of spermatogonia (Fig. 4D). No dense colonies of complete spermatogenesis were observed in any recipient testis (n = 6) transplanted with Stat3 shRNA-transduced cells. Colonies ranging from single cells to chains of no greater than 16 spermatogonia were observed, indicating that STAT3 functions at multiple levels of differentiation (Fig. 4, E and F). These results demonstrate that STAT3 plays a critical role in SSC differentiation in vivo, and confirm the role of STAT3 in SSC differentiation identified through in vitro studies with THY1+ germ cells.
FIG. 4.
Effects of impaired STAT3 expression on re-establishment of spermatogenesis following spermatogonial transplantation. A) Schematic of the experimental strategy to examine the effects of impaired STAT3 expression on spermatogonial activity in vivo. B) Quantitative RT-PCR analysis of Stat3 gene expression in cultured THY1+ germ cells stably transduced with control or Stat3 shRNA lentivirus. C) Representative image of a recipient mouse seminiferous tubule transplanted with cultured THY1+ ROSA germ cells transduced with nontargeting control shRNA lentivirus. Densely blue-stained colonies (arrow) indicate complete spermatogenesis derived from an injected SSC. D–F) Representative images of recipient mouse seminiferous tubules transplanted with cultured THY1+ ROSA germ cells transduced with Stat3 shRNA lentivirus. Colonies ranging from a single cell (asterisks) to chains of spermatogonia (arrows) were observed, but no densely stained colonies were identified. Chains of spermatogonia no greater than 16 cells were observed. These observations indicate impaired differentiation of As and Apr/Aal spermatogonia. Bars = 2 mm (C and D) and 0.5 mm (E and F).
DISCUSSION
Investigating the mechanisms that regulate SSC fate decisions in vivo is challenging due to rarity of the cells and lack of known specific markers. Use of in vitro systems that support the self-renewal and differentiation of SSCs where expression and function of specific proteins can be manipulated are ideal models for overcoming this limitation. Culture of THY1+ germ cells from mouse testes in serum-free conditions with GDNF and FGF2 supplementation only supports SSC self-renewal for extended periods of time; however, the cultures are not composed purely of SSCs, with a non-stem cell component that comprises the majority of the cell population [2]. In this study, we show that nearly all of this cell population expresses PLZF, a marker of undifferentiated spermatogonia, but not KIT, which is a marker of differentiating spermatogonia. Expression of KIT has classically been assigned to differentiating spermatogonia in mouse testes; however, studies by Morimoto et al. [30] suggest that some KIT+ cells in cultures of germ cells derived from gonocytes (termed GS cells) have stem cell capacity to regenerate spermatogenesis. Primary cultures of GS cells utilized by Morimoto et al. [30] were derived from donor mice at 0 days of age. At this stage of development, the germ cell population is composed of KIT+ and KIT− gonocytes that have not transitioned into spermatogonia [31]. Thus, KIT+ GS cells that re-establish spermatogenesis following transplantation are likely derived from KIT+ gonocytes originally seeded in culture, and these cells may not reflect the biology of KIT+ spermatogonia that are found in mouse testes after the gonocytes have transitioned into spermatogonia. In contrast, THY1+ germ cell cultures utilized in the current study were from donor mice at 6 days of age, which is a developmental stage at which all gonocytes have transitioned into spermatogonia. Findings in the current study indicate that the cultured THY1+ germ cell population consists of both SSCs and other non-stem cell undifferentiated spermatogonia. Collectively, these findings indicate that both SSC self-renewal and differentiation occurs within cultured THY1+ germ cell populations. Recently, studies by Wu et al. [32] also found that both SSC self-renewal and differentiation occurs in a culture system that supports long-term maintenance of rat SSCs. Use of these systems for rodent undifferentiated spermatogonia can provide models for making new discoveries of mechanisms regulating SSC fate decisions. However, due to the lack of known markers that distinguish SSCs from the non-stem cell spermatogonia, functional transplantation experiments must be used in conjunction with experimental manipulation of the cultured cells to confirm effects on SSC directly.
By using the culture system for mouse THY1+ spermatogonia and functional transplantation methodology, the current study provides both in vitro and in vivo evidence that STAT3 plays a role at multiple levels of differentiation in the undifferentiated spermatogonial population. In vitro experiments showed that impairment of STAT3 signaling increased SSC concentration specifically, without effecting spermatogonial proliferation overall. This finding suggests that the increase of stem cell content was not due to enhanced proliferation or survival of the total germ cell population. Thus, the effects of impaired STAT3 signaling altered the balance of SSC fate decisions in vitro, preventing differentiation in favor of a greater frequency of self-renewal. In vivo experiments showed that SSCs deficient for STAT3 expression were incapable of re-establishing spermatogenesis after transplantation, but could undergo initial colonization. Single cells within recipient testes were likely derived from stably transduced SSCs that did not progress to Apr or Aal spermatogonia. Longer cohorts could have been derived from SSCs in which STAT3 was not completely suppressed, which may be able to proceed through partial differentiation (e.g., from As to Aal), but fail to proceed beyond this point of development. Collectively, the results of these experiments indicate that STAT3 is an important regulator of undifferentiated spermatogonial differentiation in vivo. Moreover, these findings also indicate that STAT3 completely blocks further differentiation of spermatogonia to meiosis and beyond, because chains of no greater than 16 spermatogonia were observed. Thus, STAT3 is required for spermatogonial differentiation, and may block the ability of the few differentiating spermatogonia that remain from low-level STAT3 to proceed to meiosis.
In the Drosophila male germline, Stat signaling is essential for stem cell renewal [22, 23] and the phenomenon of dedifferentiation [33]. In human and mouse ES cells, activation of STAT3 signaling promotes self-renewal and maintenance of pluripotency [24, 25]. Results of the current study demonstrate that these mechanisms are not conserved in mouse SSCs in which STAT3 functions to promote differentiation. This finding highlights the differences in regulatory mechanisms of stem cell fate decisions between invertebrates and mammals, and stem cells of a pluripotent nature and tissue-specific function. While mechanisms controlling the self-renewal of stem cells have been studied, understanding of those governing differentiation are poorly defined, especially for mammalian SSCs. To our knowledge, the current study is the first to identify a specific molecular regulator of SSC differentiation, a mechanism that may be conserved in other tissue-specific stem cell populations.
Acknowledgments
We thank C. Freeman, R. Naroznowski, and M. Oatley for assistance with animal maintenance. Special thanks to Dr. X. Wu for help with experimentation.
Footnotes
Supported by National Institutes of Health grants HD044445 (to R.L.B.), HD052728 (to R.L.B.), and HD058137 (to J.M.O.), the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation (to R.L.B.), and startup funds from the Pennsylvania State University (to J.M.O.).
REFERENCES
- Oatley JM, Brinster RL.Regulation of spermatogonial stem cell self-renewal in mammals. Ann Rev Cell Dev Biol 2008; 24: 263–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL.Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A 2004; 101: 16489–16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Avarbock MR.Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A 1994; 91: 11303–11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Zimmermann JW.Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A 1994; 91: 11298–11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL.Spermatogonial stem cells. Methods Enzymol 2006; 419: 259–282. [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL.Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci U S A 2003; 100: 6487–6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM, Orwig KE.Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum Reprod 2009; 24: 1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reding SC, Stepnoski AL, Cloninger EW, Oatley JM.THY1 is a conserved marker of undifferentiated spermatogonia in the pre-pubertal bull testis. Reproduction 2010; 139: 893–903. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE.PLZF is required in adult male germ cells for stem cell self-renewal. Nat Genet 2004; 36: 647–652. [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP.Essential role of PLZF in maintenance of spermatogonial stem cells. Nat Genet 2004; 36: 653–659. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Telerant AI, Fearon DT, Brinster RL.Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci U S A 2006; 103: 9524–9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Arabock MR, Brinster RL.Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem 2007; 282: 25842–25851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T.Akt mediates self-renewal division of mouse spermatogonial stem cells. Development 2007; 134: 1853–1859. [DOI] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Morimoto H, Kazuki Y, Takashima S, Oshimura M, Toyokuni S, Shinohara T.Genetic reconstruction of mouse spermatogonial stem cell self-renewal in vitro by Ras-cyclin D2 activation. Cell Stem Cell 2009; 5: 76–86. [DOI] [PubMed] [Google Scholar]
- Sada A, Suzuki A, Suzuki H, Saga Y.The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science 2009; 325: 1394–1398. [DOI] [PubMed] [Google Scholar]
- Huckins C.The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat Rec 1971; 169: 533–557. [DOI] [PubMed] [Google Scholar]
- Oakberg EF.Spermatogonial stem-cell renewal in the mouse. Anat Rec 1971; 169: 515–531. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD.All you wanted to know about spermatogonia but were afraid to ask. J Androl 2000; 21: 776–798. [PubMed] [Google Scholar]
- Manova K, Nocka K, Besmer P, Bachvarova RF.Gonadal expression of c-KIT encoded at the W locus of the mouse. Development 1990; 110: 1057–1069. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, Fujimoto T, Nishikawa S.Role of c-KIT in mouse spermatogenesis: identification of spermatogonia as a specific site of c-KIT expression and function. Development 1991; 113: 689–699. [DOI] [PubMed] [Google Scholar]
- Schrans-Stassen BH, van de Kant HJ, de Rooij DG, van Pelt AM.Differential expression of c-KIT in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology 1999; 140: 5894–5900. [DOI] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT.Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 2001; 294: 2542–2545. [DOI] [PubMed] [Google Scholar]
- Tulina N, Matunis E.Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science 2001; 294: 2546–2549. [DOI] [PubMed] [Google Scholar]
- Niwa H, Burdon T, Chambers I, Smith A.Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev 1998; 12: 2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz R, Lee CK, Cannizzaro LA, d'Eustachio P, Levy DE.Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci U S A 1999; 96: 2846–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Carvajal L, Medico L, Pepling M.Expression of Stat3 in germ cells of developing and adult mouse ovaries and testes. Gene Expr Patterns 2005; 5: 475–482. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Miki H, Ogonuki N, Takehashi M, Morimoto T, Ogura A, Shinohara T.Clonal origin of germ cell colonies after spermatogonial transplantation in mice. Biol Reprod 2006; 75: 68–74. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Iwano T, Lee J, Kazuki Y, Inoue K, Miki H, Takehashi M, Toyokuni S, Shinkai Y, Oshimura M, Ishino F, et al. Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development 2005; 132: 4155–4163. [DOI] [PubMed] [Google Scholar]
- Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S.Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci U S A 1997; 94: 3801–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto H, Kanatsu-Shinohara M, Takashima S, Chuma S, Nakatsuji N, Takehashi M, Shinohara T.Phenotypic plasticity of mouse spermatogonial stem cells. PLoS One 2009; 4: e7909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, Yamamoto M, Suda T, Nabeshima Y.Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol 2004; 269: 447–458. [DOI] [PubMed] [Google Scholar]
- Wu Z, Luby-Phelps K, Bugde A, Molyneux LA, Denard B, Li WH, Süel GM, Garbers DL.Capacity for stochastic self-renewal and differentiation in mammalian spermatogonial stem cells. J Cell Biol 2009; 187: 513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley C, Matunis E.Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science 2004; 304: 1331–1334. [DOI] [PubMed] [Google Scholar]